Abstract
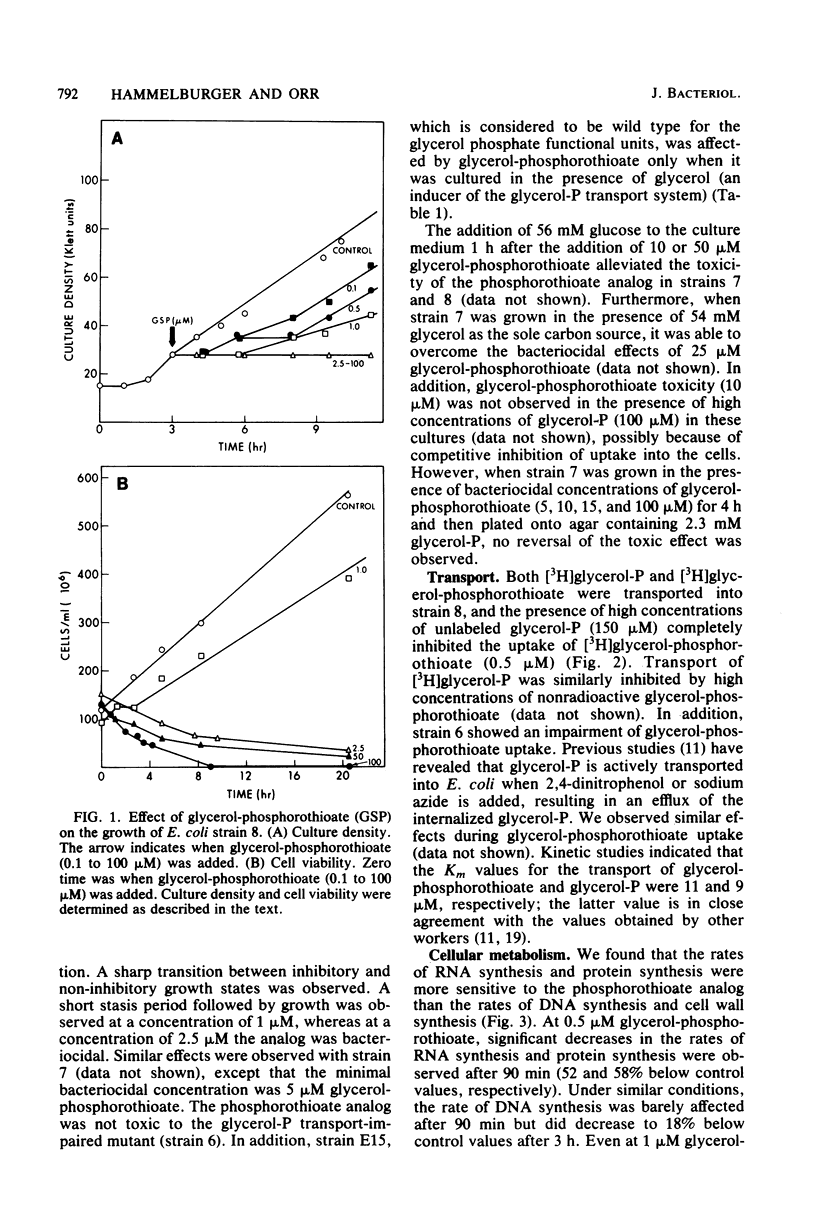
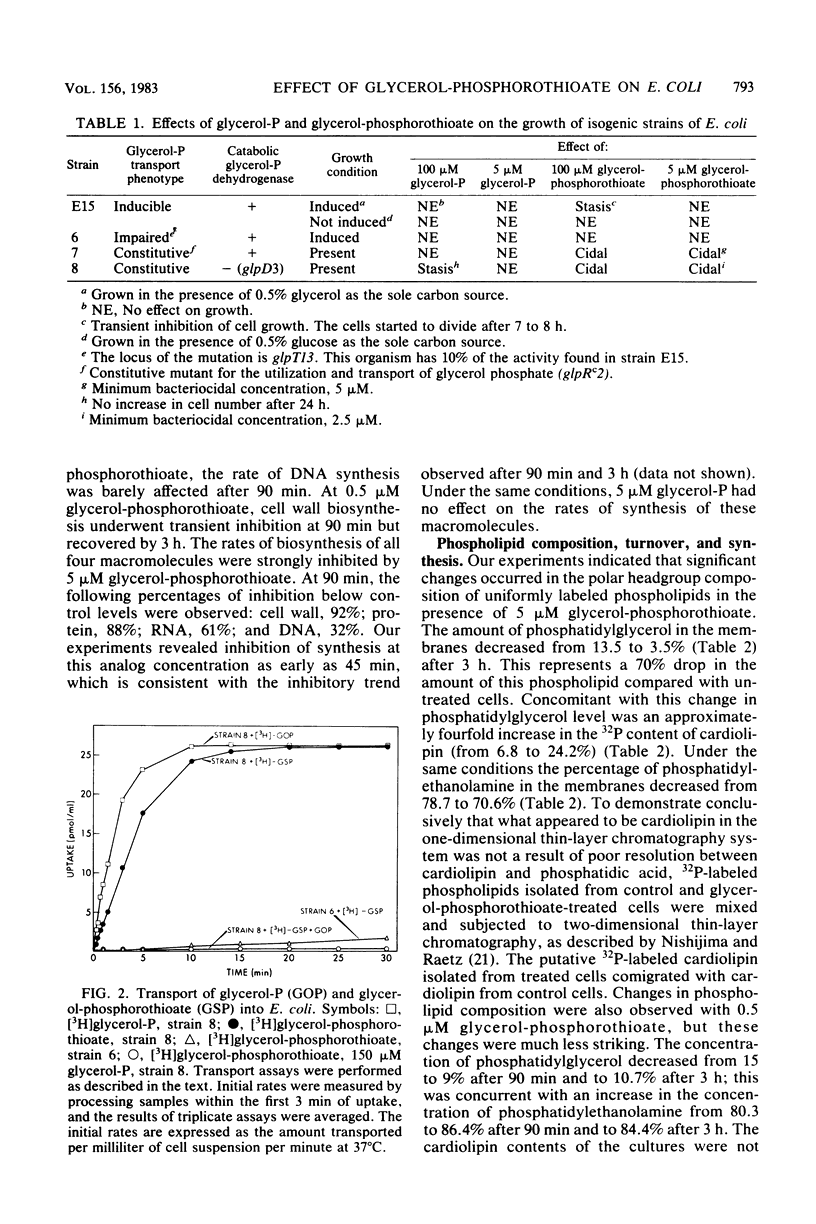
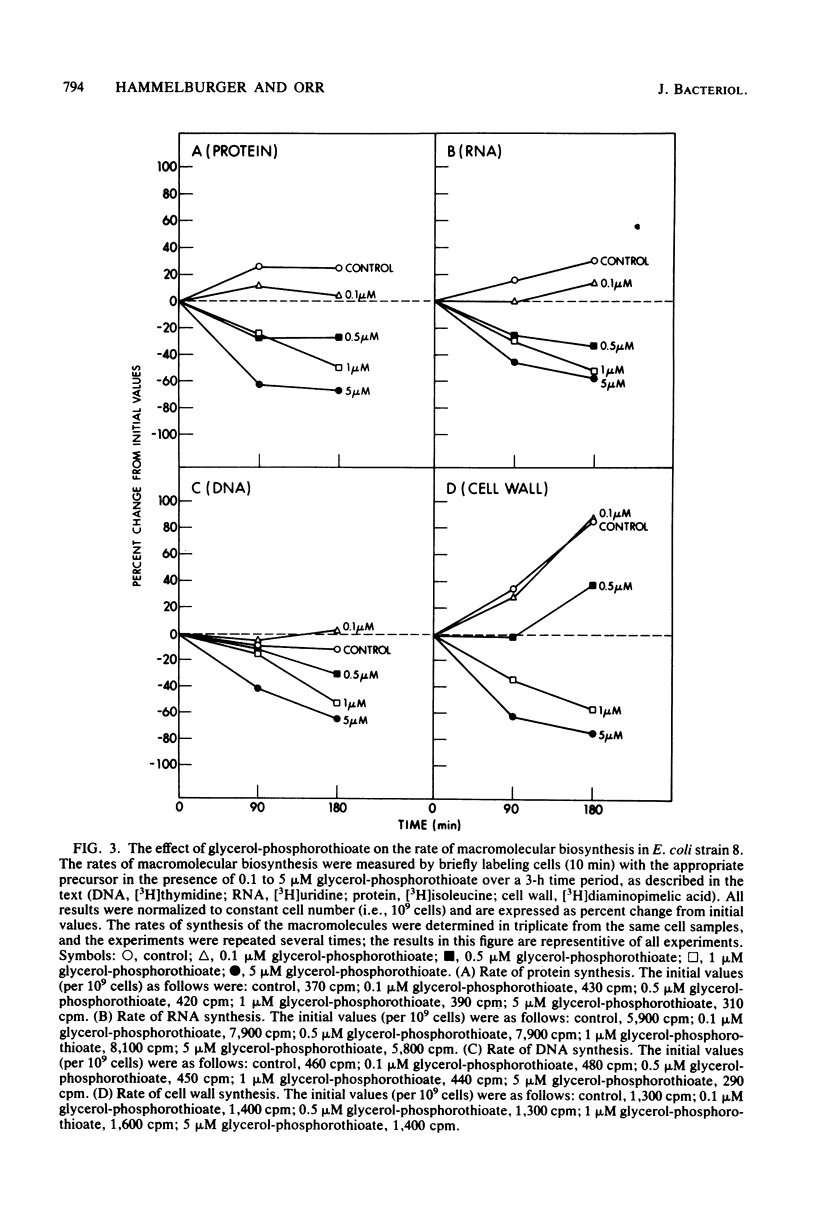
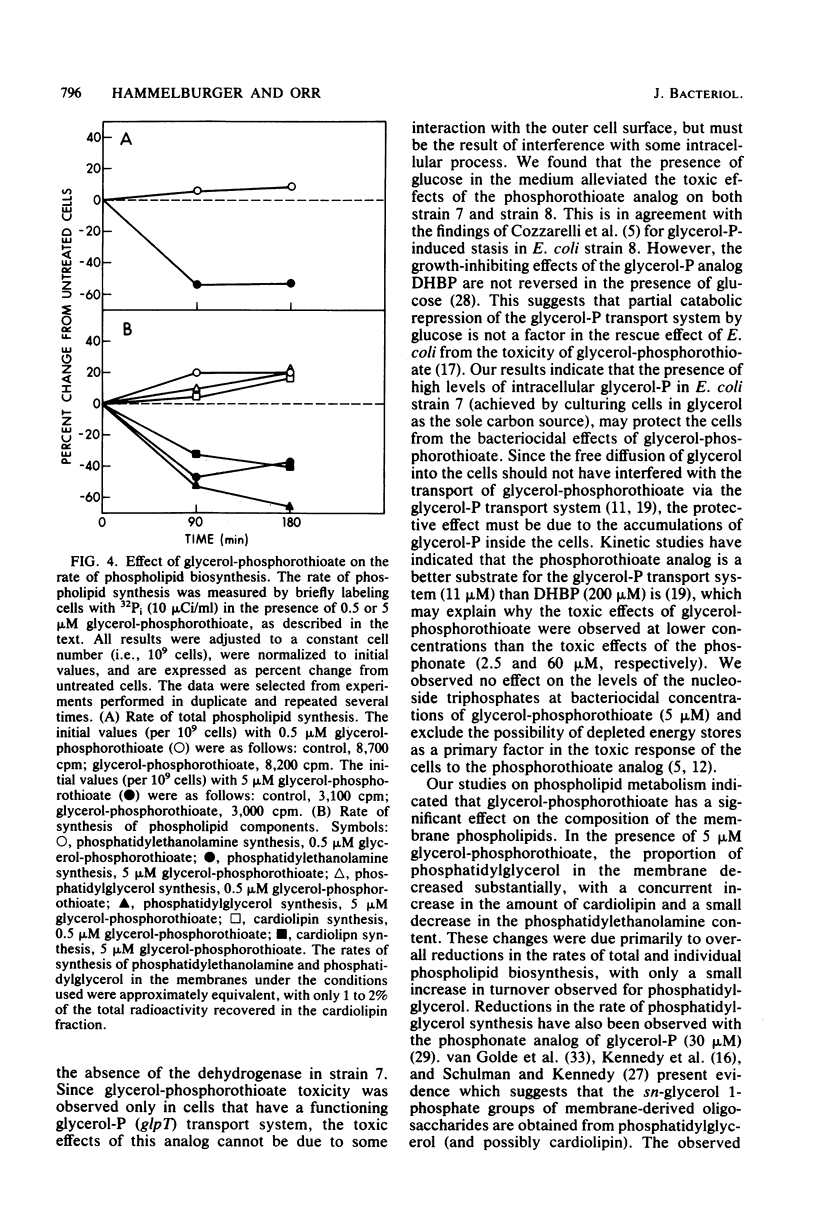
sn-Glycerol 3-phosphorothioate was found to be bacteriocidal to strains of Escherichia coli which have a functional sn-glycerol 3-phosphate transport system. This effect was manifest in strains 7 and 8, which are constitutive mutants for the utilization and transport of sn-glycerol 3-phosphate (glpRc2). Strain E15, which is considered to be wild type for the glycerol phosphate functional units, was affected by the phosphorothioate analog only under conditions that are known to induce the transport system for sn-glycerol 3-phosphate. In addition, another strain of E. coli, strain 6, which is isogenic with strain E15 but has an impaired sn-glycerol 3-phosphate transport system (glpT13), was not affected by similar concentrations of sn-glycerol 3-phosphorothioate. Transport studies in which [3H]glycerol phosphate and its phosphorothioate analog were used demonstrated that the latter compound was taken up via the specific active transport system for sn-glycerol 3-phosphate; the Km values were 9 and 11 microM, respectively. The rates of macromolecular synthesis were found to be inhibited severely by sn-glycerol 3-phosphorothioate at a concentration at which sn-glycerol 3-phosphate had no effect (5 microM). At a lower concentration of the analog (0.5 microM), the rates of protein synthesis and RNA synthesis (52 and 58% below control values after 90 min, respectively) were more sensitive than the rates of DNA synthesis and cell wall synthesis (18% below control values after 3 h for DNA; transient decrease in the cell wall values after 90 min). The levels of the nucleoside triphosphates were not affected by the presence of the phospholipid precursor or its analog at a concentration of 5 microM. The phospholipid composition was significantly altered in the presence of bacteriocidal concentrations (5 microM) of sn-glycerol 3-phosphorothioate. The amount of phosphatidylglycerol in the membranes decreased from 13.5 to 3.5%. Concomitant with this decrease in phosphatidylglycerol content was a fourfold increase in the 32P content of cardiolipin (from 6.8 to 24.2%), whereas the phosphatidylethanolamine content showed only a minor reduction (8%) after 3 h. The rates of synthesis of all of the phospholipids decreased in the presence of 5 microM sn-glycerol 3-phosphorothioate, with the most significant effects observed for phosphatidylglycerol (63% after 3 h). Phosphatidylglycerol showed increased rates of turnover after 90 min (21%) and 3 h (11%), with concomitant increases in the levels of cardiolipin of more than twofold.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames G. F. Lipids of Salmonella typhimurium and Escherichia coli: structure and metabolism. J Bacteriol. 1968 Mar;95(3):833–843. doi: 10.1128/jb.95.3.833-843.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cashel M., Lazzarini R. A., Kalbacher B. An improved method for thin-layer chromatography of nucleotide mixtures containing 32P-labelled orthophosphate. J Chromatogr. 1969 Mar 11;40(1):103–109. doi: 10.1016/s0021-9673(01)96624-5. [DOI] [PubMed] [Google Scholar]
- Contreras I., Shapiro L., Henry S. Membrane phospholipid composition of Caulobacter crescentus. J Bacteriol. 1978 Sep;135(3):1130–1136. doi: 10.1128/jb.135.3.1130-1136.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cozzarelli N. R., Koch J. P., Hayashi S., Lin E. C. Growth stasis by accumulated L-alpha-glycerophosphate in Escherichia coli. J Bacteriol. 1965 Nov;90(5):1325–1329. doi: 10.1128/jb.90.5.1325-1329.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronan J. E., Jr Molecular biology of bacterial membrane lipids. Annu Rev Biochem. 1978;47:163–189. doi: 10.1146/annurev.bi.47.070178.001115. [DOI] [PubMed] [Google Scholar]
- Cronan J. E., Jr Phospholipid alterations during growth of Escherichia coli. J Bacteriol. 1968 Jun;95(6):2054–2061. doi: 10.1128/jb.95.6.2054-2061.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fourcans B., Jain M. K. Role of phospholipids in transport and enzymic reactions. Adv Lipid Res. 1974;12(0):147–226. doi: 10.1016/b978-0-12-024912-1.50011-9. [DOI] [PubMed] [Google Scholar]
- GAREN A., LEVINTHAL C. A fine-structure genetic and chemical study of the enzyme alkaline phosphatase of E. coli. I. Purification and characterization of alkaline phosphatase. Biochim Biophys Acta. 1960 Mar 11;38:470–483. doi: 10.1016/0006-3002(60)91282-8. [DOI] [PubMed] [Google Scholar]
- HAYASHI S., KOCH J. P., LIN E. C. ACTIVE TRANSPORT OF L-ALPHA-GLYCEROPHOSPHATE IN ESCHERICHIA COLI. J Biol Chem. 1964 Sep;239:3098–3105. [PubMed] [Google Scholar]
- Hennen P. E., Carter H. B., Nunn W. D. Changes in macromolecular synthesis and nucleoside triphosphate levels during glycerol-induced growth stasis of Escherichia coli. J Bacteriol. 1978 Dec;136(3):929–935. doi: 10.1128/jb.136.3.929-935.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homma H., Nishijima M., Kobayashi T., Okuyama H., Nojima S. Incorporation and metabolism of 2-acyl lysophospholipids by Escherichia coli. Biochim Biophys Acta. 1981 Jan 26;663(1):1–13. doi: 10.1016/0005-2760(81)90189-2. [DOI] [PubMed] [Google Scholar]
- Jones N. C., Osborn M. J. Translocation of phospholipids between the outer and inner membranes of Salmonella typhimurium. J Biol Chem. 1977 Oct 25;252(20):7405–7412. [PubMed] [Google Scholar]
- KANFER J., KENNEDY E. P. METABOLISM AND FUNCTION OF BACTERIAL LIPIDS. I. METABOLISM OF PHOSPHOLIPIDS IN ESCHERICHIA COLI B. J Biol Chem. 1963 Sep;238:2919–2922. [PubMed] [Google Scholar]
- KOCH J. P., HAYASHI S., LIN E. C. THE CONTROL OF DISSIMILATION OF GLYCEROL AND L-ALPHA-GLYCEROPHOSPHATE IN ESCHERICHIA COLI. J Biol Chem. 1964 Sep;239:3106–3108. [PubMed] [Google Scholar]
- Kennedy E. P., Rumley M. K., Schulman H., Van Golde L. M. Identification of sn-glycero-1-phosphate and phosphoethanolamine residues linked to the membrane-derived Oligosaccharides of Escherichia coli. J Biol Chem. 1976 Jul 25;251(14):4208–4213. [PubMed] [Google Scholar]
- Kundig W., Roseman S. Sugar transport. II. Characterization of constitutive membrane-bound enzymes II of the Escherichia coli phosphotransferase system. J Biol Chem. 1971 Mar 10;246(5):1407–1418. [PubMed] [Google Scholar]
- Leifer Z., Engel R., Tropp B. E. Transport of 3,4-dihydroxybutyl-1-phosphonate, an analogue of sn-glycerol 3-phosphate. J Bacteriol. 1977 May;130(2):968–971. doi: 10.1128/jb.130.2.968-971.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin E. C. Glycerol dissimilation and its regulation in bacteria. Annu Rev Microbiol. 1976;30:535–578. doi: 10.1146/annurev.mi.30.100176.002535. [DOI] [PubMed] [Google Scholar]
- Nishijima M., Raetz C. R. Membrane lipid biogenesis in Escherichia coli: identification of genetic loci for phosphatidylglycerophosphate synthetase and construction of mutants lacking phosphatidylglycerol. J Biol Chem. 1979 Aug 25;254(16):7837–7844. [PubMed] [Google Scholar]
- Orr G. A., Brewer C. F., Heney G. Synthesis of the diastereoisomers of 1,2-dipalmitoyl-sn-glycero-3-thiophosphorylethanolamine and their stereospecific hydrolysis by phospholipases A2 and C. Biochemistry. 1982 Jun 22;21(13):3202–3206. doi: 10.1021/bi00256a026. [DOI] [PubMed] [Google Scholar]
- Orr G. A., Hammelburger J. W., Heney G. Interaction of sn-glycerol 3-phosphorothioate with Escherichia coli. In vitro and in vivo incorporation into phospholipids. J Biol Chem. 1983 Aug 10;258(15):9237–9244. [PubMed] [Google Scholar]
- Orr G. A., Simon J., Jones S. R., Chin G. J., Knowles J. R. Adenosine 5'-O-([gamma-18O]gamma-thio)triphosphate chiral at the gamma-phosphorus: stereochemical consequences of reactions catalyzed by pyruvate kinase, glycerol kinase, and hexokinase. Proc Natl Acad Sci U S A. 1978 May;75(5):2230–2233. doi: 10.1073/pnas.75.5.2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raetz C. R. Enzymology, genetics, and regulation of membrane phospholipid synthesis in Escherichia coli. Microbiol Rev. 1978 Sep;42(3):614–659. doi: 10.1128/mr.42.3.614-659.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randle C. L., Albro P. W., Dittmer J. C. The phosphoglyceride composition of Gram-negative bacteria and the changes in composition during growth. Biochim Biophys Acta. 1969;187(2):214–220. doi: 10.1016/0005-2760(69)90030-7. [DOI] [PubMed] [Google Scholar]
- Sandermann H., Jr Regulation of membrane enzymes by lipids. Biochim Biophys Acta. 1978 Sep 29;515(3):209–237. doi: 10.1016/0304-4157(78)90015-1. [DOI] [PubMed] [Google Scholar]
- Schulman H., Kennedy E. P. Relation of turnover of membrane phospholipids to synthesis of membrane-derived oligosaccharides of Escherichia coli. J Biol Chem. 1977 Jun 25;252(12):4250–4255. [PubMed] [Google Scholar]
- Shopsis C. S., Engel R., Tropp B. E. Effects of phosphonic acid analogues of glycerol-3-phosphate on the growth of Escherichia coli. J Bacteriol. 1972 Oct;112(1):408–412. doi: 10.1128/jb.112.1.408-412.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shopsis C. S., Engel R., Tropp B. E. The inhibition of phosphatidylglycerol synthesis in Escherichia coli by 3,4-dihydroxybutyl-1-phosphonate. J Biol Chem. 1974 Apr 25;249(8):2473–2477. [PubMed] [Google Scholar]
- Shopsis C. S., Nunn W. D., Engel R., Tropp B. E. Effects of phosphonic acid analogues of glycerol-3-phosphate on the growth of Escherichia coli: phospholipid metabolism. Antimicrob Agents Chemother. 1973 Oct;4(4):467–473. doi: 10.1128/aac.4.4.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor A. L. Current linkage map of Escherichia coli. Bacteriol Rev. 1970 Jun;34(2):155–175. doi: 10.1128/br.34.2.155-175.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yount R. G. ATP analogs. Adv Enzymol Relat Areas Mol Biol. 1975;43:1–56. doi: 10.1002/9780470122884.ch1. [DOI] [PubMed] [Google Scholar]
- van Deenen L. L. Topology and dynamics of phospholipids in membranes. FEBS Lett. 1981 Jan 12;123(1):3–15. doi: 10.1016/0014-5793(81)80007-5. [DOI] [PubMed] [Google Scholar]
- van Golde L. M. Metabolism of membrane phospholipids and its relation to a novel class of oligosaccharides in Escherichia coli. Proc Natl Acad Sci U S A. 1973 May;70(5):1368–1372. doi: 10.1073/pnas.70.5.1368. [DOI] [PMC free article] [PubMed] [Google Scholar]



