Abstract
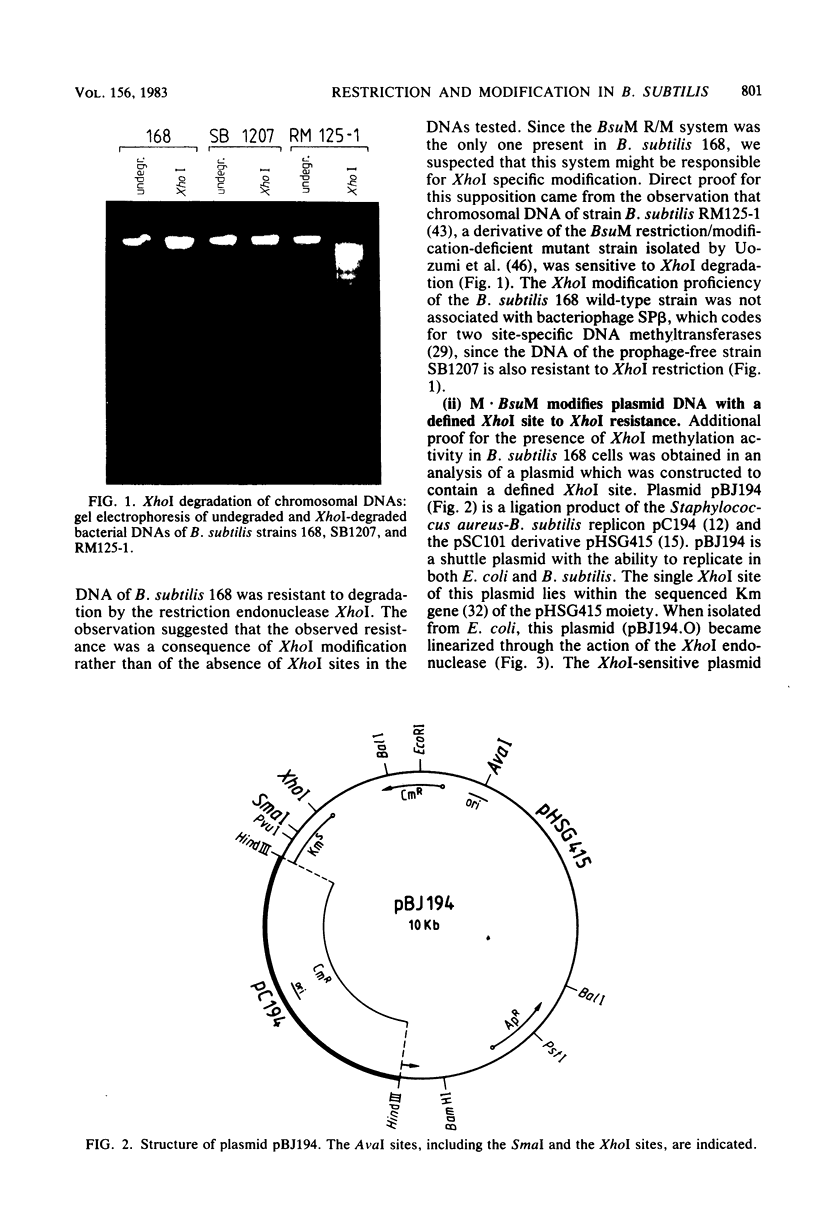
The sequence specificities of three Bacillus subtilis restriction/modification systems were established: (i) BsuM (CTCGAG), an isoschizomer to XhoI; (ii) BsuE (CGCG), an isoschizomer to FnuDII; and (iii) BsuF (CCGG), an isoschizomer to MspI, HpaII. The BsuM modification enzyme methylates the 3' cytosine of the recognition sequence. The BsuF modification enzyme methylates the 5' cytosine of the sequence, rendering such sites resistant to MspI degradation and leaving the majority of sites sensitive to HpaII degradation.
Full text
PDF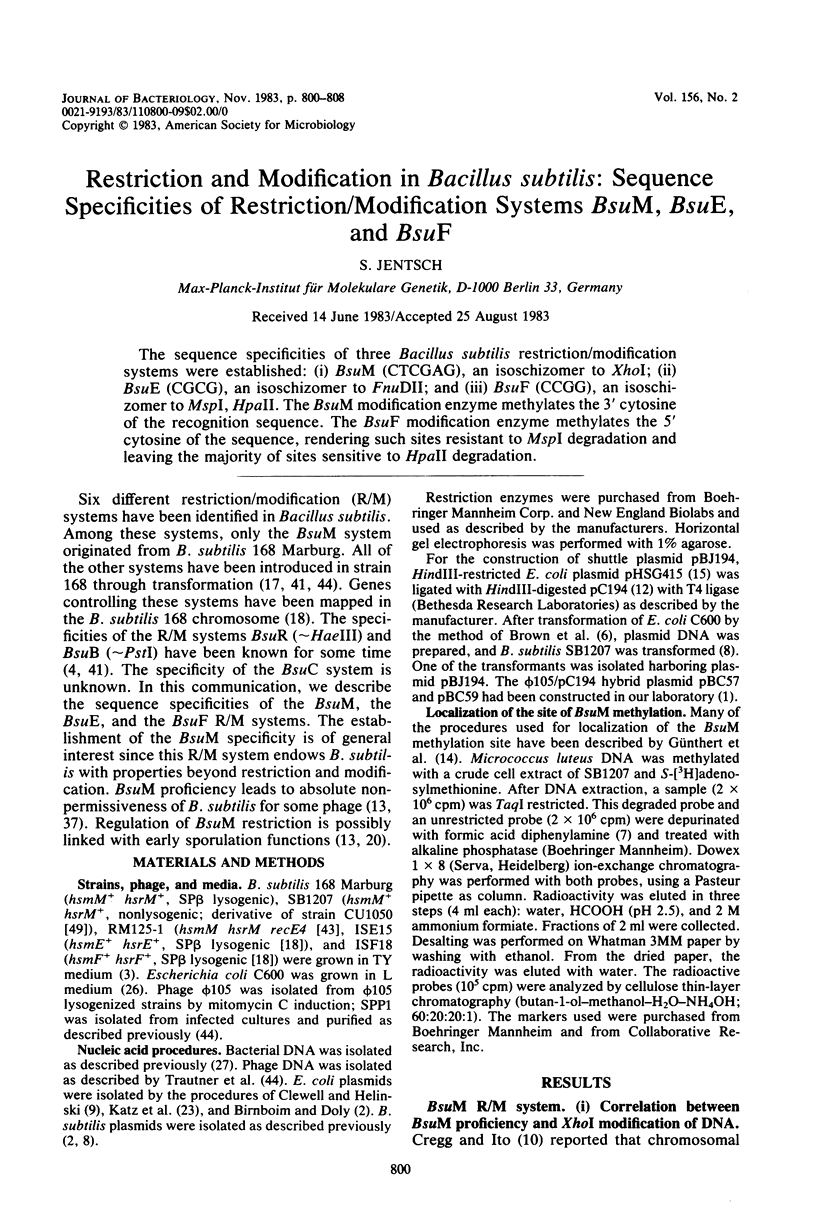







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURTON K., PETERSEN G. B. The frequencies of certain sequences of nucleotides in deoxyribonucleic acid. Biochem J. 1960 Apr;75:17–27. doi: 10.1042/bj0750017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bensi G., Iglesias A., Canosi U., Trautner T. A. Plasmid transformation in Bacillus subtilis. The significance of partial homology between plasmid and recipient cell DNAs. Mol Gen Genet. 1981;184(3):400–404. doi: 10.1007/BF00352512. [DOI] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal N., Kleinschmidt A. K., Spatz H. C., Trautner T. A. Physical properties of the DNA of bacteriophage SP50. Mol Gen Genet. 1967;100(1):39–55. doi: 10.1007/BF00425774. [DOI] [PubMed] [Google Scholar]
- Bron S., Murray K. Restriction and modification in B. subtilis. Nucleotide sequence recognised by restriction endonuclease R. Bsu R from strain R. Mol Gen Genet. 1975 Dec 30;143(1):25–33. doi: 10.1007/BF00269417. [DOI] [PubMed] [Google Scholar]
- Brooks J. E., Roberts R. J. Modification profiles of bacterial genomes. Nucleic Acids Res. 1982 Feb 11;10(3):913–934. doi: 10.1093/nar/10.3.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canosi U., Morelli G., Trautner T. A. The relationship between molecular structure and transformation efficiency of some S. aureus plasmids isolated from B. subtilis. Mol Gen Genet. 1978 Nov 9;166(3):259–267. doi: 10.1007/BF00267617. [DOI] [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. R. Supercoiled circular DNA-protein complex in Escherichia coli: purification and induced conversion to an opern circular DNA form. Proc Natl Acad Sci U S A. 1969 Apr;62(4):1159–1166. doi: 10.1073/pnas.62.4.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cregg J. M., Ito J. A physical map of the genome of temperate phage phi 3T. Gene. 1979 Jul;6(3):199–219. doi: 10.1016/0378-1119(79)90058-1. [DOI] [PubMed] [Google Scholar]
- Cregg J. M., Nguyen A. H., Ito J. DNA modification induced during infection of Bacillus subtilis by phage phi 3T. Gene. 1980 Dec;12(1-2):17–24. doi: 10.1016/0378-1119(80)90011-6. [DOI] [PubMed] [Google Scholar]
- Ehrlich S. D. Replication and expression of plasmids from Staphylococcus aureus in Bacillus subtilis. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1680–1682. doi: 10.1073/pnas.74.4.1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fucík V., Grünnerová H., Zadrazil S. Restriction and modification in Bacillus subtilis 168. Regulation of hsrM(nonB) expression in spoOA mutants and effects on permissiveness for phi15 and phi105 phages. Mol Gen Genet. 1982;186(1):118–121. doi: 10.1007/BF00422922. [DOI] [PubMed] [Google Scholar]
- Günthert U., Storm K., Bald R. Restriction and modification in Bacillus subtilis. Localization of the methylated nucleotide in the BsuRI recognition sequence. Eur J Biochem. 1978 Oct 16;90(3):581–583. doi: 10.1111/j.1432-1033.1978.tb12638.x. [DOI] [PubMed] [Google Scholar]
- Hashimoto-Gotoh T., Franklin F. C., Nordheim A., Timmis K. N. Specific-purpose plasmid cloning vectors. I. Low copy number, temperature-sensitive, mobilization-defective pSC101-derived containment vectors. Gene. 1981 Dec;16(1-3):227–235. doi: 10.1016/0378-1119(81)90079-2. [DOI] [PubMed] [Google Scholar]
- Hughes S. G., Murray K. The nucleotide sequences recognized by endonucleases AvaI and AvaII from Anabaena variabilis. Biochem J. 1980 Jan 1;185(1):65–75. doi: 10.1042/bj1850065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikawa S., Shibata T., Ando T., Saito H. Genetic studies on site-specific endodeoxyribonucleases in Bacillus subtilis: multiple modification and restriction systems in transformants of Bacillus subtilis 168. Mol Gen Genet. 1980 Feb;177(3):359–368. doi: 10.1007/BF00271474. [DOI] [PubMed] [Google Scholar]
- Ikawa S., Shibata T., Matsumoto K., Iijima T., Saito H., Ando T. Chromosomal loci of genes controlling site-specific restriction endonucleases of Bacillus subtilis. Mol Gen Genet. 1981;183(1):1–6. doi: 10.1007/BF00270129. [DOI] [PubMed] [Google Scholar]
- Ito J., Roberts R. J. Unusual base sequence arrangement in phage phi 29 DNA. Gene. 1979 Jan;5(1):1–7. doi: 10.1016/0378-1119(79)90088-x. [DOI] [PubMed] [Google Scholar]
- Ito J., Spizizen J. Abortive infection of sporulating Bacillus subtilis 168 by phi 2 bacteriophage. J Virol. 1971 Apr;7(4):515–523. doi: 10.1128/jvi.7.4.515-523.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jentsch S., Günthert U., Trautner T. A. DNA methyltransferases affecting the sequence 5'CCGG. Nucleic Acids Res. 1981 Jun 25;9(12):2753–2759. doi: 10.1093/nar/9.12.2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz L., Kingsbury D. T., Helinski D. R. Stimulation by cyclic adenosine monophosphate of plasmid deoxyribonucleic acid replication and catabolite repression of the plasmid deoxyribonucleic acid-protein relaxation complex. J Bacteriol. 1973 May;114(2):577–591. doi: 10.1128/jb.114.2.577-591.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura F., Saito H., Ikeda Y. Bacteriophage phi 1 as a gene-cloning vector in Bacillus subtilis. Mol Gen Genet. 1980;180(2):259–266. doi: 10.1007/BF00425837. [DOI] [PubMed] [Google Scholar]
- LURIA S. E., BURROUS J. W. Hybridization between Escherichia coli and Shigella. J Bacteriol. 1957 Oct;74(4):461–476. doi: 10.1128/jb.74.4.461-476.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lui A. C., McBride B. C., Vovis G. F., Smith M. Site specific endonuclease from Fusobacterium nucleatum. Nucleic Acids Res. 1979 Jan;6(1):1–15. doi: 10.1093/nar/6.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConnell D. J., Searcy D. G., Sutcliffe J. G. A restriction enzyme Tha I from the thermophilic mycoplasma Thermoplasma acidophilum. Nucleic Acids Res. 1978 Jun;5(6):1729–1739. doi: 10.1093/nar/5.6.1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noyer-Weidner M., Jentsch S., Pawlek B., Günthert U., Trautner T. A. Restriction and modification in Bacillus subtilis: DNA methylation potential of the related bacteriophages Z, SPR, SP beta, phi 3T, and rho 11. J Virol. 1983 May;46(2):446–453. doi: 10.1128/jvi.46.2.446-453.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noyer-Weidner M., Pawlek B., Jentsch S., Günthert U., Trautner T. A. Restriction and modification in Bacillus subtilis: gene coding for a BsuR-specific modification methyltransferase in the temperate bacteriophage phi 3T. J Virol. 1981 Jun;38(3):1077–1080. doi: 10.1128/jvi.38.3.1077-1080.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oda K., Marmur J. Purification and properties of deoxyribonucleic acid methylase from Bacillus subtilis. Biochemistry. 1966 Feb;5(2):761–773. doi: 10.1021/bi00866a051. [DOI] [PubMed] [Google Scholar]
- Oka A., Sugisaki H., Takanami M. Nucleotide sequence of the kanamycin resistance transposon Tn903. J Mol Biol. 1981 Apr 5;147(2):217–226. doi: 10.1016/0022-2836(81)90438-1. [DOI] [PubMed] [Google Scholar]
- Perkins J. B., Zarley C. D., Dean D. H. Restriction endonuclease mapping of bacteriophage phi105 and closely related temperate Bacillus subtilis bacteriophages rho10 and rho14. J Virol. 1978 Oct;28(1):403–407. doi: 10.1128/jvi.28.1.403-407.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pero J., Hannett N. M., Talkington C. Restriction cleavage map of SP01 DNA: general location of early, middle, and late genes. J Virol. 1979 Jul;31(1):156–171. doi: 10.1128/jvi.31.1.156-171.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratcliff S. W., Luh J., Ganesan A. T., Behrens B., Thompson R., Montenegro M. A., Morelli G., Trautner T. A. The genome of Bacillus subtilis phage SPP1: the arrangement of restriction endonuclease generated fragments. Mol Gen Genet. 1979 Jan 10;168(2):165–172. doi: 10.1007/BF00431442. [DOI] [PubMed] [Google Scholar]
- Reeve J. N., Amann E., Tailor R., Günthert U., Scholz K., Trautner T. A. Unusual behaviour of SPO1 DNA with respect to restriction and modification enzymes recognizing the sequence 5'-G-G-C-C. Mol Gen Genet. 1980 Apr;178(1):229–231. doi: 10.1007/BF00267234. [DOI] [PubMed] [Google Scholar]
- Saito H., Shibata T., Ando T. Mapping of genes determining nonpermissiveness and host-specific restriction to bacteriophages in Bacillus subtilis Marburg. Mol Gen Genet. 1979 Feb 26;170(2):117–122. doi: 10.1007/BF00337785. [DOI] [PubMed] [Google Scholar]
- Sato S., Hutchinson C. A., 3rd, Harris J. I. A thermostable sequence-specific endonuclease from Thermus aquaticus. Proc Natl Acad Sci U S A. 1977 Feb;74(2):542–546. doi: 10.1073/pnas.74.2.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata T., Ando T. Host controlled modification and restriction in Bacillus subtilis. Mol Gen Genet. 1974;131(4):275–280. doi: 10.1007/BF00264858. [DOI] [PubMed] [Google Scholar]
- Shibata T., Ikawa S., Kim C., Ando T. Site-specific deoxyribonucleases in Bacillus subtilis and other Bacillus strains. J Bacteriol. 1976 Oct;128(1):473–476. doi: 10.1128/jb.128.1.473-476.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata T., Ikawa S., Komatsu Y., Ando T., Saito H. Introduction of host-controlled modification and restriction systems of Bacillus subtilis IAM1247 into Bacillus subtilis 168. J Bacteriol. 1979 Jul;139(1):308–310. doi: 10.1128/jb.139.1.308-310.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streeck R. E. Single-strand and double-strand cleavage at half-modified and fully modified recognition sites for the restriction nucleases Sau3a and Taqi. Gene. 1980 Dec;12(3-4):267–275. doi: 10.1016/0378-1119(80)90109-2. [DOI] [PubMed] [Google Scholar]
- Tanaka T., Sakaguchi K. Construction of a recombinant plasmid composed of B. subtilis leucine genes and a B. subtilis (natto) plasmid: its use as cloning vehicle in B. subtilis 168. Mol Gen Genet. 1978 Oct 24;165(3):269–276. doi: 10.1007/BF00332526. [DOI] [PubMed] [Google Scholar]
- Trautner T. A., Pawlek B., Bron S., Anagnostopoulos C. Restriction and modification in B. subtilis. Biological aspects. Mol Gen Genet. 1974;131(3):181–191. doi: 10.1007/BF00267958. [DOI] [PubMed] [Google Scholar]
- Trautner T. A., Pawlek B., Günthert U., Canosi U., Jentsch S., Freund M. Restriction and modification in Bacillus subtilis: identification of a gene in the temperate phage SP beta coding for a BsuR specific modification methyltransferase. Mol Gen Genet. 1980;180(2):361–367. doi: 10.1007/BF00425849. [DOI] [PubMed] [Google Scholar]
- Uozumi T., Hoshino T., Miwa K., Horinouchi S., Beppu T., Arima K. Restriction and modification in Bacillus species: genetic transformation of bacteria with DNA from different species, part I. Mol Gen Genet. 1977 Mar 28;152(1):65–69. doi: 10.1007/BF00264941. [DOI] [PubMed] [Google Scholar]
- Witmer H., Franks M. Restriction and modification of bacteriophage SP10 DNA by Bacillus subtilis Marburg 168: stabilization of SP10 DNA in restricting hosts preinfected with a heterologous phage, SP18. J Virol. 1981 Jan;37(1):148–155. doi: 10.1128/jvi.37.1.148-155.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahler S. A., Korman R. Z., Rosenthal R., Hemphill H. E. Bacillus subtilis bacteriophage SPbeta: localization of the prophage attachment site, and specialized transduction. J Bacteriol. 1977 Jan;129(1):556–558. doi: 10.1128/jb.129.1.556-558.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Ploeg L. H., Flavell R. A. DNA methylation in the human gamma delta beta-globin locus in erythroid and nonerythroid tissues. Cell. 1980 Apr;19(4):947–958. doi: 10.1016/0092-8674(80)90086-0. [DOI] [PubMed] [Google Scholar]









