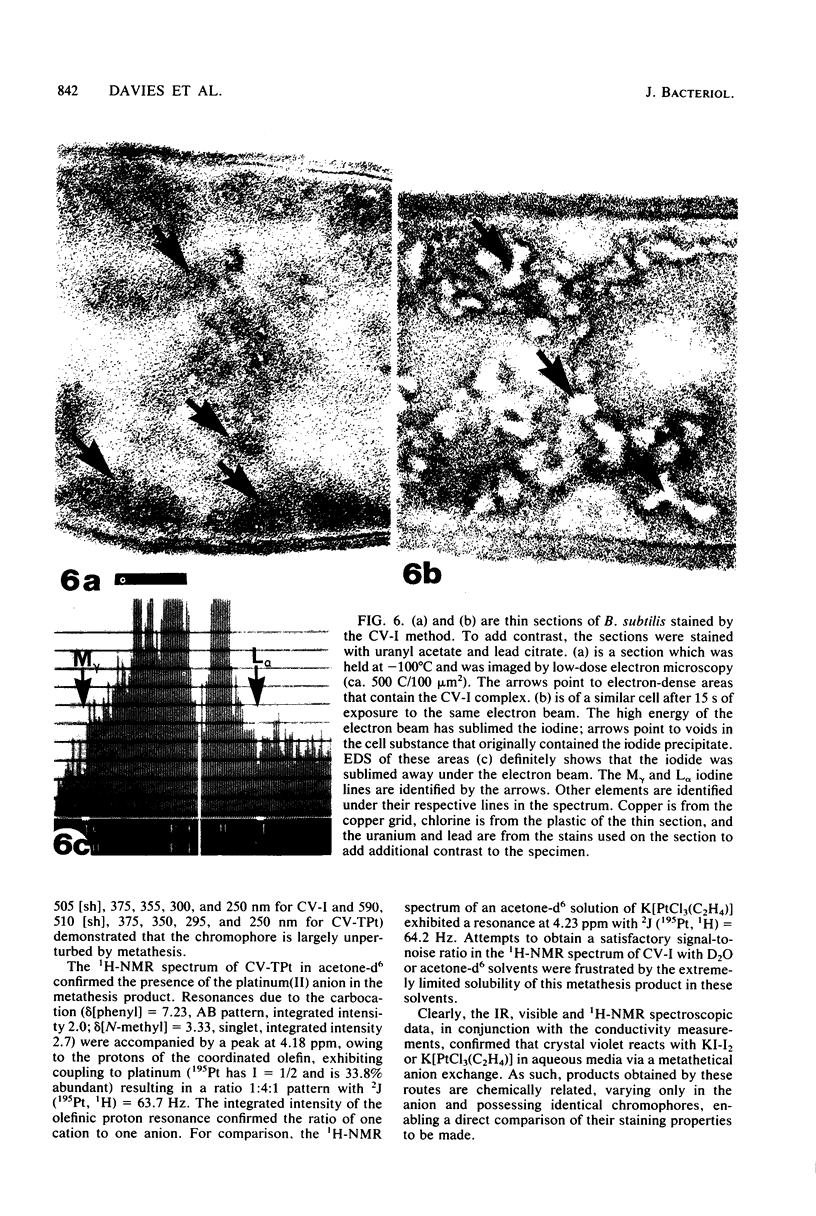
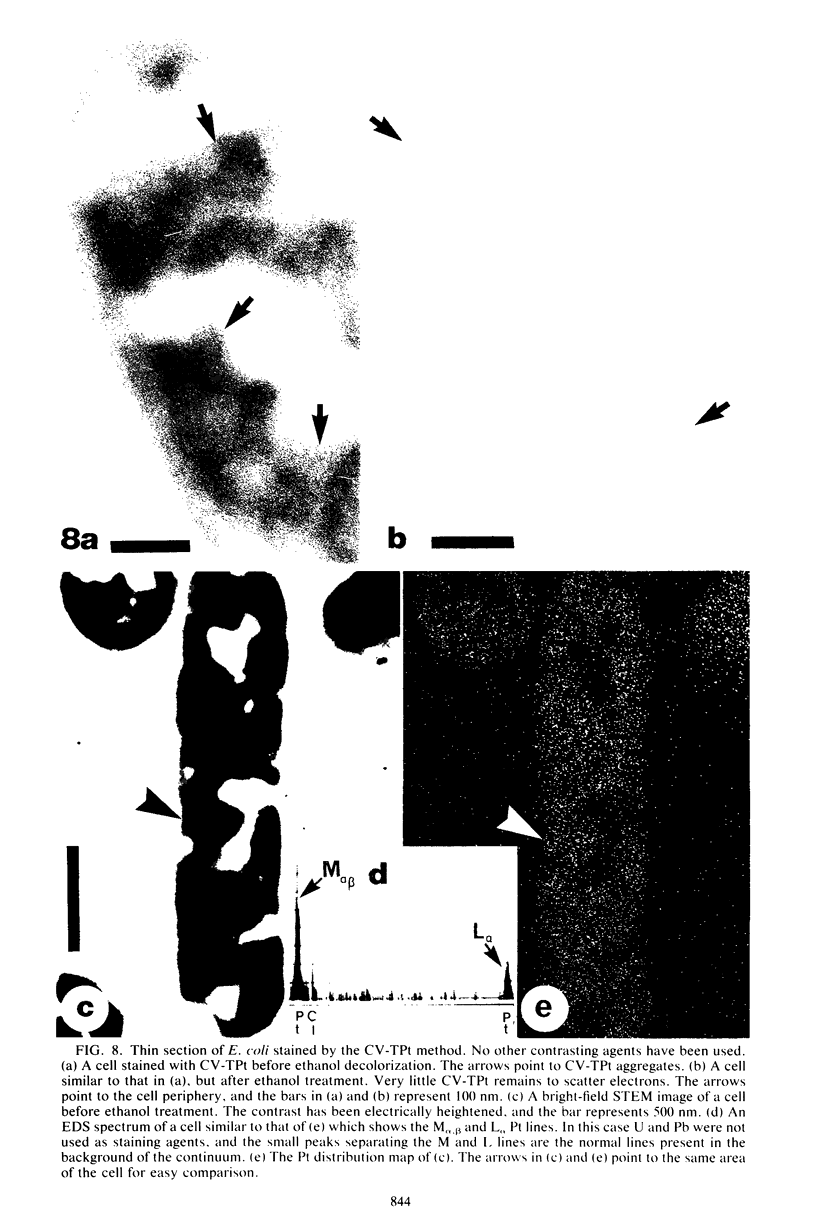
Abstract
Crystal violet (hexamethyl-para-rosaniline chloride) interacts with aqueous KI-I2 during the Gram stain via a simple metathetical anion exchange to produce a chemical precipitate. There is an apparent 1:1 stoichiometry between anion (I-) and cation (hexamethyl-para-rosaniline+) during the reaction and, since the small chloride anion is replaced by the bulkier iodide, the complex formed becomes insoluble in water. It is this same precipitate which forms in the cellular substance of bacteria (both gram-positive and gram-negative types) and which initiates the Gram reaction. Potassium trichloro(eta 2-ethylene)-platinum(II), as an electronopaque marker for electron microscopy, was chemically synthesized, and it produced an anion in aqueous solution which was compatible with crystal violet for the Gram stain. It interacted with crystal violet in a similar manner as iodide to produce an insoluble complex which was chemically and physically analogous to the dye-iodide precipitate. This platinum anion therefore allows the Gram staining mechanism to be followed by electron microscopy.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARTHOLOMEW J. W., MITTWER T. The Gram stain. Bacteriol Rev. 1952 Mar;16(1):1–29. doi: 10.1128/br.16.1.1-29.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BERTANI G. Studies on lysogenesis. I. The mode of phage liberation by lysogenic Escherichia coli. J Bacteriol. 1951 Sep;62(3):293–300. doi: 10.1128/jb.62.3.293-300.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRITT E. M., GERHARDT P., VENNES J. W. Gram reaction of isolated protoplasts and surface membranes of Bacillus megaterium. J Bacteriol. 1956 Nov;72(5):721–721. doi: 10.1128/jb.72.5.721-721.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beveridge T. J., Davies J. A. Cellular responses of Bacillus subtilis and Escherichia coli to the Gram stain. J Bacteriol. 1983 Nov;156(2):846–858. doi: 10.1128/jb.156.2.846-858.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beveridge T. J. Ultrastructure, chemistry, and function of the bacterial wall. Int Rev Cytol. 1981;72:229–317. doi: 10.1016/s0074-7696(08)61198-5. [DOI] [PubMed] [Google Scholar]
- HERBST E. J., WEAVER R. H., KEISTER D. L. The gram reaction and cell composition: diamines and polyamines. Arch Biochem Biophys. 1958 May;75(1):171–177. doi: 10.1016/0003-9861(58)90407-7. [DOI] [PubMed] [Google Scholar]
- MITCHELL P., MOYLE J. Occurrence of a phosphoric ester in certain bacteria: its relation to gram staining and penicillin sensitivity. Nature. 1950 Aug 5;166(4214):218–220. doi: 10.1038/166218a0. [DOI] [PubMed] [Google Scholar]
- MITCHELL P., MOYLE J. The Gram reaction and cell composition: nucleic acids and other phosphate fractions. J Gen Microbiol. 1954 Jun;10(3):533–540. doi: 10.1099/00221287-10-3-533. [DOI] [PubMed] [Google Scholar]
- MITCHELL P., MOYLE J. The glycerol-phospho-protein complex envelope of Micrococcus pyogenes. J Gen Microbiol. 1951 Nov;5(5 Suppl):981–992. doi: 10.1099/00221287-5-5-981. [DOI] [PubMed] [Google Scholar]
- MITTWER T., BARTHOLOMEW J. W., KALLMAN B. J. The mechanism of the gram reaction. II. The function of iodine in the gram stain. Stain Technol. 1950 Oct;25(4):169–179. doi: 10.3109/10520295009110986. [DOI] [PubMed] [Google Scholar]
- SALTON M. R. The relationship between the nature of the cell wall and the Gram stain. J Gen Microbiol. 1963 Feb;30:223–235. doi: 10.1099/00221287-30-2-223. [DOI] [PubMed] [Google Scholar]
- SCHERRER R. Cell structure and quantitative gram stain of Bacillus megaterium. J Gen Microbiol. 1963 Apr;31:135–145. doi: 10.1099/00221287-31-1-135. [DOI] [PubMed] [Google Scholar]
- SMYTH R. D., GERSHENFELD L. Iodine 131 uptake in the Gram staining technic. Stain Technol. 1960 Sep;35:237–245. doi: 10.3109/10520296009114743. [DOI] [PubMed] [Google Scholar]
- Stearn E. W., Stearn A. E. The Chemical Mechanism of Bacterial Behavior: II. A New Theory of the Gram Reaction. J Bacteriol. 1924 Sep;9(5):479–489. doi: 10.1128/jb.9.5.479-489.1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WENSINCK F., BOEVE J. J. Quantitative analysis of the Gram reaction. J Gen Microbiol. 1957 Oct;17(2):401–413. doi: 10.1099/00221287-17-2-401. [DOI] [PubMed] [Google Scholar]