Abstract
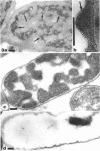
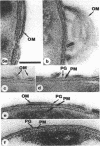
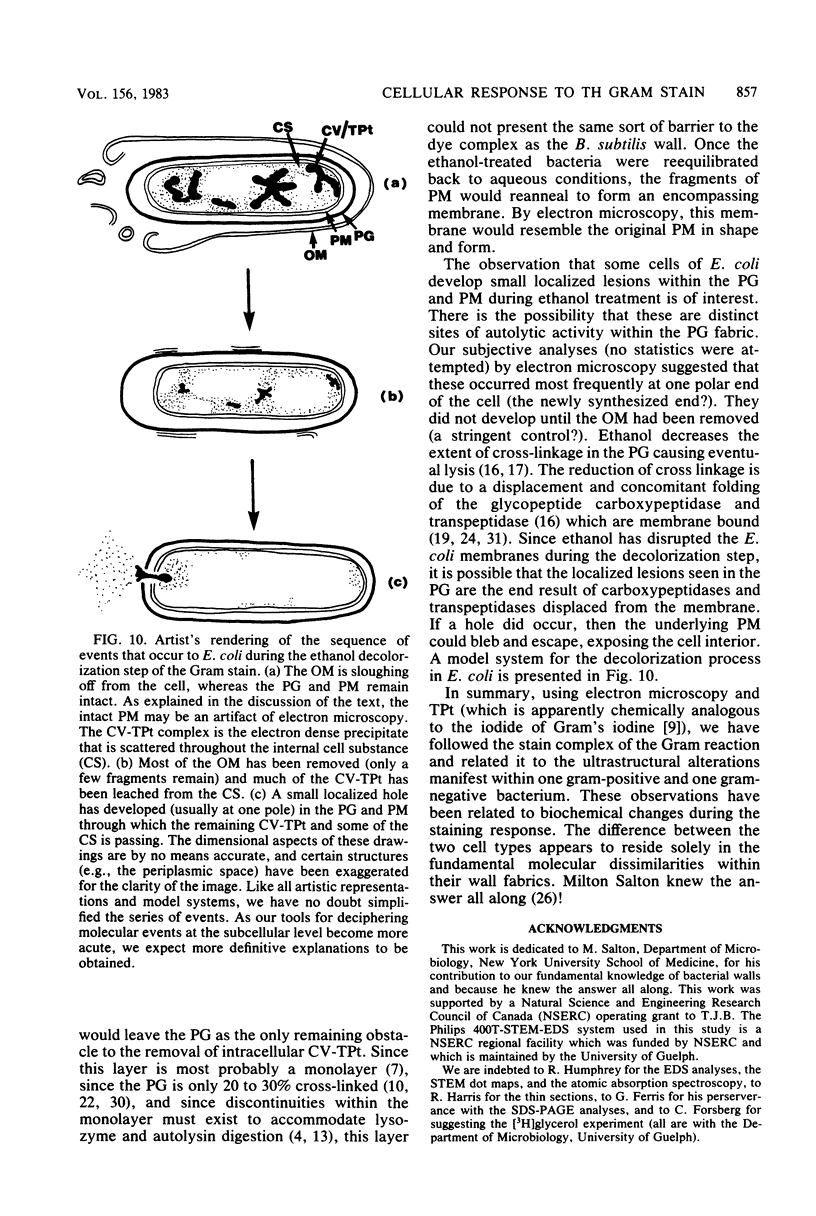
Exponentially growing cells of Bacillus subtilis and Escherichia coli were Gram stained with potassium trichloro(eta 2-ethylene)platinum(II) (TPt) in place of the usual KI-I2 mordant. This electron-dense probe allowed the staining mechanism to be followed and compared with cellular perturbations throughout the staining process. A crystal violet (CV)-TPt chemical complex was formed within the cell substance and at the cell surface of B. subtilis when the dye and Pt mordant were added. The ethanol decolorization step dissolved the precipitate from the cell surface, but the internal complex was retained by the cell wall and remained within the cell. This was not the case for E. coli; the ethanol decolorization step removed both surface-bound and cellular CV-TPt. During its removal, the outer membrane was sloughed off the cells until only the murein sacculus and plasma membrane remained. We suspect that the plasma membrane was also perturbed, but that it was retained within the cell by the murein sacculus. Occasionally, small holes within the murein and plasma membrane could be distinguished through which leaked CV-TPt and some cellular debris. Biochemical identification of distinct envelope markers confirmed the accuracy of these images.
Full text
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARTHOLOMEW J. W., MITTWER T. The Gram stain. Bacteriol Rev. 1952 Mar;16(1):1–29. doi: 10.1128/br.16.1.1-29.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BERTANI G. Studies on lysogenesis. I. The mode of phage liberation by lysogenic Escherichia coli. J Bacteriol. 1951 Sep;62(3):293–300. doi: 10.1128/jb.62.3.293-300.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beveridge T. J., Murray R. G. Sites of metal deposition in the cell wall of Bacillus subtilis. J Bacteriol. 1980 Feb;141(2):876–887. doi: 10.1128/jb.141.2.876-887.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beveridge T. J. Ultrastructure, chemistry, and function of the bacterial wall. Int Rev Cytol. 1981;72:229–317. doi: 10.1016/s0074-7696(08)61198-5. [DOI] [PubMed] [Google Scholar]
- Braun V., Gnirke H., Henning U., Rehn K. Model for the structure of the shape-maintaining layer of the Escherichia coli cell envelope. J Bacteriol. 1973 Jun;114(3):1264–1270. doi: 10.1128/jb.114.3.1264-1270.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burge R. E., Fowler A. G., Reaveley D. A. Structure of the peptidogylcan of bacterial cell walls. I. J Mol Biol. 1977 Dec 25;117(4):927–953. doi: 10.1016/s0022-2836(77)80006-5. [DOI] [PubMed] [Google Scholar]
- Davies J. A., Anderson G. K., Beveridge T. J., Clark H. C. Chemical mechanism of the Gram stain and synthesis of a new electron-opaque marker for electron microscopy which replaces the iodine mordant of the stain. J Bacteriol. 1983 Nov;156(2):837–845. doi: 10.1128/jb.156.2.837-845.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiRienzo J. M., Nakamura K., Inouye M. The outer membrane proteins of Gram-negative bacteria: biosynthesis, assembly, and functions. Annu Rev Biochem. 1978;47:481–532. doi: 10.1146/annurev.bi.47.070178.002405. [DOI] [PubMed] [Google Scholar]
- ELWORTHY P. H., McINTOSH D. S. Micelle formation by lecithin in some aliphatic alcohols. J Pharm Pharmacol. 1961 Nov;13:663–669. doi: 10.1111/j.2042-7158.1961.tb11889.x. [DOI] [PubMed] [Google Scholar]
- Formanek H. A three dimensional model of the digestion of peptidoglycan by lysozyme. Biophys Struct Mech. 1977 Dec 27;4(1):1–14. doi: 10.1007/BF00538836. [DOI] [PubMed] [Google Scholar]
- Formanek H., Formanek S., Wawra H. A three-dimensional atomic model of the murein layer of bacteria. Eur J Biochem. 1974 Jul 15;46(2):279–294. doi: 10.1111/j.1432-1033.1974.tb03620.x. [DOI] [PubMed] [Google Scholar]
- Funahara Y., Nikaido H. Asymmetric localization of lipopolysaccharides on the outer membrane of Salmonella typhimurium. J Bacteriol. 1980 Mar;141(3):1463–1465. doi: 10.1128/jb.141.3.1463-1465.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingram L. O. Mechanism of lysis of Escherichia coli by ethanol and other chaotropic agents. J Bacteriol. 1981 Apr;146(1):331–336. doi: 10.1128/jb.146.1.331-336.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingram L. O., Vreeland N. S. Differential effects of ethanol and hexanol on the Escherichia coli cell envelope. J Bacteriol. 1980 Nov;144(2):481–488. doi: 10.1128/jb.144.2.481-488.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain M. K., Gleeson J., Upreti A., Upreti G. C. Intrinsic perturbing ability of alkanols in lipid bilayers. Biochim Biophys Acta. 1978 May 4;509(1):1–8. doi: 10.1016/0005-2736(78)90002-0. [DOI] [PubMed] [Google Scholar]
- Kozarich J. W., Strominger J. L. A membrane enzyme from Staphylococcus aureus which catalyzes transpeptidase, carboxypeptidase, and penicillinase activities. J Biol Chem. 1978 Feb 25;253(4):1272–1278. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Markwell M. A., Haas S. M., Bieber L. L., Tolbert N. E. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978 Jun 15;87(1):206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- Olijhoek A. J., Klencke S., Pas E., Nanninga N., Schwarz U. Volume growth, murein synthesis, and murein cross-linkage during the division cycle of Escherichia coli PA3092. J Bacteriol. 1982 Dec;152(3):1248–1254. doi: 10.1128/jb.152.3.1248-1254.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou L. T., Marquis R. E. Coccal cell-wall compactness and the swelling action of denaturants. Can J Microbiol. 1972 May;18(5):623–629. doi: 10.1139/m72-099. [DOI] [PubMed] [Google Scholar]
- Rogers H. J. Biogenesis of the wall in bacterial morphogenesis. Adv Microb Physiol. 1979;19:1–62. doi: 10.1016/s0065-2911(08)60197-6. [DOI] [PubMed] [Google Scholar]
- SALTON M. R. The relationship between the nature of the cell wall and the Gram stain. J Gen Microbiol. 1963 Feb;30:223–235. doi: 10.1099/00221287-30-2-223. [DOI] [PubMed] [Google Scholar]
- SCHERRER R. Cell structure and quantitative gram stain of Bacillus megaterium. J Gen Microbiol. 1963 Apr;31:135–145. doi: 10.1099/00221287-31-1-135. [DOI] [PubMed] [Google Scholar]
- Sargent M. G. Membrane synthesis in synchronous cultures of Bacillus subtilis 168. J Bacteriol. 1973 Oct;116(1):397–409. doi: 10.1128/jb.116.1.397-409.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit J., Kamio Y., Nikaido H. Outer membrane of Salmonella typhimurium: chemical analysis and freeze-fracture studies with lipopolysaccharide mutants. J Bacteriol. 1975 Nov;124(2):942–958. doi: 10.1128/jb.124.2.942-958.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAKEBE I. EXTENT OF CROSS LINKAGE IN THE MUREIN SACCULUS OF ESCHERICHIA COLI B CELL WALL. Biochim Biophys Acta. 1965 Mar 1;101:124–126. doi: 10.1016/0926-6534(65)90038-2. [DOI] [PubMed] [Google Scholar]
- Tamura T., Imae Y., Strominger J. L. Purification to homogeneity and properties of two D-alanine carboxypeptidases I From Escherichia coli. J Biol Chem. 1976 Jan 25;251(2):414–423. [PubMed] [Google Scholar]
- WEISSBACH A., HURWITZ J. The formation of 2-keto-3-deoxyheptonic acid in extracts of Escherichia coli B. I. Identification. J Biol Chem. 1959 Apr;234(4):705–709. [PubMed] [Google Scholar]
- de Pedro M. A., Schwarz U. Heterogeneity of newly inserted and preexisting murein in the sacculus of Escherichia coli. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5856–5860. doi: 10.1073/pnas.78.9.5856. [DOI] [PMC free article] [PubMed] [Google Scholar]