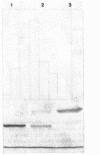
Abstract
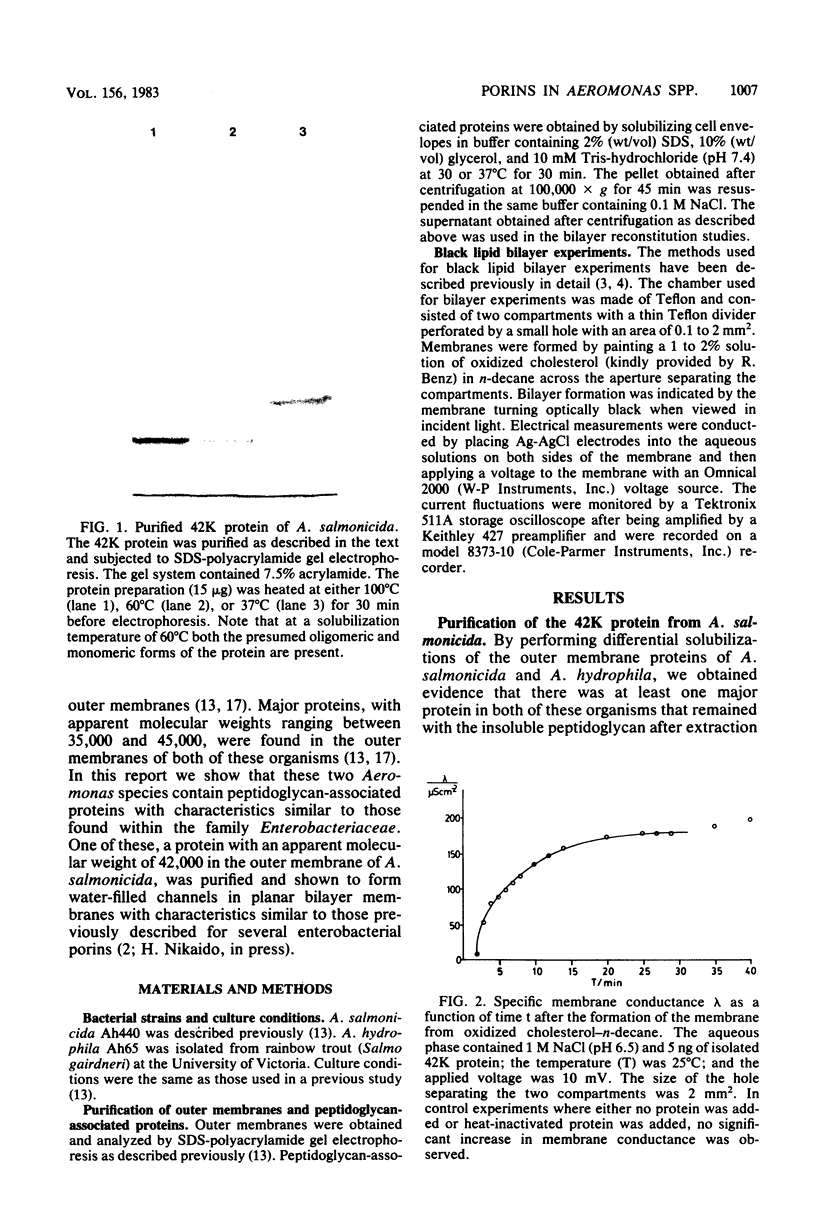
We have purified a major outer membrane protein from Aeromonas salmonicida. This 42-kilodalton protein shared several physical characteristics with enterobacterial porins in that it was noncovalently associated with the peptidoglycan, it was released from the peptidoglycan in the presence of 0.1 M NaCl and sodium dodecyl sulfate, and its mobility on sodium dodecyl sulfate-polyacrylamide gels was dependent on the solubilization temperature before electrophoresis. When added to the aqueous solution bathing a planar bilayer membrane it caused the conductance of the membrane to increase by several orders of magnitude. At lower protein concentrations, single channels with an average conductance of 1.6 nS in 1 M KCl were incorporated into the membrane in a stepwise fashion. Evidence that the protein formed a large, relatively nonselective, water-filled channel was obtained by performing single-channel experiments at different NaCl concentrations and in a variety of different salts. Current through the channel was a linear function of the applied voltage, and no evidence of voltage gating was observed. In addition, we obtained evidence for a 43-kilodalton channel-forming protein in the outer membrane of A. hydrophila with a similar single-channel conductance as the 42-kilodalton protein in 1 M NaCl.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benz R., Hancock R. E. Properties of the large ion-permeable pores formed from protein F of Pseudomonas aeruginosa in lipid bilayer membranes. Biochim Biophys Acta. 1981 Aug 20;646(2):298–308. doi: 10.1016/0005-2736(81)90336-9. [DOI] [PubMed] [Google Scholar]
- Benz R., Ishii J., Nakae T. Determination of ion permeability through the channels made of porins from the outer membrane of Salmonella typhimurium in lipid bilayer membranes. J Membr Biol. 1980 Aug 21;56(1):19–29. doi: 10.1007/BF01869348. [DOI] [PubMed] [Google Scholar]
- Benz R., Janko K., Läuger P. Ionic selectivity of pores formed by the matrix protein (porin) of Escherichia coli. Biochim Biophys Acta. 1979 Mar 8;551(2):238–247. doi: 10.1016/0005-2736(89)90002-3. [DOI] [PubMed] [Google Scholar]
- DiRienzo J. M., Nakamura K., Inouye M. The outer membrane proteins of Gram-negative bacteria: biosynthesis, assembly, and functions. Annu Rev Biochem. 1978;47:481–532. doi: 10.1146/annurev.bi.47.070178.002405. [DOI] [PubMed] [Google Scholar]
- Hancock R. E., Decad G. M., Nikaido H. Identification of the protein producing transmembrane diffusion pores in the outer membrane of Pseudomonas aeruginosa PA01. Biochim Biophys Acta. 1979 Jul 5;554(2):323–331. doi: 10.1016/0005-2736(79)90373-0. [DOI] [PubMed] [Google Scholar]
- Hancock R. E., Irvin R. T., Costerton J. W., Carey A. M. Pseudomonas aeruginosa outer membrane: peptidoglycan-associated proteins. J Bacteriol. 1981 Jan;145(1):628–631. doi: 10.1128/jb.145.1.628-631.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock R. E., Poole K., Benz R. Outer membrane protein P of Pseudomonas aeruginosa: regulation by phosphate deficiency and formation of small anion-specific channels in lipid bilayer membranes. J Bacteriol. 1982 May;150(2):730–738. doi: 10.1128/jb.150.2.730-738.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard S. P., Buckley J. T. Intracellular accumulation of extracellular proteins by pleiotropic export mutants of Aeromonas hydrophila. J Bacteriol. 1983 Apr;154(1):413–418. doi: 10.1128/jb.154.1.413-418.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugtenberg B., Bronstein H., van Selm N., Peters R. Peptidoglycan-associated outer membrane proteins in gammegatine bacteria. Biochim Biophys Acta. 1977 Mar 17;465(3):571–578. doi: 10.1016/0005-2736(77)90274-7. [DOI] [PubMed] [Google Scholar]
- MacIntyre S., Trust T. J., Buckley J. T. Identification and characterization of outer membrane fragments released by Aeromonas sp. Can J Biochem. 1980 Oct;58(10):1018–1025. doi: 10.1139/o80-138. [DOI] [PubMed] [Google Scholar]
- Mizuno T. A novel peptidoglycan-associated lipoprotein found in the cell envelope of Pseudomonas aeruginosa and Escherichia coli. J Biochem. 1979 Oct;86(4):991–1000. doi: 10.1093/oxfordjournals.jbchem.a132631. [DOI] [PubMed] [Google Scholar]
- Nakae T., Nikaido H. Outer membrane as a diffusion barrier in Salmonella typhimurium. Penetration of oligo- and polysaccharides into isolated outer membrane vesicles and cells with degraded peptidoglycan layer. J Biol Chem. 1975 Sep 25;250(18):7359–7365. [PubMed] [Google Scholar]
- Nakae T. Outer membrane of Salmonella typhimurium: reconstitution of sucrose-permeable membrane vesicles. Biochem Biophys Res Commun. 1975 Jun 16;64(4):1224–1230. doi: 10.1016/0006-291x(75)90823-2. [DOI] [PubMed] [Google Scholar]
- Nikaido H., Nakae T. The outer membrane of Gram-negative bacteria. Adv Microb Physiol. 1979;20:163–250. doi: 10.1016/s0065-2911(08)60208-8. [DOI] [PubMed] [Google Scholar]