Abstract
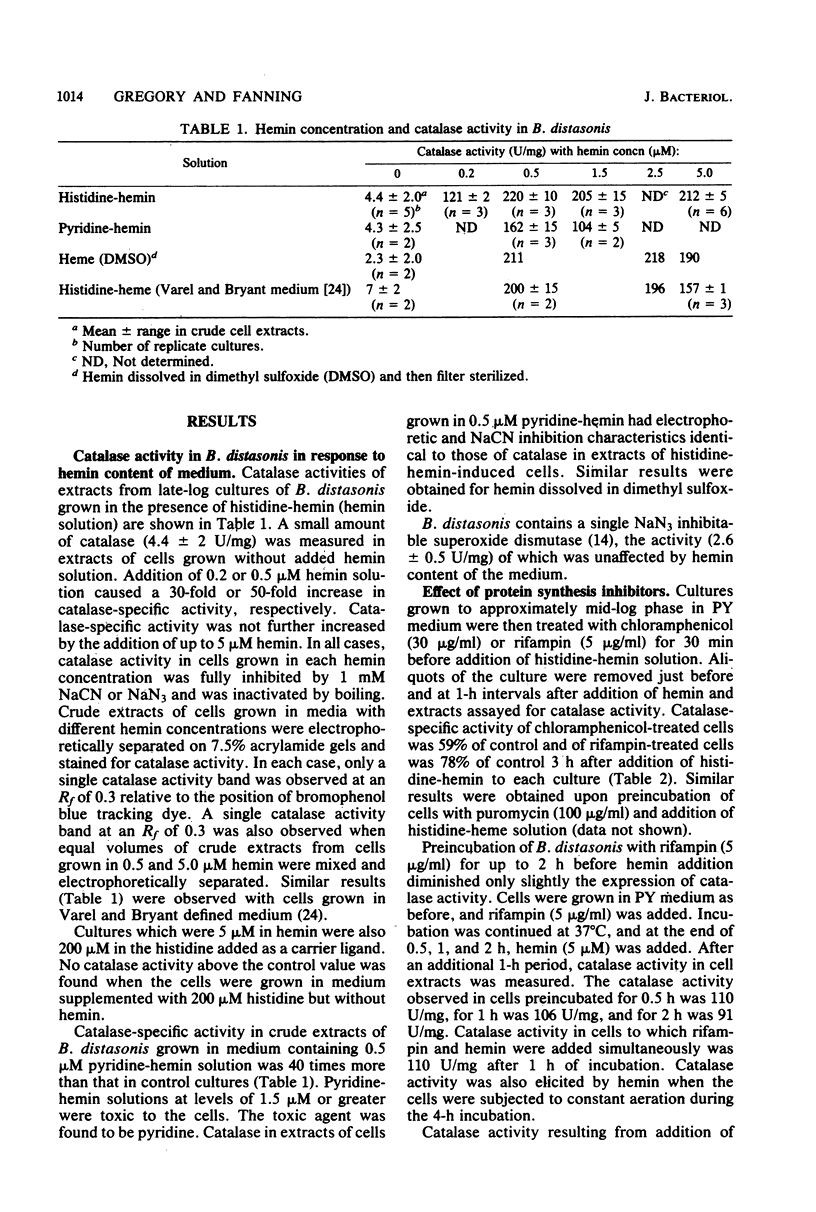
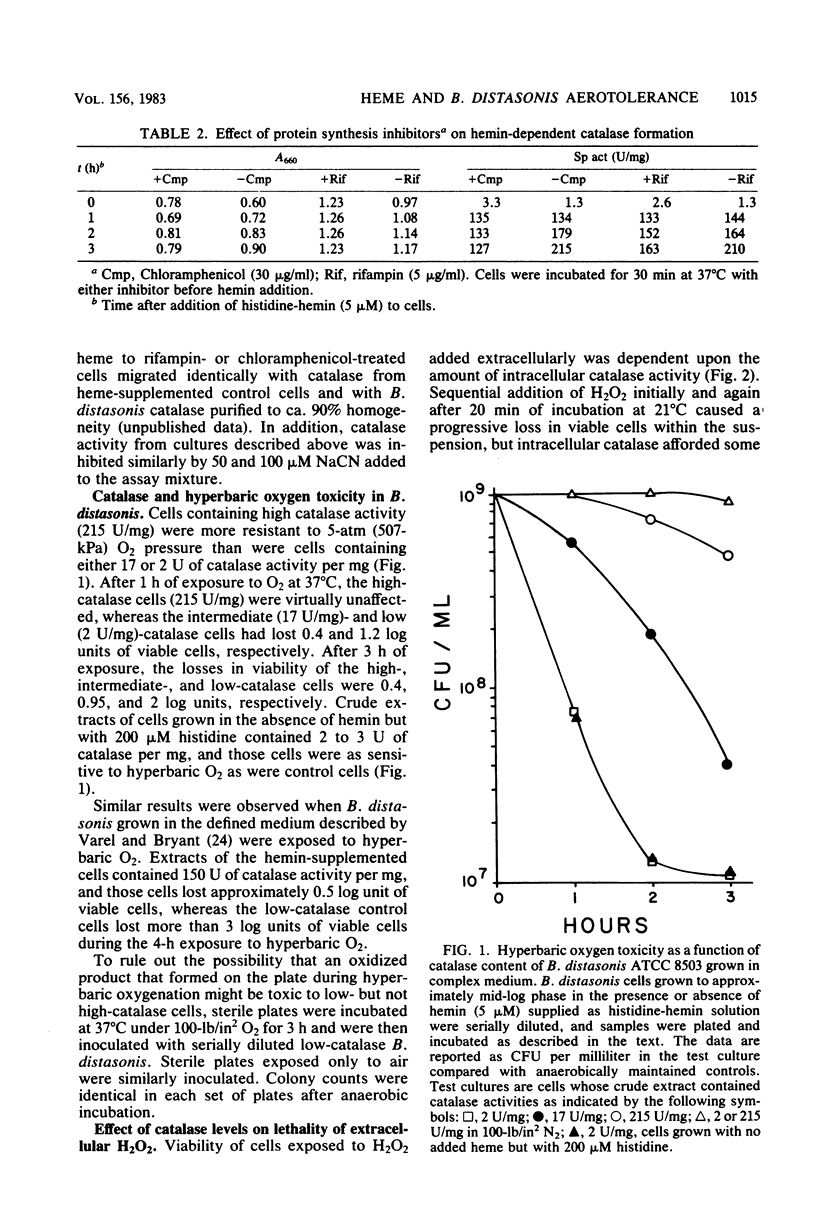
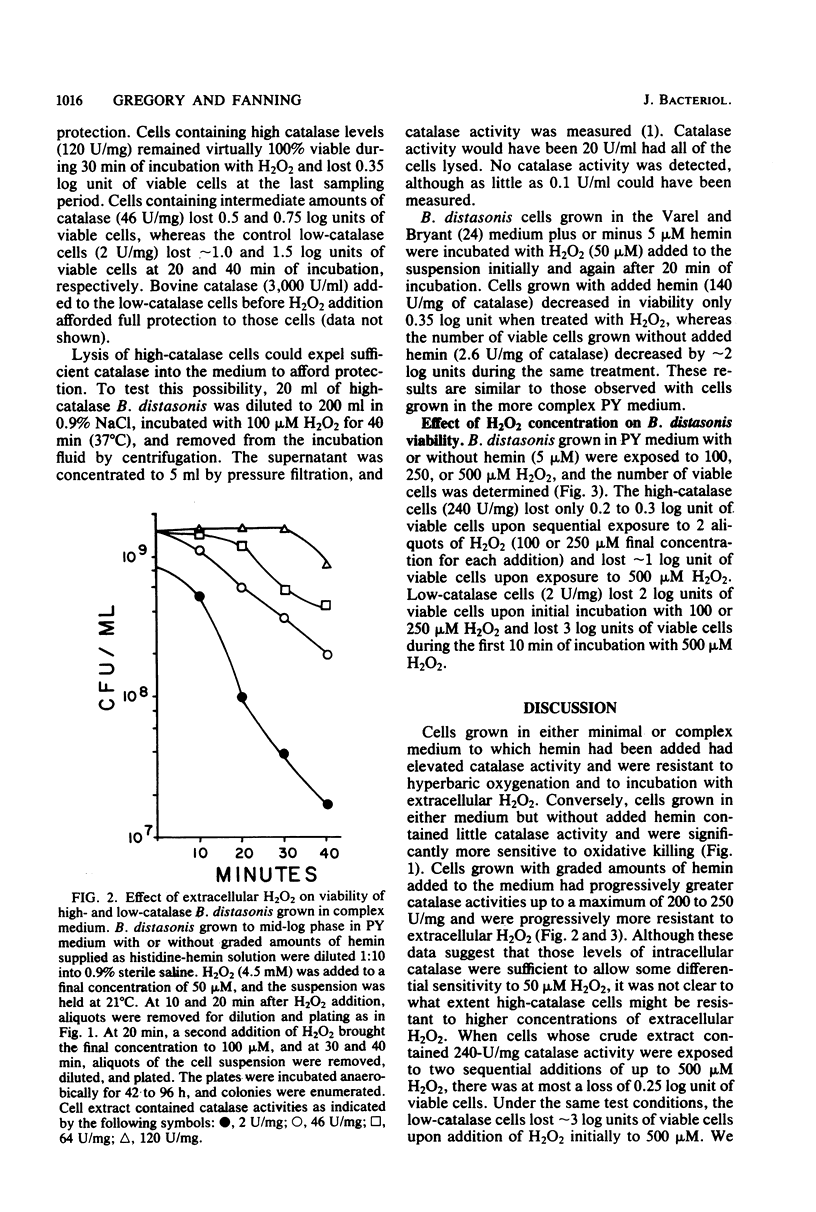
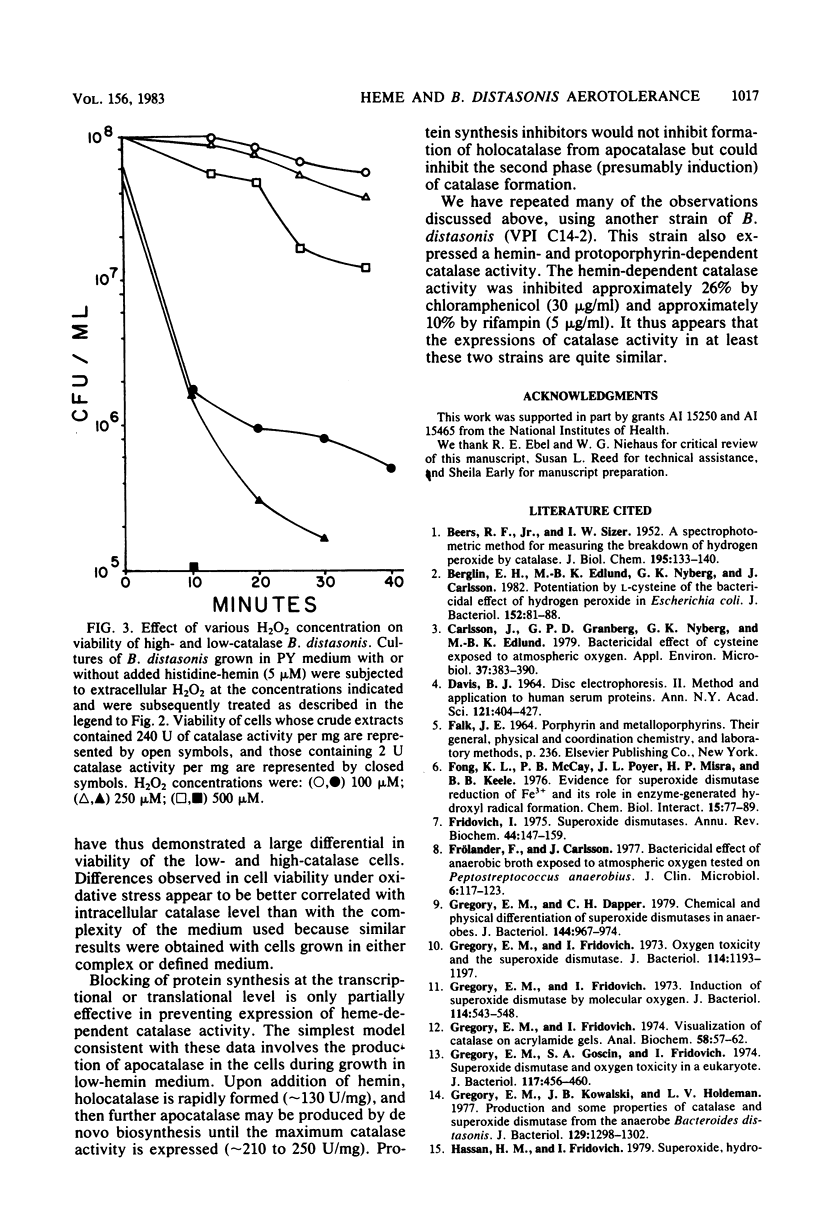
Parallel increases in intracellular catalase activity and resistance to extracellular H2O2 and to hyperbaric O2 toxicity were observed when Bacteroides distasonis VPI 4243 (ATCC 8503, type strain) was grown in either complex or defined medium containing graded amounts of hemin. Virtually all of the cells with high catalase activity (greater than 200 U/mg) remained viable upon exposure at 37 degrees C to 100-lb/in2 O2 on agar surfaces for 1 h, whereas low-catalase cells (less than 10 U/mg) lost 1.2 log units of viable cells during that treatment. Upon exposure to 500 microM H2O2, high-catalase cells lost 0.4 log units of the initial viable colonies during the same period in which low-catalase cells lost 3 log units of viable cells. The superoxide dismutase activity was the same in each test culture. These data support the role of intracellular catalase in protecting B. distasonis from oxidative damage resulting from hyperbaric oxygenation or H2O2 exposure. Catalase activity elicited by adding hemin to cells grown previously in medium lacking hemin was inhibited only 40% by prior incubation of the cells with chloramphenicol (30 micrograms/ml) and only 22% with rifampin (5 micrograms/ml). A model which is consistent with these data involves the production of an apocatalase in cells grown in low-hemin medium. Addition of hemin to the cells would result in a rapid chloramphenicolor rifampin-insensitive stimulation of catalase activity followed by further de novo biosynthesis of catalase.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BEERS R. F., Jr, SIZER I. W. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J Biol Chem. 1952 Mar;195(1):133–140. [PubMed] [Google Scholar]
- Berglin E. H., Edlund M. B., Nyberg G. K., Carlsson J. Potentiation by L-cysteine of the bactericidal effect of hydrogen peroxide in Escherichia coli. J Bacteriol. 1982 Oct;152(1):81–88. doi: 10.1128/jb.152.1.81-88.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson J., Granberg G. P., Nyberg G. K., Edlund M. B. Bactericidal effect of cysteine exposed to atmospheric oxygen. Appl Environ Microbiol. 1979 Mar;37(3):383–390. doi: 10.1128/aem.37.3.383-390.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Fong K. L., McCay P. B., Poyer J. L. Evidence for superoxide-dependent reduction of Fe3+ and its role in enzyme-generated hydroxyl radical formation. Chem Biol Interact. 1976 Sep;15(1):77–89. doi: 10.1016/0009-2797(76)90130-7. [DOI] [PubMed] [Google Scholar]
- Fridovich I. Superoxide dismutases. Annu Rev Biochem. 1975;44:147–159. doi: 10.1146/annurev.bi.44.070175.001051. [DOI] [PubMed] [Google Scholar]
- Frölander F., Carlsson J. Bactericidal effect of anaerobic broth exposed to atmospheric oxygen tested on Peptostreptococcus anaerobius. J Clin Microbiol. 1977 Aug;6(2):117–123. doi: 10.1128/jcm.6.2.117-123.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory E. M., Dapper C. H. Chemical and physical differentiation of superoxide dismutases in anaerobes. J Bacteriol. 1980 Dec;144(3):967–974. doi: 10.1128/jb.144.3.967-974.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory E. M., Fridovich I. Induction of superoxide dismutase by molecular oxygen. J Bacteriol. 1973 May;114(2):543–548. doi: 10.1128/jb.114.2.543-548.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory E. M., Fridovich I. Oxygen toxicity and the superoxide dismutase. J Bacteriol. 1973 Jun;114(3):1193–1197. doi: 10.1128/jb.114.3.1193-1197.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory E. M., Fridovich I. Visualization of catalase on acrylamide gels. Anal Biochem. 1974 Mar;58(1):57–62. doi: 10.1016/0003-2697(74)90440-0. [DOI] [PubMed] [Google Scholar]
- Gregory E. M., Goscin S. A., Fridovich I. Superoxide dismutase and oxygen toxicity in a eukaryote. J Bacteriol. 1974 Feb;117(2):456–460. doi: 10.1128/jb.117.2.456-460.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory E. M., Kowalski J. B., Holdeman L. V. Production and some properties of catalase and superoxide dismutase from the anaerobe Bacteroides distasonis. J Bacteriol. 1977 Mar;129(3):1298–1302. doi: 10.1128/jb.129.3.1298-1302.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keele B. B., Jr, McCord J. M., Fridovich I. Superoxide dismutase from escherichia coli B. A new manganese-containing enzyme. J Biol Chem. 1970 Nov 25;245(22):6176–6181. [PubMed] [Google Scholar]
- Koch A. L. Turbidity measurements of bacterial cultures in some available commercial instruments. Anal Biochem. 1970 Nov;38(1):252–259. doi: 10.1016/0003-2697(70)90174-0. [DOI] [PubMed] [Google Scholar]
- Mandell G. L. Catalase, superoxide dismutase, and virulence of Staphylococcus aureus. In vitro and in vivo studies with emphasis on staphylococcal--leukocyte interaction. J Clin Invest. 1975 Mar;55(3):561–566. doi: 10.1172/JCI107963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCord J. M., Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem. 1969 Nov 25;244(22):6049–6055. [PubMed] [Google Scholar]
- Norrod P., Morse S. A. Absence of superoxide dismutase in some strains of Neisseria gonorrhoeae. Biochem Biophys Res Commun. 1979 Oct 29;90(4):1287–1294. doi: 10.1016/0006-291x(79)91176-8. [DOI] [PubMed] [Google Scholar]
- Privalle C. T., Gregory E. M. Superoxide dismutase and O2 lethality in Bacteroides fragilis. J Bacteriol. 1979 Apr;138(1):139–145. doi: 10.1128/jb.138.1.139-145.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sels A. A., Brygier J. Inhibition studies in situ of yeast catalases. Eur J Biochem. 1980 Nov;112(2):283–291. doi: 10.1111/j.1432-1033.1980.tb07204.x. [DOI] [PubMed] [Google Scholar]
- Varel V. H., Bryant M. P. Nutritional features of Bacteroides fragilis subsp. fragilis. Appl Microbiol. 1974 Aug;28(2):251–257. doi: 10.1128/am.28.2.251-257.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterman M. R. Spectral characterization of human hemoglobin and its derivatives. Methods Enzymol. 1978;52:456–463. doi: 10.1016/s0076-6879(78)52050-8. [DOI] [PubMed] [Google Scholar]
- Wilkins T. D., Wagner D. L., Veltri B. J., Jr, Gregory E. M. Factors affecting production of catalase by Bacteroides. J Clin Microbiol. 1978 Nov;8(5):553–557. doi: 10.1128/jcm.8.5.553-557.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yost F. J., Jr, Fridovich I. An iron-containing superoxide dismutase from Escherichia coli. J Biol Chem. 1973 Jul 25;248(14):4905–4908. [PubMed] [Google Scholar]



