Abstract
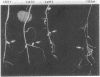
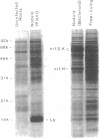
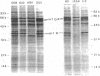
To investigate the expression of specific symbiotic genes during the development of nitrogen-fixing root nodules, we conducted a systematic analysis of nodule-specific proteins and RNAs produced after the inoculation of alfalfa roots with a series of Rhizobium meliloti mutants generated by site-directed transposon Tn5 mutagenesis. The mutagenized region of the Rhizobium genome covered approximately 10 kilobases and included the region encoding the nitrogenase polypeptides. All mutant strains that were analyzed produced nodules, but with several strains the nodules failed to fix nitrogen (Nod+ Fix- phenotype). All Fix- nodules accumulated reduced levels of the host plant protein leghemoglobin. In addition, Tn5 insertions in the nitrogenase operon (nifHDK genes) eliminated some or all of the nitrogenase polypeptides and nifHDK RNA transcripts, depending on the site of insertion. Finally, mutation of a region approximately 5 kilobases upstream from the nitrogenase operon resulted in the absence of all three nitrogenase polypeptides and their corresponding RNAs, suggesting that this region may serve a regulatory function during nitrogen fixation. The studies presented here indicate that site-directed mutagenesis coupled with biochemical analysis of nodule proteins and RNAs allows the identification of products of specific gene regions as well as the assignment of specific functions to previously unidentified regions of the R. meliloti genome.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berg D. E., Weiss A., Crossland L. Polarity of Tn5 insertion mutations in Escherichia coli. J Bacteriol. 1980 May;142(2):439–446. doi: 10.1128/jb.142.2.439-446.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger S. L., Birkenmeier C. S. Inhibition of intractable nucleases with ribonucleoside--vanadyl complexes: isolation of messenger ribonucleic acid from resting lymphocytes. Biochemistry. 1979 Nov 13;18(23):5143–5149. doi: 10.1021/bi00590a018. [DOI] [PubMed] [Google Scholar]
- Buchanan-Wollaston V., Cannon M. C., Beynon J. L., Cannon F. C. Role of the nifA gene product in the regulation of nif expression in Klebsiella pneumoniae. Nature. 1981 Dec 24;294(5843):776–778. doi: 10.1038/294776a0. [DOI] [PubMed] [Google Scholar]
- Buikema W. J., Long S. R., Brown S. E., van den Bos R. C., Earl C., Ausubel F. M. Physical and genetic characterization of Rhizobium meliloti symbiotic mutants. J Mol Appl Genet. 1983;2(3):249–260. [PubMed] [Google Scholar]
- Bánfalvi Z., Sakanyan V., Koncz C., Kiss A., Dusha I., Kondorosi A. Location of nodulation and nitrogen fixation genes on a high molecular weight plasmid of R. meliloti. Mol Gen Genet. 1981;184(2):318–325. doi: 10.1007/BF00272925. [DOI] [PubMed] [Google Scholar]
- Corbin D., Barran L., Ditta G. Organization and expression of Rhizobium meliloti nitrogen fixation genes. Proc Natl Acad Sci U S A. 1983 May;80(10):3005–3009. doi: 10.1073/pnas.80.10.3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbin D., Ditta G., Helinski D. R. Clustering of nitrogen fixation (nif) genes in Rhizobium meliloti. J Bacteriol. 1982 Jan;149(1):221–228. doi: 10.1128/jb.149.1.221-228.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dilworth M. J. The plant as the genetic determinant of leghaemoglobin production in the legume root nodule. Biochim Biophys Acta. 1969 Jul 30;184(2):432–441. doi: 10.1016/0304-4165(69)90047-6. [DOI] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Forrai T., Vincze E., Bánfalvi Z., Kiss G. B., Randhawa G. S., Kondorosi A. Localization of symbiotic mutations in Rhizobium meliloti. J Bacteriol. 1983 Feb;153(2):635–643. doi: 10.1128/jb.153.2.635-643.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godson G. N., Vapnek D. A simple method of preparing large amounts of phiX174 RF 1 supercoiled DNA. Biochim Biophys Acta. 1973 Apr 11;299(4):516–520. doi: 10.1016/0005-2787(73)90223-2. [DOI] [PubMed] [Google Scholar]
- Jackson A. O., Larkins B. A. Influence of Ionic Strength, pH, and Chelation of Divalent Metals on Isolation of Polyribosomes from Tobacco Leaves. Plant Physiol. 1976 Jan;57(1):5–10. doi: 10.1104/pp.57.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legocki R. P., Verma D. P. Identification of "nodule-specific" host proteins (nodoulins) involved in the development of rhizobium-legume symbiosis. Cell. 1980 May;20(1):153–163. doi: 10.1016/0092-8674(80)90243-3. [DOI] [PubMed] [Google Scholar]
- Lienhard G. E., Secemski I. I., Koehler K. A., Lindquist R. N. Enzymatic catalysis and the transition state theory of reaction rates: transition state analogs. Cold Spring Harb Symp Quant Biol. 1972;36:45–51. doi: 10.1101/sqb.1972.036.01.009. [DOI] [PubMed] [Google Scholar]
- MacNeil T., MacNeil D., Roberts G. P., Supiano M. A., Brill W. J. Fine-structure mapping and complementation analysis of nif (nitrogen fixation) genes in Klebsiella pneumoniae. J Bacteriol. 1978 Oct;136(1):253–266. doi: 10.1128/jb.136.1.253-266.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magasanik B. Genetic control of nitrogen assimilation in bacteria. Annu Rev Genet. 1982;16:135–168. doi: 10.1146/annurev.ge.16.120182.001031. [DOI] [PubMed] [Google Scholar]
- Maizels N. Dictyostelium 17S, 25S, and 5S rDNAs lie within a 38,000 base pair repeated unit. Cell. 1976 Nov;9(3):431–438. doi: 10.1016/0092-8674(76)90088-x. [DOI] [PubMed] [Google Scholar]
- Meade H. M., Long S. R., Ruvkun G. B., Brown S. E., Ausubel F. M. Physical and genetic characterization of symbiotic and auxotrophic mutants of Rhizobium meliloti induced by transposon Tn5 mutagenesis. J Bacteriol. 1982 Jan;149(1):114–122. doi: 10.1128/jb.149.1.114-122.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Roberts G. P., MacNeil T., MacNeil D., Brill W. J. Regulation and characterization of protein products coded by the nif (nitrogen fixation) genes of Klebsiella pneumoniae. J Bacteriol. 1978 Oct;136(1):267–279. doi: 10.1128/jb.136.1.267-279.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg C., Boistard P., Dénarié J., Casse-Delbart F. Genes controlling early and late functions in symbiosis are located on a megaplasmid in Rhizobium meliloti. Mol Gen Genet. 1981;184(2):326–333. doi: 10.1007/BF00272926. [DOI] [PubMed] [Google Scholar]
- Ruvkun G. B., Ausubel F. M. A general method for site-directed mutagenesis in prokaryotes. Nature. 1981 Jan 1;289(5793):85–88. doi: 10.1038/289085a0. [DOI] [PubMed] [Google Scholar]
- Ruvkun G. B., Ausubel F. M. Interspecies homology of nitrogenase genes. Proc Natl Acad Sci U S A. 1980 Jan;77(1):191–195. doi: 10.1073/pnas.77.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruvkun G. B., Long S. R., Meade H. M., van den Bos R. C., Ausubel F. M. ISRm1: A Rhizobium meliloti insertion sequence that transposes preferentially into nitrogen fixation genes. J Mol Appl Genet. 1982;1(5):405–418. [PubMed] [Google Scholar]
- Ruvkun G. B., Sundaresan V., Ausubel F. M. Directed transposon Tn5 mutagenesis and complementation analysis of Rhizobium meliloti symbiotic nitrogen fixation genes. Cell. 1982 Jun;29(2):551–559. doi: 10.1016/0092-8674(82)90171-4. [DOI] [PubMed] [Google Scholar]
- Sundaresan V., Jones J. D., Ow D. W., Ausubel F. M. Klebsiella pneumoniae nifA product activates the Rhizobium meliloti nitrogenase promoter. Nature. 1983 Feb 24;301(5902):728–732. doi: 10.1038/301728a0. [DOI] [PubMed] [Google Scholar]
- Sundaresan V., Ow D. W., Ausubel F. M. Activation of Klebsiella pneumoniae and Rhizobium meliloti nitrogenase promoters by gln (ntr) regulatory proteins. Proc Natl Acad Sci U S A. 1983 Jul;80(13):4030–4034. doi: 10.1073/pnas.80.13.4030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma D. P., Bal A. K. Intracellular site of synthesis and localization of leghemoglobin in root nodules. Proc Natl Acad Sci U S A. 1976 Nov;73(11):3843–3847. doi: 10.1073/pnas.73.11.3843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma D. P., Haugland R., Brisson N., Legocki R. P., Lacroix L. Regulation of the expression of leghaemoglobin genes in effective and ineffective root nodules of soybean. Biochim Biophys Acta. 1981 Mar 26;653(1):98–107. doi: 10.1016/0005-2787(81)90108-8. [DOI] [PubMed] [Google Scholar]