Abstract
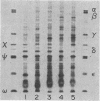
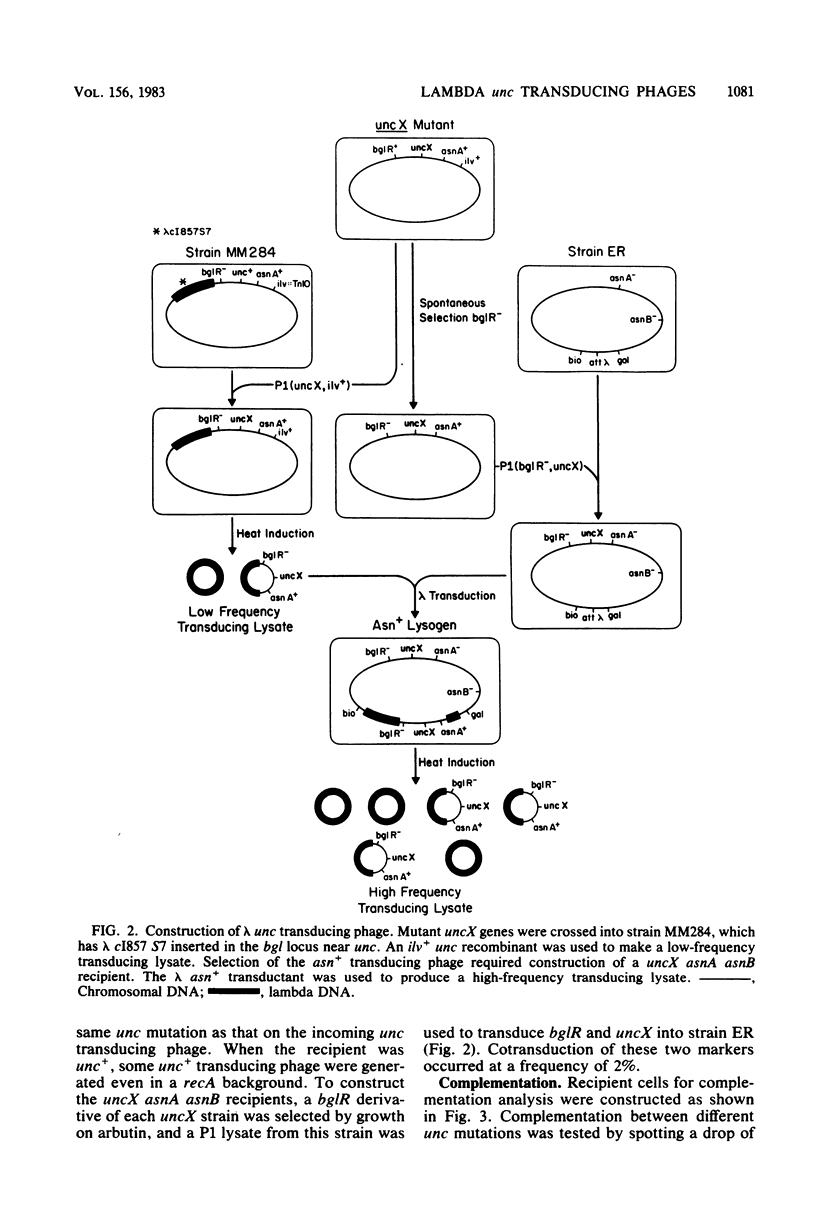
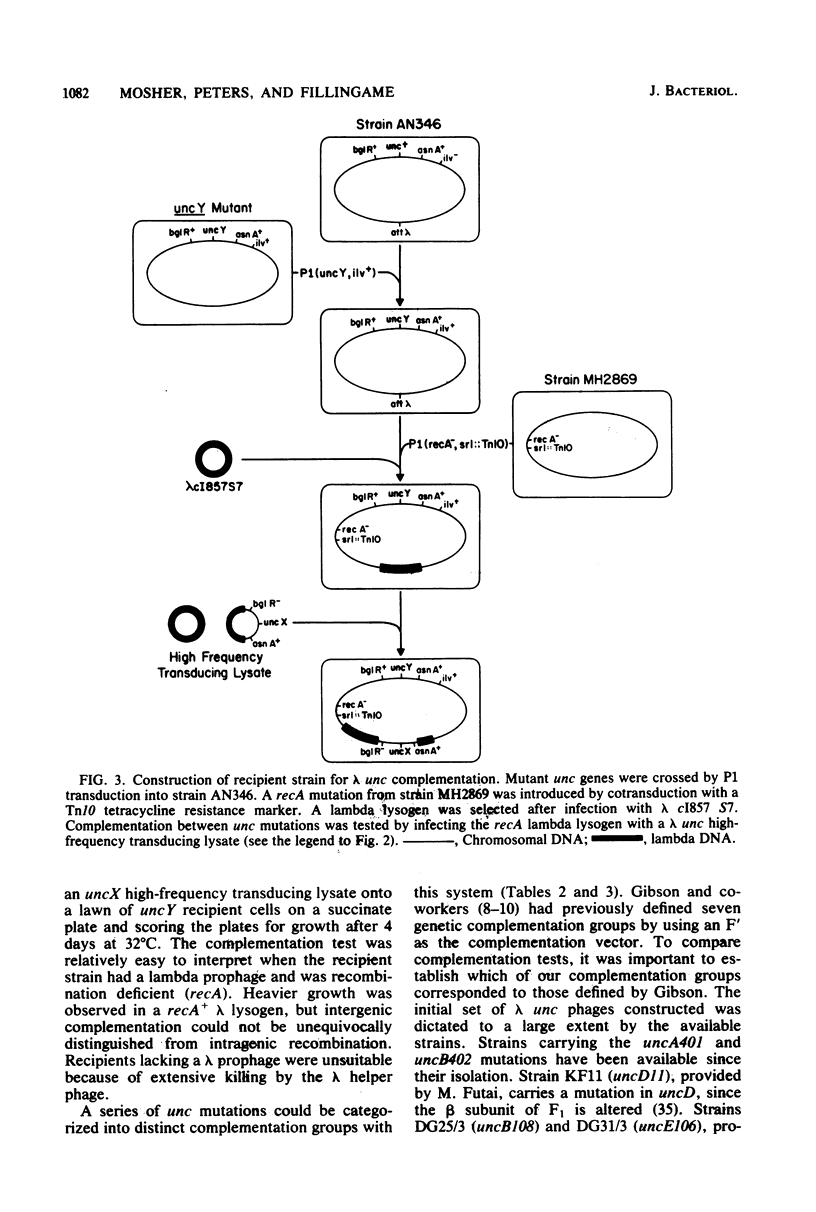
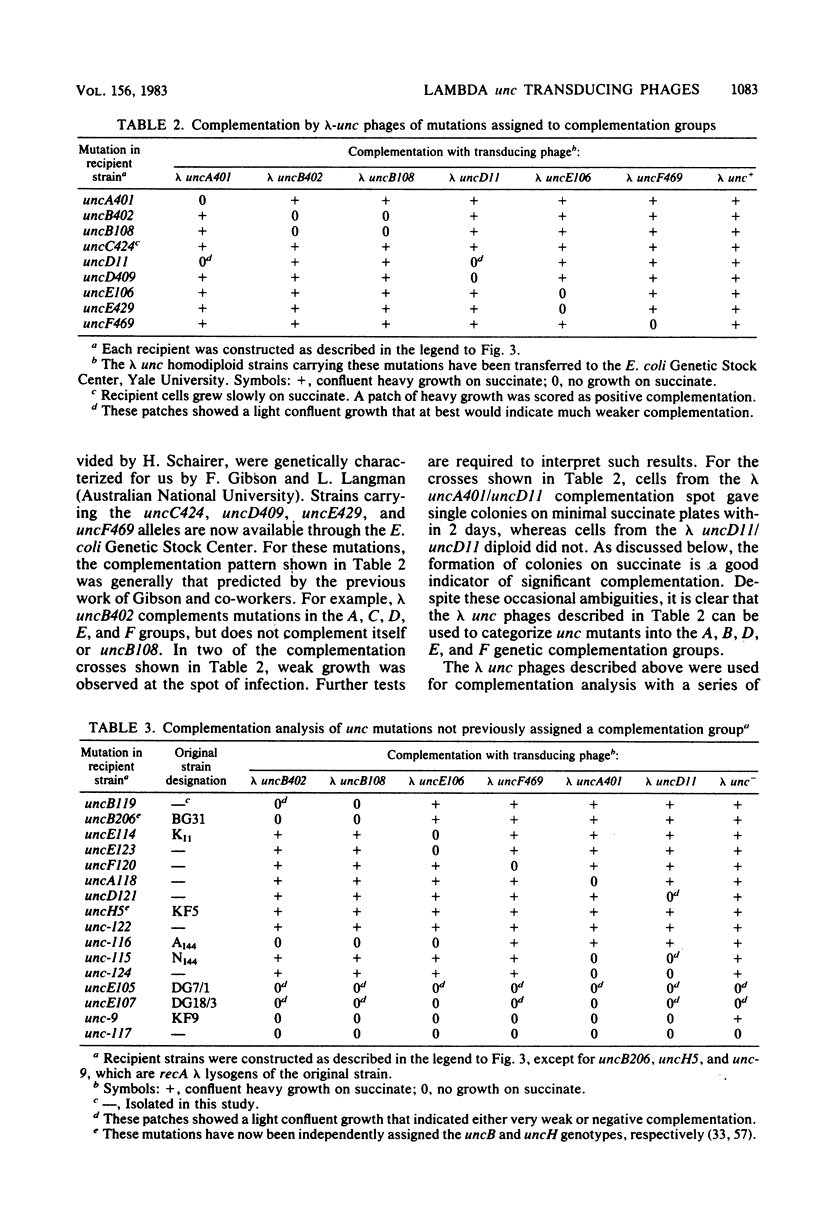
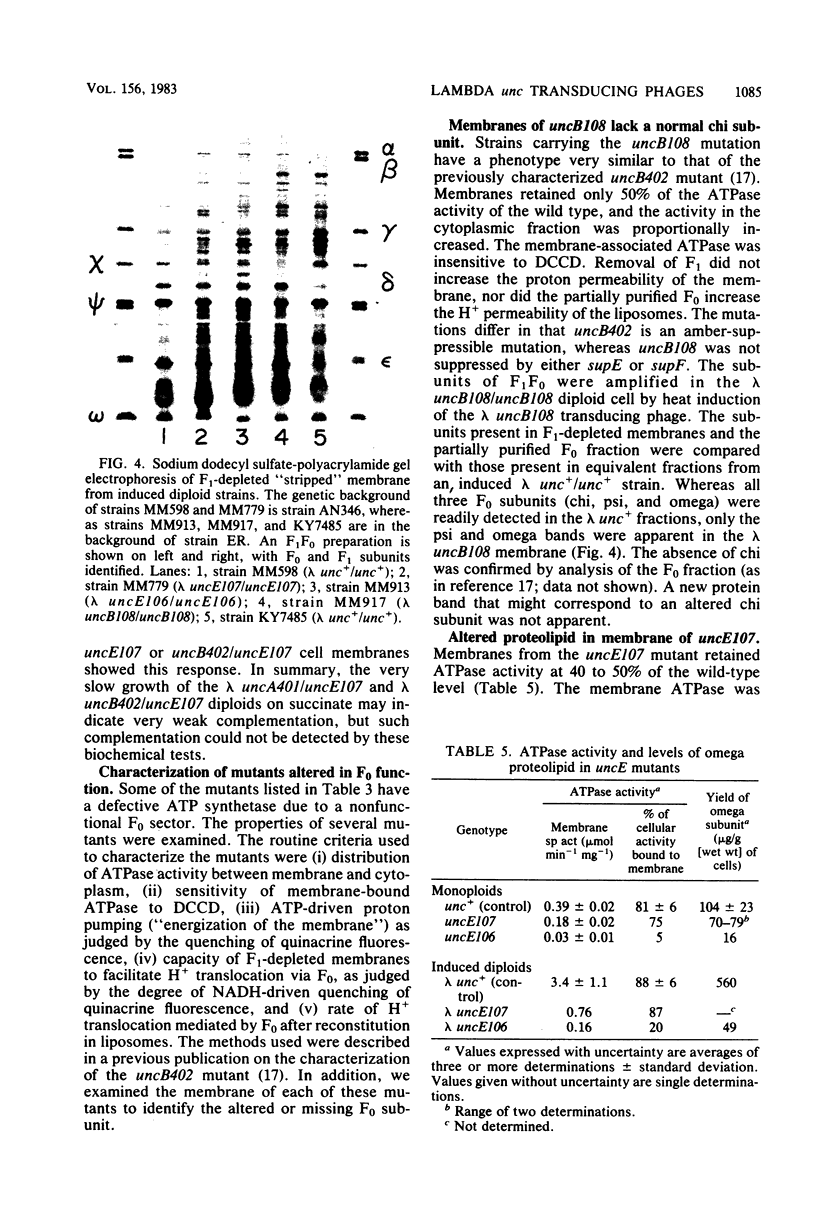
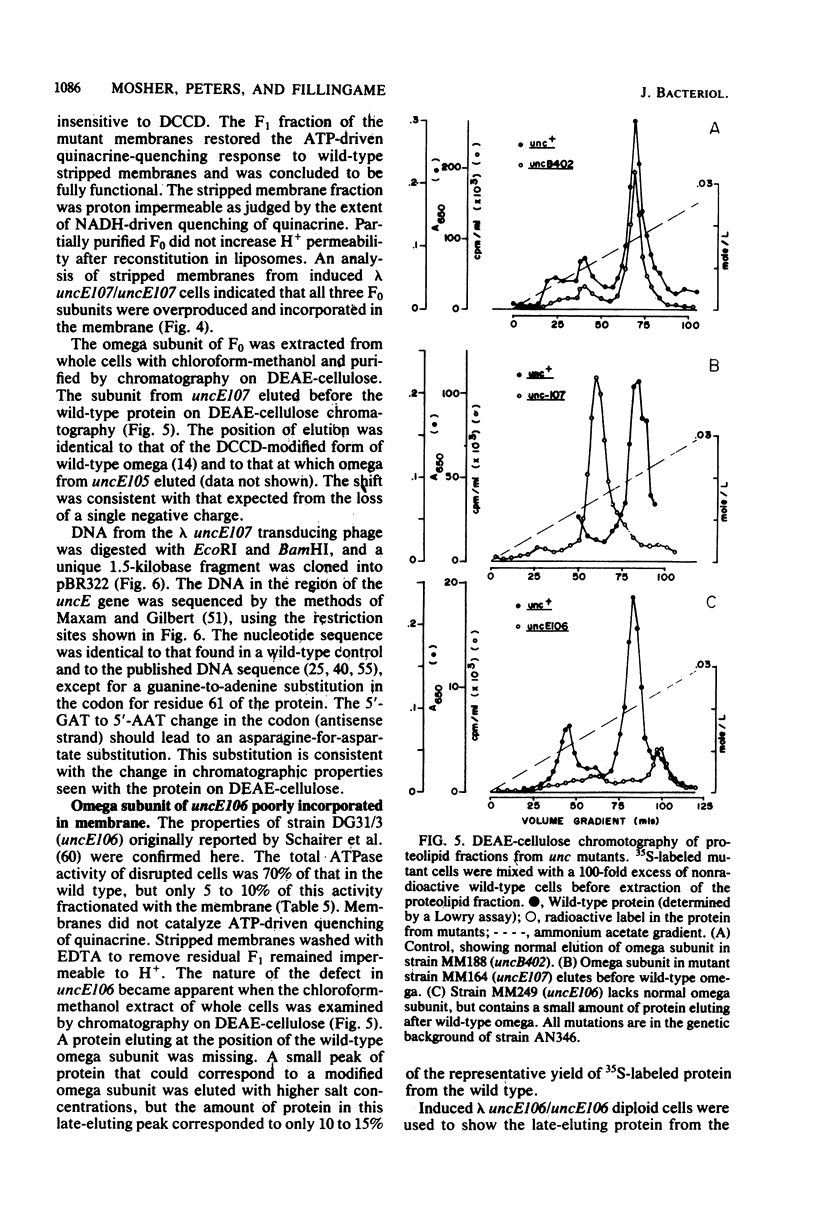
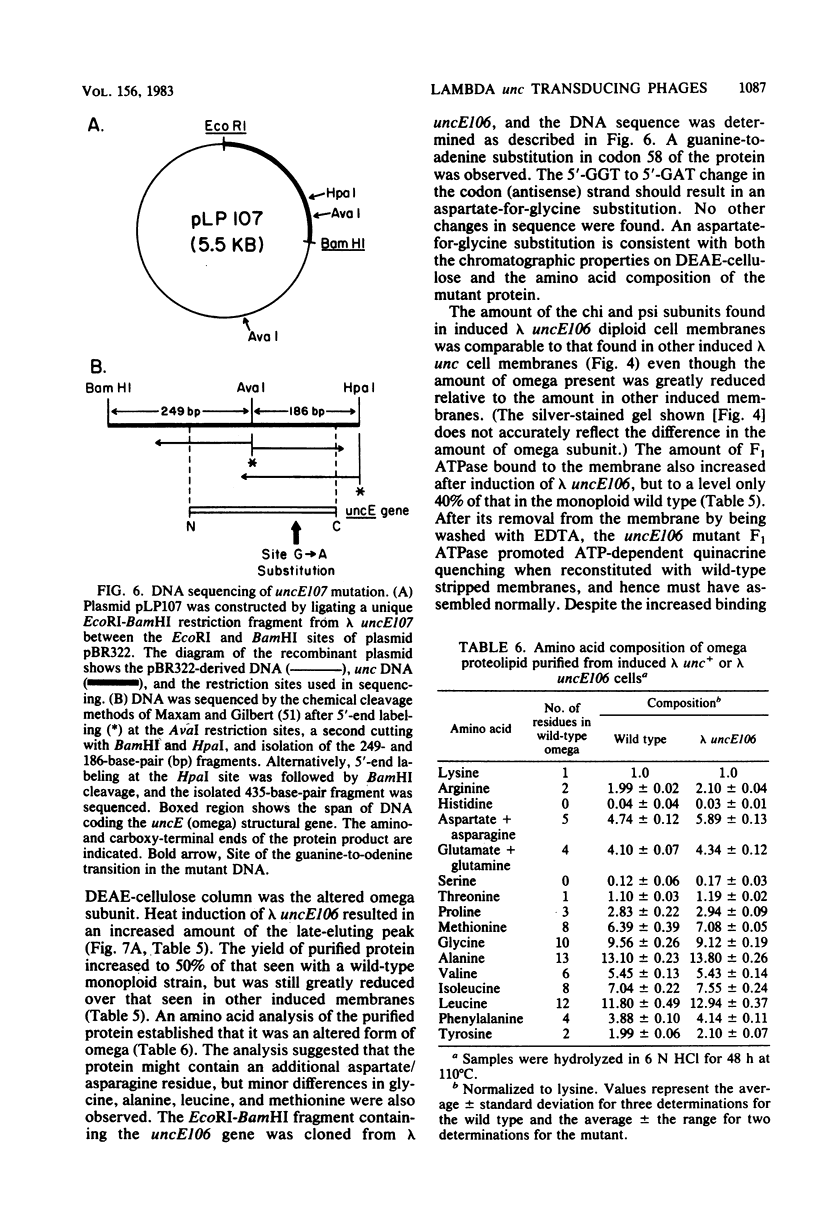
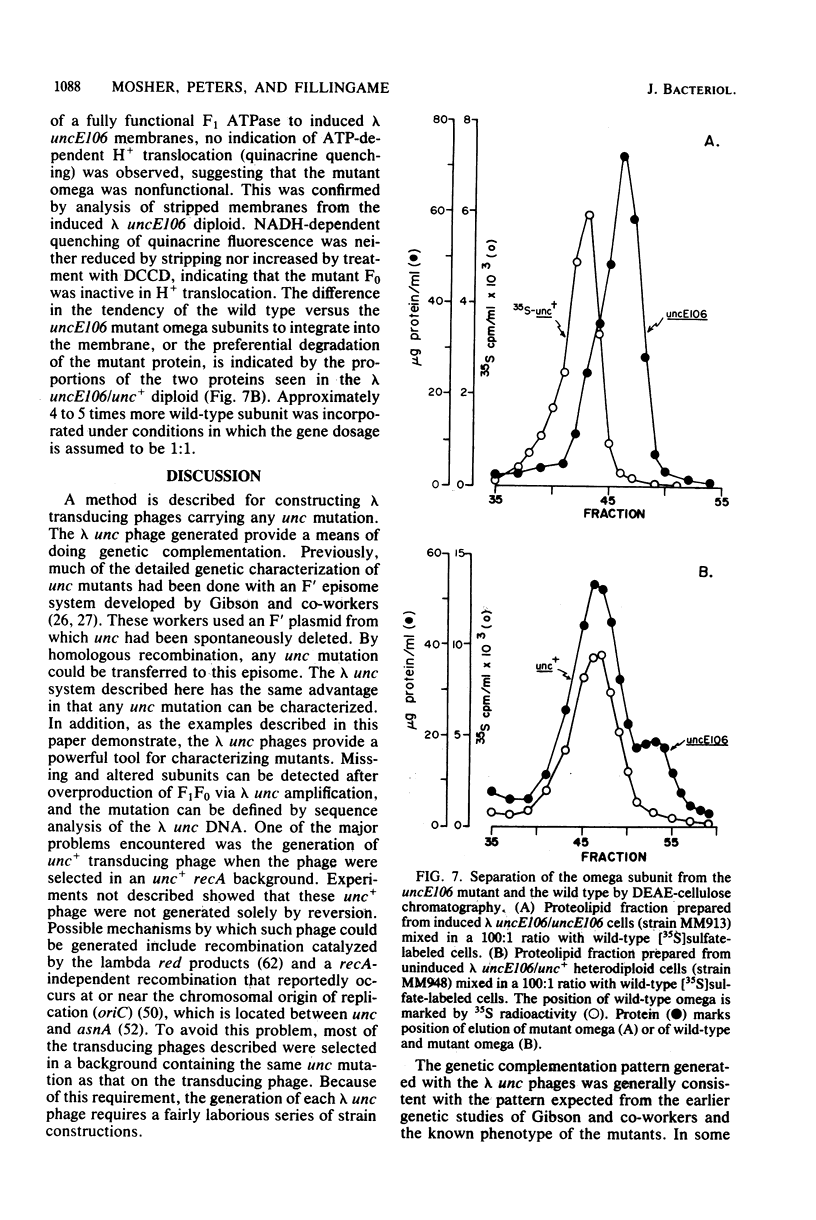
The eight subunits of the H+-ATPase of Escherichia coli are coded by the genes of the unc operon, which maps between bglB and asnA. A collection of unc mutations were transferred via P1 transduction into a strain in which lambda cI857 S7 was inserted into bglB. The lambda phage was induced, and asnA+ transducing phage that carried unc were selected. Transducing phage carrying mutations in the uncA, B, D, E, and F genes were used for complementation analysis with a collection of unc mutants, including mutants which had been reported previously but not genetically characterized. Some mutations gave a simple complementation pattern, indicating a single defective gene, whereas other mutations gave more complex patterns. Two mutants (uncE105 and uncE107) altered in the proteolipid (omega) subunit of F0 were not complemented by any of the lambda unc phage, even though both mutants had a fully functional F1 ATPase and therefore normal A and D genes. Hence, only limited conclusions can be drawn from genetic complementation alone, since it cannot distinguish normal from abnormal genes in certain classes of unc mutants. The lambda unc phage proved to be essential in characterizing several mutants defective in F0-mediated H+ translocation. The unc gene products were overproduced by heat induction of the lysogenized lambda unc phage to determine whether all the F0 subunits were in the membrane. Two mutants that gave a simple complementation pattern, indicative of one defective gene, did not assemble a three-subunit F0. The uncB108 mutant was shown to lack the chi subunit of F0 but to retain psi and omega. Trace amounts of an altered omega subunit and normal amounts of chi and psi were found in the uncE106 mutant. A substitution of aspartate for glycine at residue 58 of the protein was determined by DNA sequence analysis of the uncE gene cloned from the lambda uncE106 phage DNA. One of the omega-defective, noncomplementing mutants (uncE107) was shown to retain all three F0 subunits. The uncE gene from this mutant was also sequenced to confirm an asparagine-for-aspartate substitution at position 61 (the dicyclohexylcarbodiimide-binding site) of the omega subunit.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blattner F. R., Williams B. G., Blechl A. E., Denniston-Thompson K., Faber H. E., Furlong L., Grunwald D. J., Kiefer D. O., Moore D. D., Schumm J. W. Charon phages: safer derivatives of bacteriophage lambda for DNA cloning. Science. 1977 Apr 8;196(4286):161–169. doi: 10.1126/science.847462. [DOI] [PubMed] [Google Scholar]
- Bragg P. D., Hou C. Subunit composition, function, and spatial arrangement in the Ca2+-and Mg2+-activated adenosine triphosphatases of Escherichia coli and Salmonella typhimurium. Arch Biochem Biophys. 1975 Mar;167(1):311–321. doi: 10.1016/0003-9861(75)90467-1. [DOI] [PubMed] [Google Scholar]
- Butlin J. D., Cox G. B., Gibson F. Oxidative phosphorylation in Escherichia coli K-12: the genetic and biochemical characterisations of a strain carrying a mutation in the uncB gene. Biochim Biophys Acta. 1973 Feb 22;292(2):366–375. doi: 10.1016/0005-2728(73)90043-1. [DOI] [PubMed] [Google Scholar]
- Butlin J. D., Cox G. B., Gibson F. Oxidative phosphorylation in Escherichia coli K12. Mutations affecting magnesium ion- or calcium ion-stimulated adenosine triphosphatase. Biochem J. 1971 Aug;124(1):75–81. doi: 10.1042/bj1240075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox G. B., Downie J. A., Gibson F., Radik J. Genetic complementation between two mutant unc alleles (unc A401 and unc D409) affecting the Fl portion of the magnesium ion-stimulated adenosine triphosphatase of Escherichia coli K12. Biochem J. 1978 Mar 15;170(3):593–598. doi: 10.1042/bj1700593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox G. B., Downie J. A., Langman L., Senior A. E., Ash G., Fayle D. R., Gibson F. Assembly of the adenosine triphosphatase complex in Escherichia coli: assembly of F0 is dependent on the formation of specific F1 subunits. J Bacteriol. 1981 Oct;148(1):30–42. doi: 10.1128/jb.148.1.30-42.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downie J. A., Cox G. B., Langman L., Ash G., Becker M., Gibson F. Three genes coding for subunits of the membrane sector (F0) of the Escherichia coli adenosine triphosphatase complex. J Bacteriol. 1981 Jan;145(1):200–210. doi: 10.1128/jb.145.1.200-210.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downie J. A., Gibson F., Cox G. B. Membrane adenosine triphosphatases of prokaryotic cells. Annu Rev Biochem. 1979;48:103–131. doi: 10.1146/annurev.bi.48.070179.000535. [DOI] [PubMed] [Google Scholar]
- Downie J. A., Langman L., Cox G. B., Yanofsky C., Gibson F. Subunits of the adenosine triphosphatase complex translated in vitro from the Escherichia coli unc operon. J Bacteriol. 1980 Jul;143(1):8–17. doi: 10.1128/jb.143.1.8-17.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downie J. A., Senior A. E., Gibson F., Cox G. B. A fifth gene (uncE) in the operon concerned with oxidative phosphorylation in Escherichia coli. J Bacteriol. 1979 Feb;137(2):711–718. doi: 10.1128/jb.137.2.711-718.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fayle D. R., Downie J. A., Cox G. B., Gibson F., Radik J. Characterization of the mutant-unc D-gene product in a strain of Escherichia coli K12. An altered beta-subunit of the magnesium ion-stimulated adenosine triphosphatase. Biochem J. 1978 Jun 15;172(3):523–531. doi: 10.1042/bj1720523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felton J., Michaelis S., Wright A. Mutations in two unlinked genes are required to produce asparagine auxotrophy in Escherichia coli. J Bacteriol. 1980 Apr;142(1):221–228. doi: 10.1128/jb.142.1.221-228.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillingame R. H., Mosher M. E., Negrin R. S., Peters L. K. H+-ATPase of Escherichia coli uncB402 mutation leads to loss of chi subunit of subunit of F0 sector. J Biol Chem. 1983 Jan 10;258(1):604–609. [PubMed] [Google Scholar]
- Fillingame R. H. Purification of the carbodiimide-reactive protein component of the ATP energy-transducing system of Escherichia coli. J Biol Chem. 1976 Nov 10;251(21):6630–6637. [PubMed] [Google Scholar]
- Fillingame R. H. The proton-translocating pumps of oxidative phosphorylation. Annu Rev Biochem. 1980;49:1079–1113. doi: 10.1146/annurev.bi.49.070180.005243. [DOI] [PubMed] [Google Scholar]
- Foster D. L., Fillingame R. H. Energy-transducing H+-ATPase of Escherichia coli. Purification, reconstitution, and subunit composition. J Biol Chem. 1979 Sep 10;254(17):8230–8236. [PubMed] [Google Scholar]
- Foster D. L., Fillingame R. H. Stoichiometry of subunits in the H+-ATPase complex of Escherichia coli. J Biol Chem. 1982 Feb 25;257(4):2009–2015. [PubMed] [Google Scholar]
- Foster D. L., Mosher M. E., Futai M., Fillingame R. H. Subunits of the H+-ATPase of Escherichia coli. Overproduction of an eight-subunit F1F0-ATPase following induction of a lambda-transducing phage carrying the unc operon. J Biol Chem. 1980 Dec 25;255(24):12037–12041. [PubMed] [Google Scholar]
- Friedl P., Friedl C., Schairer H. U. F0 of Escherichia coli ATP-synthase containing mutant and wild-type carbodiimide-binging proteins is impaired in H+ -conduction. FEBS Lett. 1980 Oct 6;119(2):254–256. doi: 10.1016/0014-5793(80)80265-1. [DOI] [PubMed] [Google Scholar]
- Friedl P., Schairer H. U. The isolated F0 of Escherichia coli aTP-synthase is reconstitutively active in H+-conduction and ATP-dependent energy-transduction. FEBS Lett. 1981 Jun 15;128(2):261–264. doi: 10.1016/0014-5793(81)80094-4. [DOI] [PubMed] [Google Scholar]
- Gay N. J., Walker J. E. The atp operon: nucleotide sequence of the promoter and the genes for the membrane proteins, and the delta subunit of Escherichia coli ATP-synthase. Nucleic Acids Res. 1981 Aug 25;9(16):3919–3926. doi: 10.1093/nar/9.16.3919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gay N. J., Walker J. E. The atp operon: nucleotide sequence of the region encoding the alpha-subunit of Escherichia coli ATP-synthase. Nucleic Acids Res. 1981 May 11;9(9):2187–2194. doi: 10.1093/nar/9.9.2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson F., Cox G. B., Downie J. A., Radik J. A mutation affecting a second component of the F0 portion of the magnesium ion-stimulated adenosine triphosphatase of Escherichia coli K12. The uncC424 allele. Biochem J. 1977 Apr 15;164(1):193–198. doi: 10.1042/bj1640193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson F., Cox G. B., Downie J. A., Radik J. Partial diploids of Escherichia coli carrying normal and mutant alleles affecting oxidative phosphorylation. Biochem J. 1977 Mar 15;162(3):665–670. doi: 10.1042/bj1620665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giulian G. G., Moss R. L., Greaser M. Improved methodology for analysis and quantitation of proteins on one-dimensional silver-stained slab gels. Anal Biochem. 1983 Mar;129(2):277–287. doi: 10.1016/0003-2697(83)90551-1. [DOI] [PubMed] [Google Scholar]
- Goldberg A. R., Howe M. New mutations in the S cistron of bacteriophage lambda affecting host cell lysis. Virology. 1969 May;38(1):200–202. doi: 10.1016/0042-6822(69)90148-2. [DOI] [PubMed] [Google Scholar]
- Gunsalus R. P., Brusilow W. S., Simoni R. D. Gene order and gene-polypeptide relationships of the proton-translocating ATPase operon (unc) of Escherichia coli. Proc Natl Acad Sci U S A. 1982 Jan;79(2):320–324. doi: 10.1073/pnas.79.2.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoppe J., Schairer H. U., Friedl P., Sebald W. An Asp-Asn substitution in the proteolipid subunit of the ATP-synthase from Escherichia coli leads to a non-functional proton channel. FEBS Lett. 1982 Aug 16;145(1):21–29. doi: 10.1016/0014-5793(82)81198-8. [DOI] [PubMed] [Google Scholar]
- Hoppe J., Schairer H. U., Sebald W. The proteolipid of a mutant ATPase from Escherichia coli defective in H+-conduction contains a glycine instead of the carbodiimide-reactive aspartyl residue. FEBS Lett. 1980 Jan 1;109(1):107–111. doi: 10.1016/0014-5793(80)81321-4. [DOI] [PubMed] [Google Scholar]
- Humbert R., Brusilow W. S., Gunsalus R. P., Klionsky D. J., Simoni R. D. Escherichia coli mutants defective in the uncH gene. J Bacteriol. 1983 Jan;153(1):416–422. doi: 10.1128/jb.153.1.416-422.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JACOB F., WOLLMAN E. L. Etude génétique d'un bactériophage tempéré d'Escherichia coli. l. Le système genétique du bactériophage. Ann Inst Pasteur (Paris) 1954 Dec;87(6):653–673. [PubMed] [Google Scholar]
- Kanazawa H., Horiuchi Y., Takagi M., Ishino Y., Futai M. Coupling factor F1 ATPase with defective beta subunit from a mutant of Escherichia coli. J Biochem. 1980 Sep;88(3):695–703. doi: 10.1093/oxfordjournals.jbchem.a133022. [DOI] [PubMed] [Google Scholar]
- Kanazawa H., Kayano T., Kiyasu T., Futai M. Nucleotide sequence of the genes for beta and epsilon subunits of proton-translocating ATPase from Escherichia coli. Biochem Biophys Res Commun. 1982 Apr 29;105(4):1257–1264. doi: 10.1016/0006-291x(82)90922-6. [DOI] [PubMed] [Google Scholar]
- Kanazawa H., Kayano T., Mabuchi K., Futai M. Nucleotide sequence of the genes coding for alpha, beta and gamma subunits of the proton-translocating ATPase of Escherichia coli. Biochem Biophys Res Commun. 1981 Nov 30;103(2):604–612. doi: 10.1016/0006-291x(81)90494-0. [DOI] [PubMed] [Google Scholar]
- Kanazawa H., Mabuchi K., Futai M. Nucleotide sequence of the promoter region of the gene cluster for proton-translocating ATPase from Escherichia coli and identification of the active promotor. Biochem Biophys Res Commun. 1982 Jul 30;107(2):568–575. doi: 10.1016/0006-291x(82)91529-7. [DOI] [PubMed] [Google Scholar]
- Kanazawa H., Mabuchi K., Kayano T., Noumi T., Sekiya T., Futai M. Nucleotide sequence of the genes for F0 components of the proton-translocating ATPase from Escherichia coli: prediction of the primary structure of F0 subunits. Biochem Biophys Res Commun. 1981 Nov 30;103(2):613–620. doi: 10.1016/0006-291x(81)90495-2. [DOI] [PubMed] [Google Scholar]
- Kanazawa H., Mabuchi K., Kayano T., Tamura F., Futai M. Nucleotide sequence of genes coding for dicyclohexylcarbodiimide-binding protein and the alpha subunit of proton-translocating ATPase of Escherichia coli. Biochem Biophys Res Commun. 1981 May 15;100(1):219–225. doi: 10.1016/s0006-291x(81)80085-x. [DOI] [PubMed] [Google Scholar]
- Kanazawa H., Saito S., Futai M. Coupling factor ATPase from Escherichia coli. An uncA mutant (uncA401) with defective alpha subunit. J Biochem. 1978 Dec;84(6):1513–1517. doi: 10.1093/oxfordjournals.jbchem.a132276. [DOI] [PubMed] [Google Scholar]
- Kanazawa H., Tamura F., Mabuchi K., Miki T., Futai M. Organization of unc gene cluster of Escherichia coli coding for proton-translocating ATPase of oxidative phosphorylation. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7005–7009. doi: 10.1073/pnas.77.12.7005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanner B. I., Gutnick D. L. Use of neomycin in the isolation of mutants blocked in energy conservation in Escherichia coli. J Bacteriol. 1972 Jul;111(1):287–289. doi: 10.1128/jb.111.1.287-289.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanner B. I., Nelson N., Gutnick D. L. Differentiation between mutants of Escherichia coli K defective in oxidative phosphorylation. Biochim Biophys Acta. 1975 Sep 8;396(3):347–359. doi: 10.1016/0005-2728(75)90141-3. [DOI] [PubMed] [Google Scholar]
- Loo T. W., Bragg P. D. The DCCD-binding polypeptide alone is insufficient for proton translocation through F0 in membranes of Escherichia coli. Biochem Biophys Res Commun. 1981 Nov 16;103(1):52–59. doi: 10.1016/0006-291x(81)91659-4. [DOI] [PubMed] [Google Scholar]
- Mabuchi K., Kanazawa H., Kayano T., Futai M. Nucleotide sequence of the gene coding for the delta subunit of proton translocating ATPase of Escherichia coli. Biochem Biophys Res Commun. 1981 Sep 16;102(1):172–179. doi: 10.1016/0006-291x(81)91504-7. [DOI] [PubMed] [Google Scholar]
- Maeda M., Futai M., Anraku Y. Biochemical characterization of the uncA phenotype of Escherichia coli. Biochem Biophys Res Commun. 1976 May 23;76(2):331–338. doi: 10.1016/0006-291x(77)90729-x. [DOI] [PubMed] [Google Scholar]
- Mao D., Wachter E., Wallace B. A. Folding of the mitochondrial proton adenosinetriphosphatase proteolipid channel in phospholipid vesicles. Biochemistry. 1982 Sep 28;21(20):4960–4968. doi: 10.1021/bi00263a020. [DOI] [PubMed] [Google Scholar]
- Masters M., Andresdottir V., Wolf-Watz H. Plasmids carrying oriC can integrate at or near the chromosome origin of Escherichia coli in the absence of a functional recA product. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 2):1069–1072. doi: 10.1101/sqb.1979.043.01.118. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Miki T., Hiraga S., Nagata T., Yura T. Bacteriophage lambda carrying the Escherichia coli chromosomal region of the replication origin. Proc Natl Acad Sci U S A. 1978 Oct;75(10):5099–5103. doi: 10.1073/pnas.75.10.5099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murgola E. J., Yanofsky C. Structural interactions between amino acid residues at positions 22 and 211 in the tryptophan synthetase alpha chain of Escherichia coli. J Bacteriol. 1974 Feb;117(2):444–448. doi: 10.1128/jb.117.2.444-448.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen J., Hansen F. G., Hoppe J., Friedl P., von Meyenburg K. The nucleotide sequence of the atp genes coding for the F0 subunits a, b, c and the F1 subunit delta of the membrane bound ATP synthase of Escherichia coli. Mol Gen Genet. 1981;184(1):33–39. doi: 10.1007/BF00271191. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis F. J., Kanner B. I., Gutnick D. L., Postma P. W., van Dam K. Energy conservation in membranes of mutants of Escherichia coli defective in oxidative phosphorylation. Biochim Biophys Acta. 1973 Oct 19;325(1):62–71. doi: 10.1016/0005-2728(73)90151-5. [DOI] [PubMed] [Google Scholar]
- Noumi T., Kanazawa H. Mutants of Escherichia coli H+-ATPase defective in the delta subunit of F1 and the b subunit of F0. Biochem Biophys Res Commun. 1983 Feb 28;111(1):143–149. doi: 10.1016/s0006-291x(83)80128-4. [DOI] [PubMed] [Google Scholar]
- Rosner J. L. Formation, induction, and curing of bacteriophage P1 lysogens. Virology. 1972 Jun;48(3):679–689. doi: 10.1016/0042-6822(72)90152-3. [DOI] [PubMed] [Google Scholar]
- Saraste M., Gay N. J., Eberle A., Runswick M. J., Walker J. E. The atp operon: nucleotide sequence of the genes for the gamma, beta, and epsilon subunits of Escherichia coli ATP synthase. Nucleic Acids Res. 1981 Oct 24;9(20):5287–5296. doi: 10.1093/nar/9.20.5287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schairer H. U., Friedl P., Schmid B. I., Vogel G. The use of several energy-coupling reactions in characterizing mutants of Escherichia coli K12 defective in oxidative phosphorylation. Eur J Biochem. 1976 Jul 1;66(2):257–268. doi: 10.1111/j.1432-1033.1976.tb10515.x. [DOI] [PubMed] [Google Scholar]
- Signer E. R., Weil J. Recombination in bacteriophage lambda. I. Mutants deficient in general recombination. J Mol Biol. 1968 Jul 14;34(2):261–271. doi: 10.1016/0022-2836(68)90251-9. [DOI] [PubMed] [Google Scholar]
- Simoni R. D., Shandell A. Energy transduction in Escherichia coli. Genetic alteration of a membrane polypeptide of the (Ca2+,Mg2+)-ATPase. J Biol Chem. 1975 Dec 25;250(24):9421–9427. [PubMed] [Google Scholar]
- Slater E. C. Mechanism of oxidative phosphorylation. Annu Rev Biochem. 1977;46:1015–1026. doi: 10.1146/annurev.bi.46.070177.005055. [DOI] [PMC free article] [PubMed] [Google Scholar]