Abstract
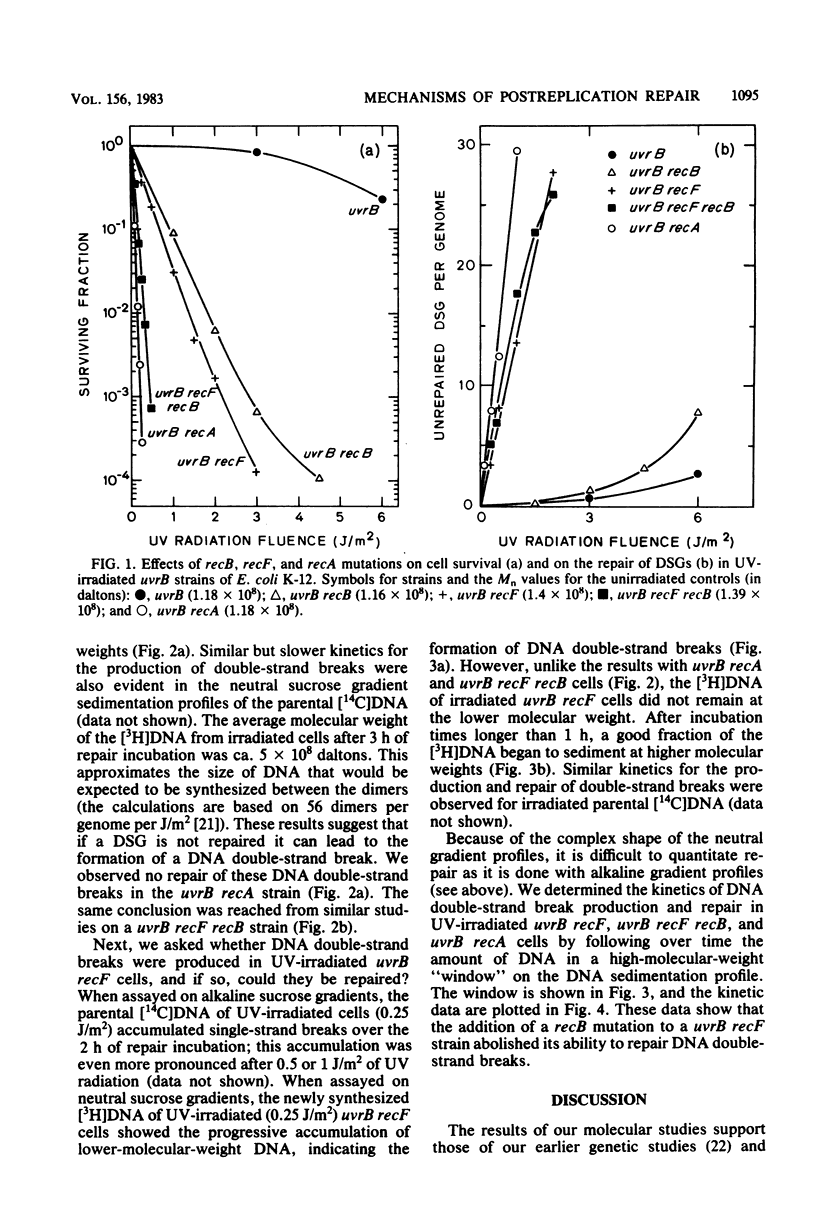
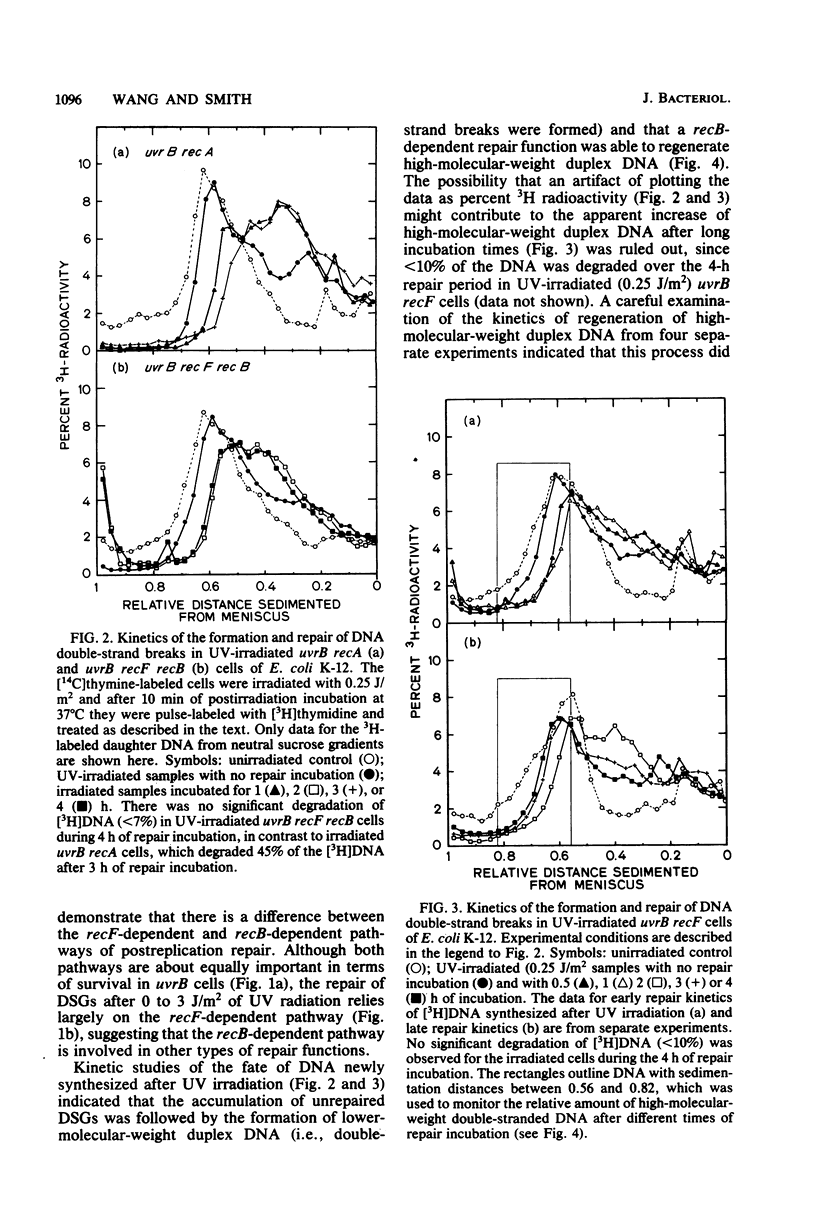
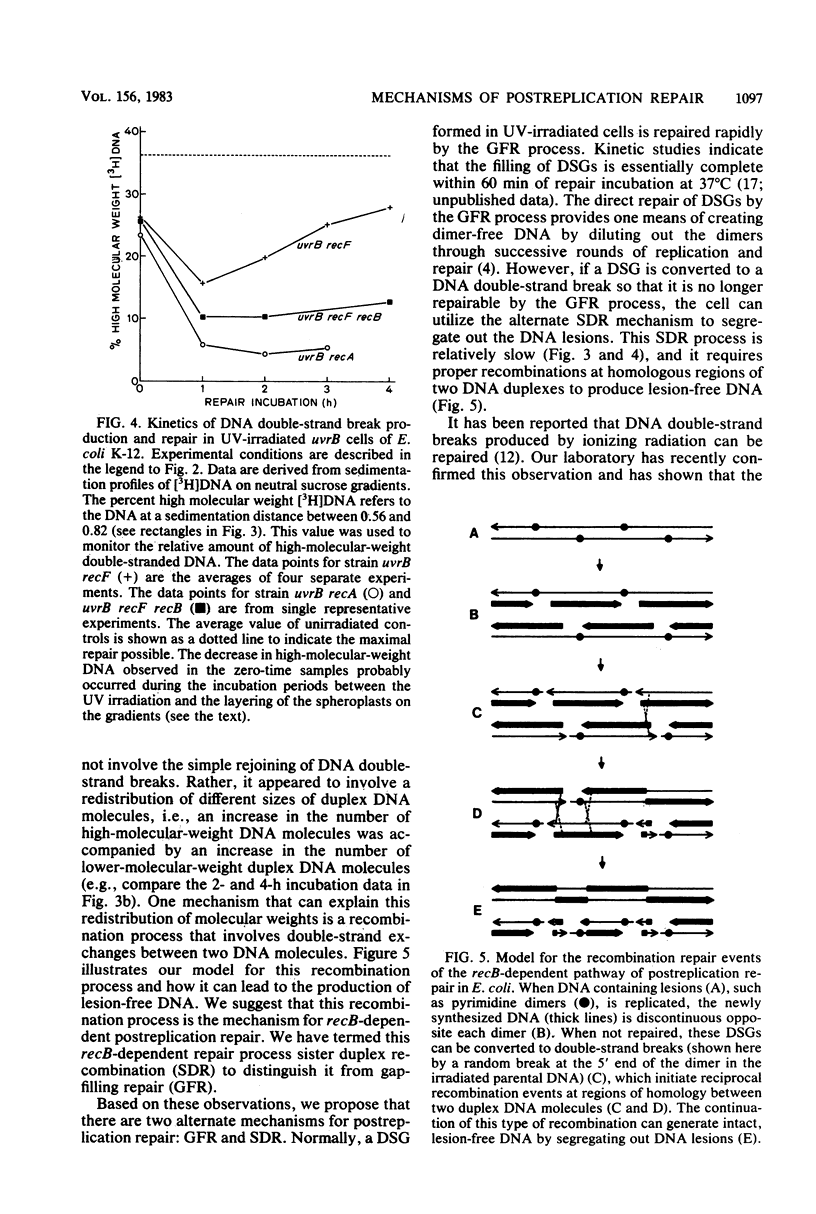
The molecular mechanisms for the recF-dependent and recB-dependent pathways of postreplication repair were studied by sedimentation analysis of DNA from UV-irradiated Escherichia coli cells. When the ability to repair DNA daughter strand gaps was compared, uvrB recF cells showed a gross deficiency, whereas uvrB recB cells showed only a small deficiency. Nevertheless, the uvrB recF cells were able to perform some limited repair of daughter strand gaps compared with a "repairless" uvrB recA strain. The introduction of a recB mutation into the uvrB recF strain greatly increased its UV radiation sensitivity, yet decreased only slightly its ability to repair daughter strand gaps. Kinetic studies of DNA repair with alkaline and neutral sucrose gradients indicated that the accumulation of unrepaired daughter strand gaps led to the formation of low-molecular-weight DNA duplexes (i.e., DNA double-strand breaks were formed). The uvrB recF cells were able to regenerate high-molecular-weight DNA from these low-molecular-weight DNA duplexes, whereas the uvrB recF recB and uvrB recA cells were not. A model for the recB-dependent pathway of postreplication repair is presented.
Full text
PDF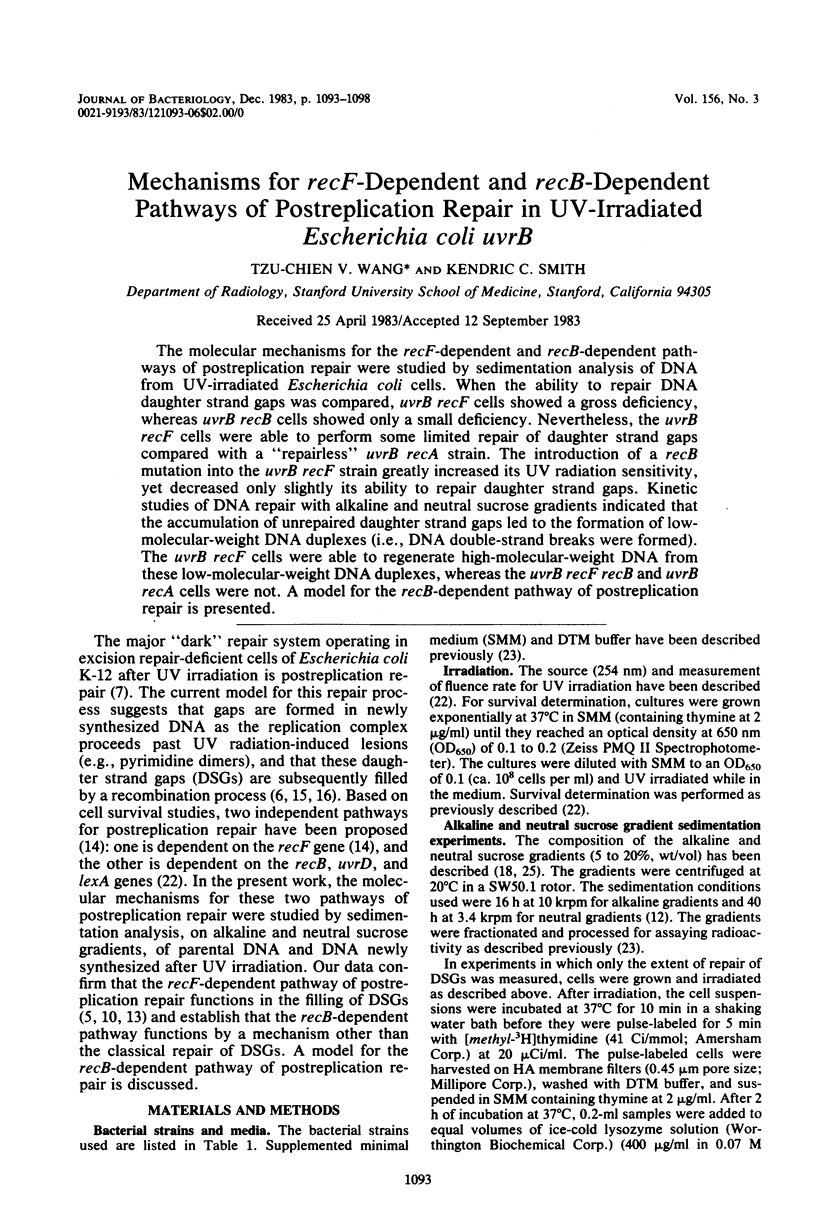


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachmann B. J., Low K. B. Linkage map of Escherichia coli K-12, edition 6. Microbiol Rev. 1980 Mar;44(1):1–56. doi: 10.1128/mr.44.1.1-56.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caillet-Fauquet P., Defais M., Radman M. Molecular mechanisms of induced mutagenesis. Replication in vivo of bacteriophage phiX174 single-stranded, ultraviolet light-irradiated DNA in intact and irradiated host cells. J Mol Biol. 1977 Nov 25;117(1):95–110. doi: 10.1016/0022-2836(77)90025-0. [DOI] [PubMed] [Google Scholar]
- Ganesan A. K. Persistence of pyrimidine dimers during post-replication repair in ultraviolet light-irradiated Escherichia coli K12. J Mol Biol. 1974 Jul 25;87(1):103–119. doi: 10.1016/0022-2836(74)90563-4. [DOI] [PubMed] [Google Scholar]
- Ganesan A. K., Seawell P. C. The effect of lexA and recF mutations on post-replication repair and DNA synthesis in Escherichia coli K-12. Mol Gen Genet. 1975 Dec 1;141(3):189–205. doi: 10.1007/BF00341799. [DOI] [PubMed] [Google Scholar]
- Hanawalt P. C., Cooper P. K., Ganesan A. K., Smith C. A. DNA repair in bacteria and mammalian cells. Annu Rev Biochem. 1979;48:783–836. doi: 10.1146/annurev.bi.48.070179.004031. [DOI] [PubMed] [Google Scholar]
- Howard-Flanders P. DNA repair. Annu Rev Biochem. 1968;37:175–200. doi: 10.1146/annurev.bi.37.070168.001135. [DOI] [PubMed] [Google Scholar]
- Kato H. Induction of sister chromatid exchanges by UV light and its inhibition by caffeine. Exp Cell Res. 1973 Dec;82(2):383–390. doi: 10.1016/0014-4827(73)90356-x. [DOI] [PubMed] [Google Scholar]
- Kato H. Induction of sister chromatid exchanges by chemical mutagens and its possible relevance to DNA repair. Exp Cell Res. 1974 Apr;85(2):239–247. doi: 10.1016/0014-4827(74)90123-2. [DOI] [PubMed] [Google Scholar]
- Kato T. Effects of chloramphenicol and caffeine on postreplication repair in uvr A- umuC- und uvrA- recF- strains of Escherichia coli K-12. Mol Gen Genet. 1977 Nov 14;156(2):115–120. doi: 10.1007/BF00283483. [DOI] [PubMed] [Google Scholar]
- Kato T., Rothman R. H., Clark A. J. Analysis of the role of recombination and repair in mutagenesis of Escherichia coli by UV irradiation. Genetics. 1977 Sep;87(1):1–18. doi: 10.1093/genetics/87.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasin F., Hutchinson F. Repair of DNA double-strand breaks in Escherichia coli, which requires recA function and the presence of a duplicate genome. J Mol Biol. 1977 Oct 15;116(1):81–98. doi: 10.1016/0022-2836(77)90120-6. [DOI] [PubMed] [Google Scholar]
- Rothman R. H., Clark A. J. The dependence of postreplication repair on uvrB in a recF mutant of Escherichia coli K-12. Mol Gen Genet. 1977 Oct 24;155(3):279–286. doi: 10.1007/BF00272806. [DOI] [PubMed] [Google Scholar]
- Rothman R. H., Kato T., Clark A. J. The beginning of an investigation of the role of recF in the pathways of metabolism of ultraviolet-irradiated DNA in Escherichia coli. Basic Life Sci. 1975;5A:283–291. doi: 10.1007/978-1-4684-2895-7_37. [DOI] [PubMed] [Google Scholar]
- Rupp W. D., Howard-Flanders P. Discontinuities in the DNA synthesized in an excision-defective strain of Escherichia coli following ultraviolet irradiation. J Mol Biol. 1968 Jan 28;31(2):291–304. doi: 10.1016/0022-2836(68)90445-2. [DOI] [PubMed] [Google Scholar]
- Rupp W. D., Wilde C. E., 3rd, Reno D. L., Howard-Flanders P. Exchanges between DNA strands in ultraviolet-irradiated Escherichia coli. J Mol Biol. 1971 Oct 14;61(1):25–44. doi: 10.1016/0022-2836(71)90204-x. [DOI] [PubMed] [Google Scholar]
- Smith K. C., Meun D. H. Repair of radiation-induced damage in Escherichia coli. I. Effect of rec mutations on post-replication repair of damage due to ultraviolet radiation. J Mol Biol. 1970 Aug;51(3):459–472. doi: 10.1016/0022-2836(70)90001-x. [DOI] [PubMed] [Google Scholar]
- Tang M. S., Smith K. C. The effects of lexA101, recB21, recF143 and uvrD3 mutations on liquid-holding recovery in ultraviolet-irradiated Escherichia coli K12 recA56. Mutat Res. 1981 Jan;80(1):15–25. doi: 10.1016/0027-5107(81)90174-3. [DOI] [PubMed] [Google Scholar]
- Town C. D., Smith K. C., Kaplan H. S. Influence of ultrafast repair processes (independent of DNA polymerase I) on the yield of DNA single-strand breaks in Escherichia coli K-12 x-irradiated in the presence of or absence of oxygen. Radiat Res. 1972 Oct;52(1):99–114. [PubMed] [Google Scholar]
- Town C. D., Smith K. C., Kaplan H. S. Production and repair of radiochemical damage in Escherichia coli deoxyribonucleic acid; its modification by culture conditions and relation to survival. J Bacteriol. 1971 Jan;105(1):127–135. doi: 10.1128/jb.105.1.127-135.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unrau P., Wheatcroft R., Cox B., Olive T. The formation of pyrimidine dimers in the DNA of fungi and bacteria. Biochim Biophys Acta. 1973 Jul 27;312(4):626–632. doi: 10.1016/0005-2787(73)90065-8. [DOI] [PubMed] [Google Scholar]
- Wang T. C., Smith K. C. Effects of the ssb-1 and ssb-113 mutations on survival and DNA repair in UV-irradiated delta uvrB strains of Escherichia coli K-12. J Bacteriol. 1982 Jul;151(1):186–192. doi: 10.1128/jb.151.1.186-192.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T. V., Smith K. C. Effect of recB21, uvrD3, lexA101 and recF143 mutations on ultraviolet radiation sensitivity and genetic recombination in delta uvrB strains of Escherichia coli K-12. Mol Gen Genet. 1981;183(1):37–44. doi: 10.1007/BF00270135. [DOI] [PubMed] [Google Scholar]
- Wolff S., Bodycote J., Painter R. B. Sister chromatid exchanges induced in Chinese hamster cells by UV irradiation of different stages of the cell cycle: the necessity for cells to pass through S. Mutat Res. 1974 Oct;25(1):73–81. doi: 10.1016/0027-5107(74)90220-6. [DOI] [PubMed] [Google Scholar]
- Youngs D. A., Smith K. C. Evidence for the control by exrA and polA genes of two branches of the uvr gene-dependent excision repair pathway in Escherichia coli K-12. J Bacteriol. 1973 Oct;116(1):175–182. doi: 10.1128/jb.116.1.175-182.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]



