Abstract
We report on an observation that the orientation of cell division is directed by small, applied electric fields (EFs). Cultured human corneal epithelial cells were exposed to a direct-current EF of physiological magnitude. Cells divided while attached to the culture dish, and most did so with a cleavage plane perpendicular to the EF vector. There are many instances in which cell divisions in vivo occur in the presence of direct-current physiological EF, for example, during embryonic morphogenesis, neuronal and epithelial differentiation, wound healing, or tumor formation. Endogenous physiological EFs may play important roles in some or all of these processes by regulating the axis of cell division and, hence, the positioning of daughter cells.
Keywords: orientation, cleavage plane, corneal epithelium
Controlling cell division is fundamental to normal development, and the regulation of the axes of cell division is considered to have major morphogenetic impact. For example, as mouse epidermis develops, a single sheet of cells is transformed into a stratified epithelium by altering the axis of cleavage by 90° (1). Cleavage perpendicular to the sheet keeps the daughter cells in the sheet, and cleavage parallel to the sheet produces a second layer of cells. In the developing mouse central nervous system (CNS), choices regarding the axis of cleavage also are made and determine the fate of the daughter cells (2). Daughter cells may remain within the germinative layer of cells in contact with the basement membrane, or, if cleavage is parallel to this plane, one cell is released from the germinative layer and differentiates to become a migratory neuron. This involves differential inheritance of several key cytoplasmic proteins (e.g., numb, notch, prospero, miranda, staufen) that become distributed asymmetrically to the two poles before cytokinesis and that subsequently influence differentiation (3–5). Indeed, there is evidence both from zebra fish neuroblasts and in corneal epithelium that clonally related groups of cells may divide in the same plane and at the same time and undergo subsequently similar migrations, indicating that the cell cycle may have a profound influence on morphogenesis in the CNS and the cornea (6, 7). Potentially linked to this are the observations that certain major morphogenetic events involve marked shortening of the cell cycle, for example, during formation of the primitive streak and during neurulation and neural folding, where the period between cell divisions can drop to 3–5 hr (8, 9). Even at the same developmental time but in different parts of the neural tube, substantial variations in cell cycle length are found (10).
The extrinsic controls over cell division (and over the cell cycle) are not well understood. In Caenorhabditis elegans, the orientation of some cell divisions is determined by cell–cell contact during early embryonic development (11–13), whereas in fucus, the plane of division of zygotes is directed by several natural, environmental vectors. These include influences from other zygotes up to several egg diameters away (the so-called group effects), remarkably unspecific influences from adult plants in the intertidal zone up to 10 mm or more away, and weak unilateral light (14). In addition, the second and third cleavage planes of frog eggs recently have been shown to be affected by strong magnetic fields (15). Here, we have tested the hypothesis that a small dc electric field may be one other extrinsic factor that can orient cell division. Part of the rationale is that primitive streak formation (16) and neurulation and maturation of the neural tube (17, 18) are both associated with the transient appearance of spatially restricted small dc electric fields (EFs), and both events require tight control of cell cycle length and the axes of cell divisions. Commonly, these endogenous physiological EFs arise from spatial differences in epithelial ion transport or from spatially separated leaky and tight areas of epithelium (19). Given the spatial and developmental conjunction of directed cell divisions and endogenous dc EFs in vivo (see Discussion), we asked whether small dc physiological EFs can direct the axis of mammalian cell division by using a model tissue culture system. We found that corneal epithelial cells (CECs) divided with a cleavage furrow perpendicular to the EF vector.
MATERIALS AND METHODS
Cell Cultures and EF Exposure.
Primary and transformed human CECs were cultured as described previously (20). The chamber for EF application was formed by two parallel strips of cover glass fixed to the base of a tissue culture dish, 1 cm apart. Once cells adhered within the shallow trough, a third cover glass was added as a lid to create a thin, wafer-like chamber (22-mm long × 10-mm wide × 0.5-mm deep). The EF was applied from a dc power source via Ag/AgCl electrodes in beakers of saline, which were connected to the ends of the galvanic chamber by using agar salt bridges. This isolates the cultures from electrode products. For some experiments, the galvanic chambers (see figure 1 of ref. 21 for details) were moved from the CO2 incubator to the microscope stage about 20 hr after seeding. At this stage cultures possessed mostly single cells. Cultures with a sparse cell population were selected to ensure that individual cells were not affected by neighbor–neighbor interactions. Cell division was monitored continuously and photographs were stored in an image analyzer. The temperature was maintained at 37°C during the experiments by using a microscope stage incubator. Some experiments were carried out in a 37°C, 5% CO2 incubator. In these cases, immediately after termination of EF exposure, cells were viewed, all double cells with clear evidence of a cleavage plane were photographed, and images were stored. The field strength used was 150 mV/mm unless otherwise stated and is within the range of those measured in many developing and regenerating systems (22).
Quantification of Cleavage Orientation.
The orientation of dividing cells with respect to the EF was defined as a function of cos [2(θ-90)], where θ is the angle between a line drawn through the cleavage plane and the EF vector. This gives a polarization index that varies from −1 to 1. A cell with its plane of cleavage parallel to the vector of the EF will have a polarization of −1, and a cell that divided with a cleavage plane exactly perpendicular to the EF vector will have a polarization of 1. A randomly oriented population of dividing cells will have an average polarization (defined by Σ n cos [2 (θ-90)]/n, where n is the number of measurements) of 0. A population of dividing cells with cleavage planes, on average, perpendicular rather than parallel to the field direction will have a polarization value between 0 and 1; the higher the value, the more perpendicular cleavage was to the applied EFs. A population of dividing cells with cleavage planes, on average, parallel rather than perpendicular to the field direction will have a polarization value between 0 and −1, with −1 indicating all cells with a cleavage furrow exactly parallel to the EF.
The significance of this two-dimensional orientation distribution against randomness was calculated by using Rayleigh’s distribution (21, 23). A probability level of 0.001 was used as the limit for significant polarization.
Confocal Microscopy.
Cells for immunofluorescence study were prepared as before (24). Bovine CECs cultured on acid-washed glass slides within a galvanic chamber were exposed to EFs for ≈18 hr in a 5% CO2 incubator, washed, and stained as before (24). Filamentous actin (F-actin) was stained with rhodamine phalloidin from Molecular Probes, and TGFR II (transforming growth factor β receptor type II) was stained with a polyclonal rabbit anti-human TGFR II from Upstate Biotechnology (cat. 06–277; Lake Placid, NY).
Cells were viewed with a Bio-Rad MRC 1024 confocal microscope. Cells stained with FITC or rhodamine were scanned by using a Krypton/Argon laser (all line) with emission maximum at 520 and 590, respectively, with selective filters. Oil-immersion objectives (×60 or ×100) were used in conjunction with an iris setting of 2–3.5 mm for image collection. Serial and vertical sections were collected and analyzed with Bio-Rad MRC-1024 lasersharp, Version 2.1a.
Statistical analyses were made by using unpaired, two-tailed Student’s t test or Welch’s unpaired t test, when SDs were significantly different from each other. Data are expressed as mean ± SEM, unless stated otherwise.
RESULTS AND DISCUSSION
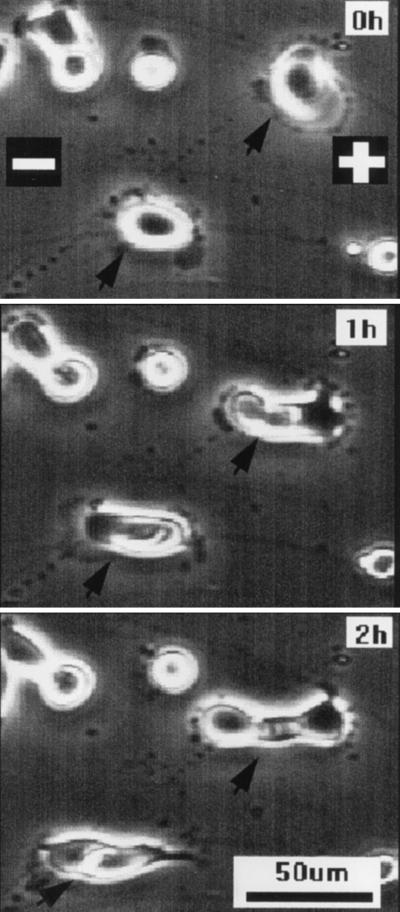
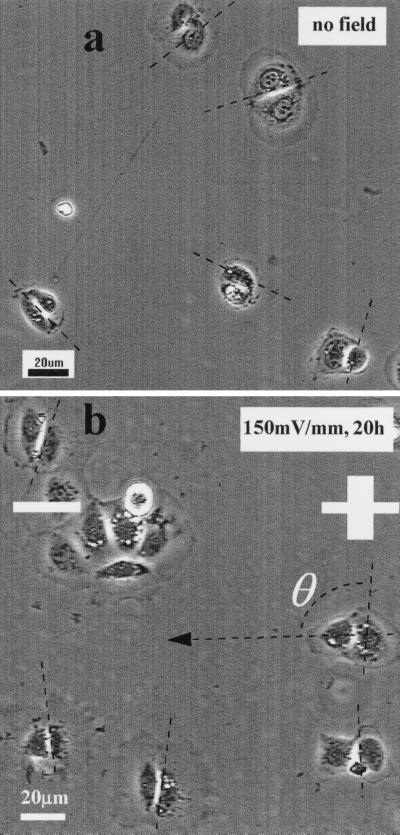
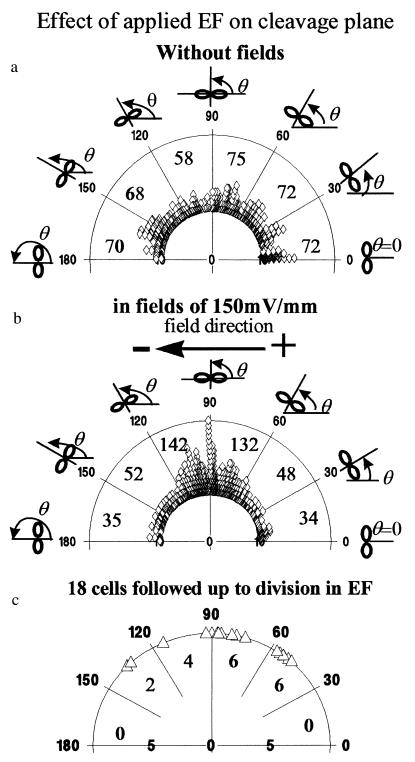
Nondividing epithelial cells normally established a long axis perpendicular to an applied EF (20, 21). Fig. 1 shows a representative sequence of human CECs in primary culture, which divided in a physiological EF (150 mV/mm). When these cells divided, they did so with a cleavage plane perpendicular to the field vector (Fig. 1, arrow). This also was the case for a transformed human CEC line. The cleavage plane of cells cultured with no EF was oriented randomly (Fig. 2a); those of cells cultured in EFs lay predominantly perpendicular to the field vector (Fig. 2b). This was analyzed further and quantified by measuring the angle θ formed between the cleavage furrow and the field vector (Figs. 2 and 3). For cells in control cultures (no EF; Fig. 2a), an angle distribution (Fig. 3a) and a polarization value (see next paragraph) indicating random orientation of the cleavage furrow were obtained. However, cells cultured in EFs at 150 mV/mm for 20 hr showed preferential orientation, with the cleavage plane aligned most often around 90° to the field vector (Figs. 2b and 3b). We followed 18 dividing cells continuously on the microscope stage in EFs (nine experiments). Almost all of the cells aligned themselves with the division axis parallel to the field vector, thus with the cleavage angle perpendicular to the field vector (Fig. 3c).
Figure 1.
Two human corneal epithelial cells (arrow) dividing in an EF of 150 mV/mm. The field vector is horizontal, and cleavage occurred roughly perpendicular to the applied EF. The cells were a primary culture from donor cornea grown in DMEM/15% FBS.
Figure 2.
Orientation of the cleavage plane of transformed human corneal epithelial cells also was affected by applied EFs (strength and polarity as shown). (a) No field control. Cleavage furrows are evident and oriented randomly. (b) Dividing cells in an applied EF showed preferential orientation of the cleavage furrow. The angle formed by the cleavage plane and the vector of the electric field was used to calculate the polarization of cleavage angle (for details see Materials and Methods). The cells were cultured in DMEM/F12 with 15% heat-inactivated FBS.
Figure 3.
Angles of cleavage plane of transformed human corneal epithelial cells expressed as a polar diagram, where each symbol represents one cell. (a) Angles of cleavage plane of 415 dividing cells cultured without electric fields. (b) Polarized orientation of the cleavage plane of 443 cells dividing in a small physiological EF (in EFs for 20 hr in 5% CO2 incubator/37°C). Clearly, a high proportion of cells divided with a cleavage plane orthogonal to the EFs. (c) Polarized cleavage plane orientation of 18 dividing cells followed continuously in EF on the microscope stage. The total number of cells in each range of angles is indicated, both numerically and by compression of the symbols.
The cleavage orientation of dividing cells was analyzed by using Rayleigh’s distribution (Materials and Methods). This yielded a mean polarization value (Σ n cos [2 (θ-90)]/n; see Materials and Methods) of −0.029 ± 0.03 for control cells dividing without an applied EF (n = 415; 15 experiments), indicating random cleavage orientation, and a remarkable 0.41 ± 0.04 for cells dividing in a physiological EF of 150 mV/mm (n = 443; 18 experiments), indicating perpendicular cleavage orientation. For cells observed continuously throughout division in EF, the polarization value was even higher: 0.61 ± 0.09 (n = 18; nine experiments). Statistical analysis indicated a random distribution of the cleavage plane for control cells (without EF) and extreme significance of perpendicular cleavage plane formation in a physiological EF. (Rayleigh’s two-dimensional distribution analysis: probabilities of random orientation were 0.70 for no EF, 6.7E-33 in the field, and 0.0004 for the 18 cells followed continuously up to completion of cytokinesis in EFs.)
Thus, the orientation of cell cleavage was perpendicular to the EF vector, suggesting that the mitotic spindle had become aligned parallel to the field vector. How might this occur?
The mitotic spindle dictates the position of the cleavage plane, which occurs between the two asters at the poles of the spindle (12, 25). Recently, in rat cortical neurons, the spindle was shown to rotate continually before settling in a particular plane (26). Cell–cell contact can dictate the orientation of the mitotic spindle (13), and G proteins are required for correct spatial orientation of some early cleavages in C. elegans (27). One interpretation is that an unidentified surface receptor becomes localized/clustered by cell contact and signals through G proteins to create a “cortical trapping zone,” which captures either one centrosome or some portion of the cortical microtubule asters, thus defining the position of the mitotic apparatus. There is also substantial movement of cortical cytoplasm and a coordinated flow toward the developing cleavage furrow of preformed actin filaments; of actin-binding proteins, for instance, the ezrin, radixin, moesin family of proteins; and of surface receptors, e.g., for the lectin con A, integrins, and CD43 (28–34). Intracellular calcium is elevated in areas that predict the site of first/second cleavage in the fertilized medaka fish eggs (35) and zebra fish eggs (36), and calcium buffer injections inhibit furrow formation in Xenopus eggs (37). In addition to accumulating circumferentially at the furrow, actin filaments also elongate along the axis of the spindle microtubules (33). Despite this detail, there is no clear picture of how these events are integrated to determine the axis of spindle orientation.
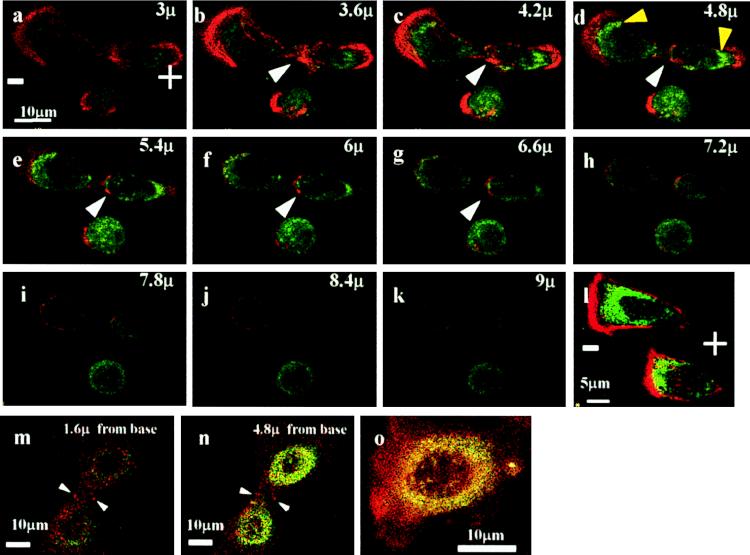
In several cell types, physiological EFs redistribute surface receptors to one pole of the cell (24, 38, 39) and secondarily induce asymmetry of cortical, receptor-associated F-actin (40, 41). How such events integrate with the surface and intracellular rearrangements occurring as cytokinesis proceeds is unclear. Shifting a particular class of receptor to one pole of a cell as it prepares to divide could be sufficient to induce a patch of cortical membrane that trapped one centrosome, or certain aster microtubules, and, therefore, determined the axis of spindle alignment. We have begun to investigate this possibility. TGF-β controls proliferation in CECs (42), and the distribution of its receptors and of F-actin was studied in cells exposed to a small EF. In nondividing cells, staining for both TGFR II and F-actin was concentrated at the cathodal-facing side of cells cultured in EFs. In dividing cells, F-actin and TGFR II accumulated strongly at the cleavage site and at both poles of the daughter cells (Fig. 4 a–k). By contrast, in both dividing (Fig. 4 m and n) and nondividing cells (Fig. 4o) in control cultures (no EF), staining for F-actin and TGF-β type II receptors was more diffuse and less polarized, although there was some accumulation of F-actin in the cleavage furrow (Fig. 4m).
Figure 4.
(a–k) Confocal optical sections of a dividing bovine corneal epithelial cell in an applied EF (at 150 mV/mm in DMEM/10% FBS). F-actin is concentrated at the cleavage plane (white arrowhead). Additionally, there is prominent accumulation of F-actin (red) and of TGFR II (green) at either poles of the two daughter cells (especially b–e). Although this segregation of F-actin and TGFR II to the two poles is evident, a nondividing cell beneath and two other nondividing cells (l) showed predominant accumulation of F-actin and TGFR II on the cathodal side. (m–o) Dividing cells (m and n) and nondividing cells (o) cultured without an applied EF are shown. Staining in both cases is more diffuse than for EF-treated cells, and, with the exception of some F-actin accumulation in the cleavage furrow, there was little polarization of F-actin or of TGFR II.
Aster microtubules also are potential downstream targets (secondary to surface receptor rearrangements) whose polarization and function may be affected by a physiological EF. In Xenopus epithelial melanocytes, pigment granules, which are transported in a microtubule-dependent manner, moved rapidly toward a focal micropipette acting as a cathodal dc electrode (C.D.M., unpublished data).
Two issues are relevant regarding the cellular targets of endogenous EFs in dividing cells. First, there is a transient 30% increase in cell surface negativity as cells prepare to divide (43). Additionally, tumor cells with short cell cycles show a sustained 100% increase in surface negativity, a part of which may be due to high levels of expression of the linear, negatively charged polysialic acid/neural cell adhesion molecule (43–45). In addition to altering receptor mobility, dynamic or static alterations to cell surface charge may induce local EFs around dividing single cells or groups of cells (e.g., tumors).
Physiological Significance.
Several examples argue for a physiological role for EF-induced regulation of the axis of cell division in vivo. During neurulation in zebra fish, rat, and chick, neuroepithelial cell divisions occur either along a rostrocaudally oriented spindle axis or at 90° to this, mediolaterally (46–48). Intriguingly, by measuring spatial variations in the trans-epidermal potential difference across the developing amphibian neural plate, two orthogonal EFs with rostrocaudal and mediolateral vectors also have been demonstrated (18). Perhaps they are linked causally. In adult myelinated nerve, Schwann cells divide along a mitotic spindle oriented parallel to the nerve (49). Mitosis is controlled, in part, by electrical activity of the nerve, because when this is inhibited by using tetrodotoxin, cell division is inhibited (50). One element of action potential activity in myelinated nerves is that saltatory conduction between nodes involves extracellular current flow and will establish pulsed, extracellular EFs oriented parallel to the nerve, which could influence Schwann cell mitotic spindle orientation. A third example occurs during corneal epithelial wound healing. DNA synthesis and mitotic activity near a wound edge increased markedly during the first 12 hr (51). During in vitro corneal wound healing, dividing cells were observed at the leading edge (52). Corneal epithelial wounds generate dc EFs because of the local collapse of the trans-epithelial potential difference, which is steepest across the cells within the first few hundred micrometers of the wound edge (53). In similar applied EFs, cultured CECs show directed migration (20, 21, 54). The present results indicate that the direction of cell division also may be oriented toward the wound edge by wound-induced dc EF.
Acknowledgments
We thank Dr. E. Pels at the Netherlands Ophthalmic Research Institute for providing donor eye materials and Dr. K. Araki-Sasaki at the Kinki Central Hospital, Japan, for providing human corneal cell line. This work and M.Z. were supported by the Dr. James Alexander Mearns’ Trust, Scotland, and by The Wellcome Trust.
ABBREVIATIONS
- CEC
corneal epithelial cell
- EF
electric field
- TGF-β
transforming growth factor type β
- TGFRII
TGF-β type II receptor
- F-actin
filamentous actin
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
References
- 1.Smart I H M. Br J Derm. 1970;82:276–282. doi: 10.1111/j.1365-2133.1970.tb12437.x. [DOI] [PubMed] [Google Scholar]
- 2.Chenn A, McConnell S K. Cell. 1995;82:631–641. doi: 10.1016/0092-8674(95)90035-7. [DOI] [PubMed] [Google Scholar]
- 3.Knoblich J A, Jan L Y, Jan Y N. Nature (London) 1995;377:624–627. doi: 10.1038/377624a0. [DOI] [PubMed] [Google Scholar]
- 4.Li P, Yang X, Wasser M, Cai Y, Chia W. Cell. 1997;90:437–447. doi: 10.1016/s0092-8674(00)80504-8. [DOI] [PubMed] [Google Scholar]
- 5.Shen C-P, Jan L Y, Jan Y N. Cell. 1997;90:449–458. doi: 10.1016/s0092-8674(00)80505-x. [DOI] [PubMed] [Google Scholar]
- 6.Kimmel C B, Warga R M, Kane D A. Development. 1994;120:265–276. doi: 10.1242/dev.120.2.265. [DOI] [PubMed] [Google Scholar]
- 7.Beebe D C, Masters B R. Invest Ophthalmol Visual Sci. 1996;37:1815–1825. [PubMed] [Google Scholar]
- 8.Mac Auley A, Werb Z, Mirkes P E. Development. 1993;117:873–883. doi: 10.1242/dev.117.3.873. [DOI] [PubMed] [Google Scholar]
- 9.Wilson D B. Dev Br Res. 1982;2:420–424. [Google Scholar]
- 10.Martin A H. Nature (London) 1967;216:1133–1134. doi: 10.1038/2161133a0. [DOI] [PubMed] [Google Scholar]
- 11.Strome S, Wood W B. Cell. 1983;35:15–25. doi: 10.1016/0092-8674(83)90203-9. [DOI] [PubMed] [Google Scholar]
- 12.Strome S. Cell. 1993;72:3–6. doi: 10.1016/0092-8674(93)90041-n. [DOI] [PubMed] [Google Scholar]
- 13.Goldstein B. J Cell Biol. 1995;129:1071–1080. doi: 10.1083/jcb.129.4.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jaffe L F. Adv Morph. 1968;7:295–328. doi: 10.1016/b978-1-4831-9954-2.50012-4. [DOI] [PubMed] [Google Scholar]
- 15.Denegre M M, Valles J M, Jr, Lin K, Jordan W B, Mowry K L. Proc Natl Acad Sci USA. 1998;95:14729–14732. doi: 10.1073/pnas.95.25.14729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jaffe L F, Stern C. Science. 1979;206:569–571. doi: 10.1126/science.573921. [DOI] [PubMed] [Google Scholar]
- 17.Shi R, Borgens R B. Dev Dynamics. 1995;202:101–114. doi: 10.1002/aja.1002020202. [DOI] [PubMed] [Google Scholar]
- 18.Borgens R B, Shi R. Dev Dynamics. 1995;203:456–467. doi: 10.1002/aja.1002030408. [DOI] [PubMed] [Google Scholar]
- 19.Robinson K R, Messerli M A. In: Nerve Growth and Nerve Guidance. McCaig C D, editor. London: Portland Press; 1996. pp. 131–150. [Google Scholar]
- 20.Zhao M, McCaig C D, Agius-Fernandez A, Forrester J V, Araki-Sasaki K. Curr Eye Res. 1997;16:973–984. doi: 10.1076/ceyr.16.10.973.9014. [DOI] [PubMed] [Google Scholar]
- 21.Zhao M, Agius-Fernandez A, Forrester J V, McCaig C D. J Cell Sci. 1996;109:1405–1414. doi: 10.1242/jcs.109.6.1405. [DOI] [PubMed] [Google Scholar]
- 22.Nuccitelli R. Adv Cell Biol. 1988;2:213–233. [Google Scholar]
- 23.Curray J R. J Geol. 1956;64:117–130. [Google Scholar]
- 24.Zhao, M., Dick, A., Forrester, J. V. & McCaig, C. D. (1999) Mol. Biol. Cell, in press. [DOI] [PMC free article] [PubMed]
- 25.Rappaport R. J Exp Zool. 1961;148:81–89. doi: 10.1002/jez.1401480107. [DOI] [PubMed] [Google Scholar]
- 26.Adams R J. J Neurosci. 1996;16:7610–7618. doi: 10.1523/JNEUROSCI.16-23-07610.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zwaal R R, Ahringer J, van Leunen H G A M, Rushforth A, Anderson P, Plasterk R H A. Cell. 1996;86:619–629. doi: 10.1016/s0092-8674(00)80135-x. [DOI] [PubMed] [Google Scholar]
- 28.Hird S N, White J G. J Cell Biol. 1993;121:1343–1355. doi: 10.1083/jcb.121.6.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cao L-g, Wang Y-l. J Cell Biol. 1990;110:1089–1095. doi: 10.1083/jcb.110.4.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cao L-g, Wang Y-l. Mol Biol Cell. 1996;7:225–232. doi: 10.1091/mbc.7.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yonemura S, Nagafuchi A, Sato N, Tsukita S. J Cell Biol. 1993;120:437–449. doi: 10.1083/jcb.120.2.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McCaig C D, Robinson K R. Dev Biol. 1982;92:197–202. doi: 10.1016/0012-1606(82)90163-4. [DOI] [PubMed] [Google Scholar]
- 33.Fishkind D J, Wang Y-l. J Cell Biol. 1993;123:837–848. doi: 10.1083/jcb.123.4.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang Y-l, Silverman J D, Cao L-g. J Cell Biol. 1994;127:963–971. doi: 10.1083/jcb.127.4.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fluck R A, Miller A L, Jaffe L F. J Cell Biol. 1991;115:1259–1265. doi: 10.1083/jcb.115.5.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chang D C, Meng C. J Cell Biol. 1995;131:1539–1545. doi: 10.1083/jcb.131.6.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miller A L, Fluck R A, McLaughlin J A, Jaffe L F. J Cell Sci. 1993;106:523–534. doi: 10.1242/jcs.106.2.523. [DOI] [PubMed] [Google Scholar]
- 38.Poo M-m, Robinson K R. Nature (London) 1977;265:602–605. doi: 10.1038/265602a0. [DOI] [PubMed] [Google Scholar]
- 39.Patel N, Poo M-m. J Neurosci. 1982;2:483–496. doi: 10.1523/JNEUROSCI.02-04-00483.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Luther P W, Peng H B, Lin J J-C. Nature (London) 1983;303:61–64. doi: 10.1038/303061a0. [DOI] [PubMed] [Google Scholar]
- 41.McCaig C D, Dover P J. J Cell Sci. 1991;98:497–506. doi: 10.1242/jcs.98.4.497. [DOI] [PubMed] [Google Scholar]
- 42.Honma Y, Nishida K, Sotozono C, Kinoshita S. Exp Eye Res. 1997;65:391–396. doi: 10.1006/exer.1997.0338. [DOI] [PubMed] [Google Scholar]
- 43.Brent T P, Forrester J A. Nature (London) 1967;215:92–93. doi: 10.1038/215092a0. [DOI] [PubMed] [Google Scholar]
- 44.Ambrose E J, James A M, Lowick J H B. Nature (London) 1956;177:576–577. doi: 10.1038/177576a0. [DOI] [PubMed] [Google Scholar]
- 45.Elul R, Brons J, Kravitz K. Nature (London) 1975;258:616–617. doi: 10.1038/258616a0. [DOI] [PubMed] [Google Scholar]
- 46.Concha M L, Adams R J. Development. 1998;125:983–994. doi: 10.1242/dev.125.6.983. [DOI] [PubMed] [Google Scholar]
- 47.Tuckett F, Morris-Kay G M. J Embryol Exp Morphol. 1985;85:111–119. [PubMed] [Google Scholar]
- 48.Sausedo R A, Smith J L, Schoenwolf G C. J Comp Neurol. 1997;381:473–488. [PubMed] [Google Scholar]
- 49.Martin J R, Webster H D. Dev Biol. 1973;32:417–431. doi: 10.1016/0012-1606(73)90251-0. [DOI] [PubMed] [Google Scholar]
- 50.Barres B A, Raff M C. Nature (London) 1993;361:258–260. doi: 10.1038/361258a0. [DOI] [PubMed] [Google Scholar]
- 51.Thompson H W, Malter J S, Steinmann T L, Benerman R W. Invest Ophthalmol Visual Sci. 1991;32:433–436. [PubMed] [Google Scholar]
- 52.Chan K Y, Patton D L, Cosgrove Y T. Invest Ophthalmol Visual Sci. 1989;30:2488–2498. [PubMed] [Google Scholar]
- 53.Chiang M, Robinson K R, Vanable J W., Jr Exp Eye Res. 1992;54:999–1003. doi: 10.1016/0014-4835(92)90164-n. [DOI] [PubMed] [Google Scholar]
- 54.Zhao M, Agius-Fernandez A, Forrester J V, McCaig C D. Invest Ophthalmol Visual Sci. 1996;37:2548–2558. [PubMed] [Google Scholar]