Abstract
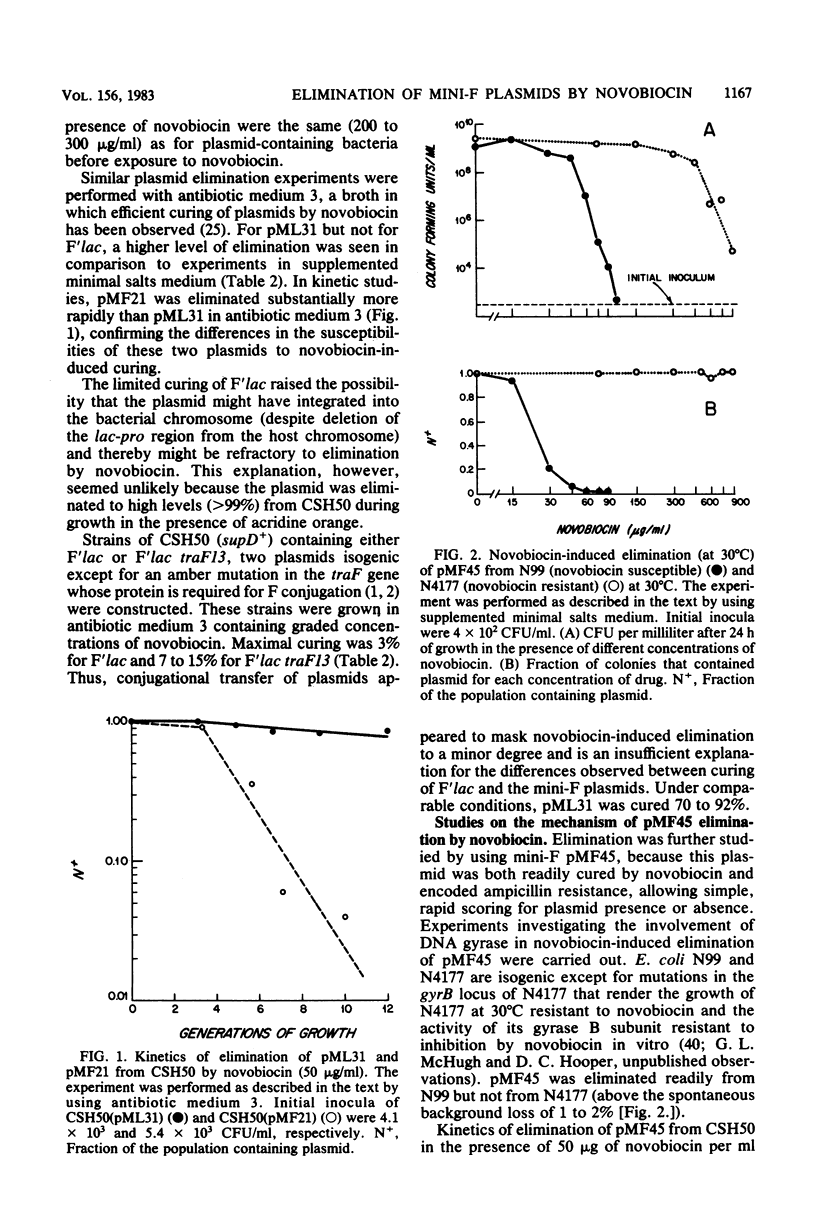
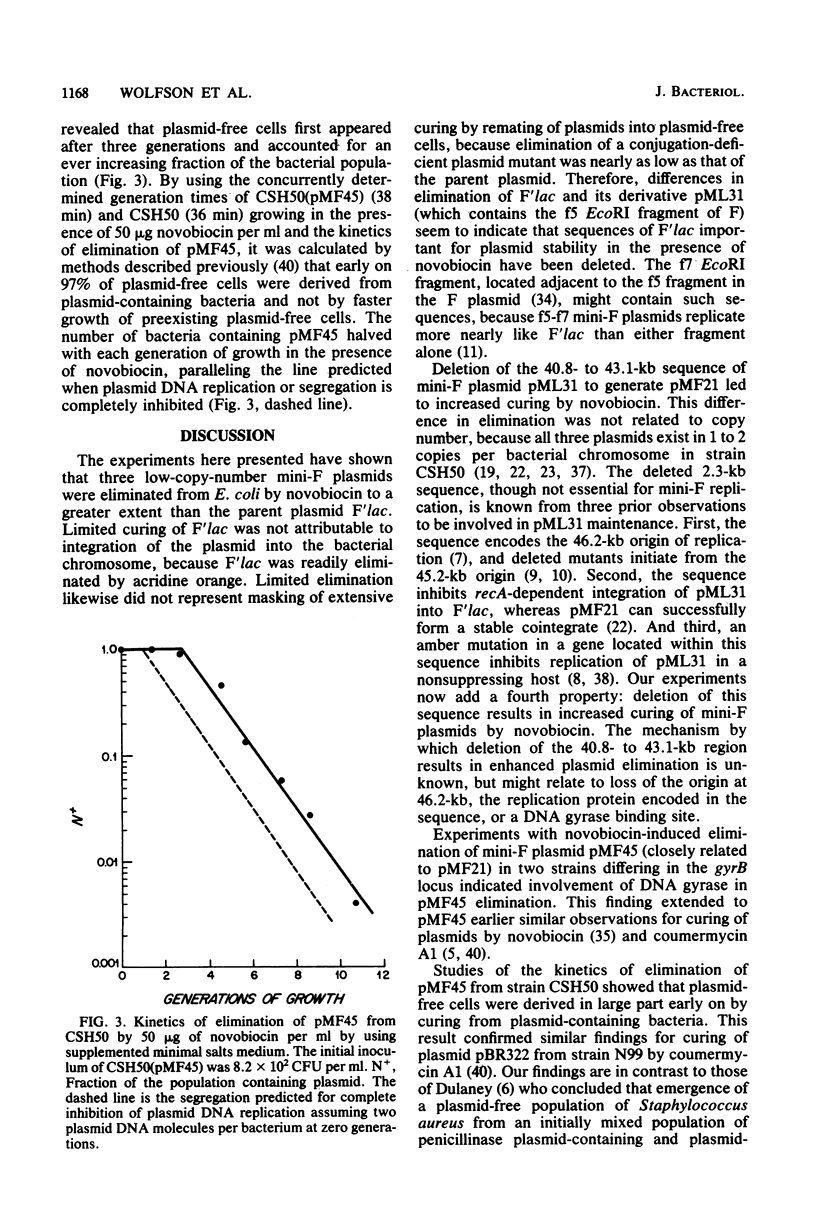
Novobiocin eliminated (cured) F'lac and three low-copy-number mini-F plasmids (pML31, pMF21, and pMF45) from Escherichia coli to different extents. F'lac was cured 0 to 3%. pML31, whose replication region is contained on the 9-kilobase f5 EcoRI restriction enzyme fragment of F, was eliminated 10 to 92%. pMF21, deleted of the origin of mini-F replication at 42.6 kilobases on the F map and known to initiate from an origin at 45.1 kilobases, and its closely related derivative pMF45 were cured to the greatest extent (greater than 97%). pMF45 was eliminated from a wild-type bacterial strain but not from an isogenic novobiocin-resistant gyrB mutant strain, indicating involvement of the B subunit of DNA gyrase in the curing phenomenon. The number of bacteria containing pMF45 halved with each generation of growth in the presence of novobiocin, as is predicted for complete inhibition of plasmid DNA replication.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Achtman M., Willetts N., Clark A. J. Beginning a genetic analysis of conjugational transfer determined by the F factor in Escherichia coli by isolation and characterization of transfer-deficient mutants. J Bacteriol. 1971 May;106(2):529–538. doi: 10.1128/jb.106.2.529-538.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achtman M., Willetts N., Clark A. J. Conjugational complementation analysis of transfer-deficient mutants of Flac in Escherichia coli. J Bacteriol. 1972 Jun;110(3):831–842. doi: 10.1128/jb.110.3.831-842.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cejka K., Holubová I., Hubácek J. Curing effect of clorobiocin on Escherichia coli plasmids. Mol Gen Genet. 1982;186(1):153–155. doi: 10.1007/BF00422928. [DOI] [PubMed] [Google Scholar]
- Danilevskaya O. N., Gragerov A. I. Curing of Escherichia coli K12 plasmids by coumermycin. Mol Gen Genet. 1980 Apr;178(1):233–235. doi: 10.1007/BF00267235. [DOI] [PubMed] [Google Scholar]
- Eichelaub R., Wehlmann H. Amber-mutants of plasmid mini-F defective in replication. Mol Gen Genet. 1980;180(1):201–204. doi: 10.1007/BF00267370. [DOI] [PubMed] [Google Scholar]
- Eichenlaub R., Figurski D., Helinski D. R. Bidirection replication from a unique origin in a mini-F plasmid. Proc Natl Acad Sci U S A. 1977 Mar;74(3):1138–1141. doi: 10.1073/pnas.74.3.1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner R. C., Caughey P. A., Lane D., Bergquist P. L. Replication mutants of the F-plasmid of Escherichia coli. II. Cloned replication regions of temperature-sensitive mutants. Plasmid. 1980 Mar;3(2):179–192. doi: 10.1016/0147-619x(80)90108-0. [DOI] [PubMed] [Google Scholar]
- Gellert M., O'Dea M. H., Itoh T., Tomizawa J. Novobiocin and coumermycin inhibit DNA supercoiling catalyzed by DNA gyrase. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4474–4478. doi: 10.1073/pnas.73.12.4474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafsson P., Dreisig H., Molin S., Nordström K., Uhlin B. E. DNA replication control: studies of plasmid R1. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 1):419–425. doi: 10.1101/sqb.1979.043.01.048. [DOI] [PubMed] [Google Scholar]
- Hahn F. E. Experimental elimination of R factors. Antibiot Chemother (1971) 1975;20:196–226. doi: 10.1159/000398468. [DOI] [PubMed] [Google Scholar]
- Hirota Y. THE EFFECT OF ACRIDINE DYES ON MATING TYPE FACTORS IN ESCHERICHIA COLI. Proc Natl Acad Sci U S A. 1960 Jan;46(1):57–64. doi: 10.1073/pnas.46.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan J. E., Kline B. C., Levy S. B. Regions on the F plasmid which affect plasmid maintenance and the ability to segregate into Escherichia coli minicells. Plasmid. 1982 Jul;8(1):36–44. doi: 10.1016/0147-619x(82)90039-7. [DOI] [PubMed] [Google Scholar]
- Itoh T., Tomizawa J. Initiation of replication of plasmid ColE1 DNA by RNA polymerase, ribonuclease H, and DNA polymerase I. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 1):409–417. doi: 10.1101/sqb.1979.043.01.047. [DOI] [PubMed] [Google Scholar]
- Lederberg E. M., Cohen S. N. Transformation of Salmonella typhimurium by plasmid deoxyribonucleic acid. J Bacteriol. 1974 Sep;119(3):1072–1074. doi: 10.1128/jb.119.3.1072-1074.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low K. B. Escherichia coli K-12 F-prime factors, old and new. Bacteriol Rev. 1972 Dec;36(4):587–607. doi: 10.1128/br.36.4.587-607.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAY J. W., HOUGHTON R. H., PERRET C. J. THE EFFECT OF GROWTH AT ELEVATED TEMPERATURES ON SOME HERITABLE PROPERTIES OF STAPHYLOCOCCUS AUREUS. J Gen Microbiol. 1964 Nov;37:157–169. doi: 10.1099/00221287-37-2-157. [DOI] [PubMed] [Google Scholar]
- Manis J. J., Kline B. C. F plasmid incompatibility and copy number genes: their map locations and interactions. Plasmid. 1978 Sep;1(4):492–507. doi: 10.1016/0147-619x(78)90007-0. [DOI] [PubMed] [Google Scholar]
- Manis J. J., Kline B. C. Restriction endonuclease mapping and mutagenesis of the F sex factor replication region. Mol Gen Genet. 1977 Apr 29;152(3):175–182. doi: 10.1007/BF00268815. [DOI] [PubMed] [Google Scholar]
- Manis J., Kline B. Recombination between an Flac and a mini-FKmr plasmid deleted for an origin of replication. Plasmid. 1978 Sep;1(4):480–491. doi: 10.1016/0147-619x(78)90006-9. [DOI] [PubMed] [Google Scholar]
- McHugh G. L., Swartz M. N. Elimination of plasmids from several bacterial species by novobiocin. Antimicrob Agents Chemother. 1977 Sep;12(3):423–426. doi: 10.1128/aac.12.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick R. P. Extrachromosomal inheritance in bacteria. Bacteriol Rev. 1969 Jun;33(2):210–263. doi: 10.1128/br.33.2.210-263.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogasawara N., Seiki M., Yoshikawa H. Initiation of DNA replication in Bacillus subtilis. V. Role of DNA gyrase and superhelical structure in initiation. Mol Gen Genet. 1981;181(3):332–337. doi: 10.1007/BF00425607. [DOI] [PubMed] [Google Scholar]
- Orr E., Fairweather N. F., Holland I. B., Pritchard R. H. Isolation and characterisation of a strain carrying a conditional lethal mutation in the cou gene of Escherichia coli K12. Mol Gen Genet. 1979;177(1):103–112. doi: 10.1007/BF00267259. [DOI] [PubMed] [Google Scholar]
- Orr E., Staudenbauer W. L. An Escherichia coli mutant thermosensitive in the B subunit of DNA gyrase: effect on the structure and replication of the colicin E1 plasmid in vitro. Mol Gen Genet. 1981;181(1):52–56. doi: 10.1007/BF00339004. [DOI] [PubMed] [Google Scholar]
- Riise E., Stougaard P., Bindslev B., Nordström K., Molin S. Molecular cloning and functional characterization of a copy number control gene (copB) of plasmid R1. J Bacteriol. 1982 Sep;151(3):1136–1145. doi: 10.1128/jb.151.3.1136-1145.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seelke R. W., Kline B. C., Trawick J. D., Ritts G. D. Genetic studies of F plasmid maintenance genes involved in copy number control, incompatability, and partitioning. Plasmid. 1982 Mar;7(2):163–179. doi: 10.1016/0147-619x(82)90075-0. [DOI] [PubMed] [Google Scholar]
- Sharp P. A., Cohen S. N., Davidson N. Electron microscope heteroduplex studies of sequence relations among plasmids of Escherichia coli. II. Structure of drug resistance (R) factors and F factors. J Mol Biol. 1973 Apr 5;75(2):235–255. doi: 10.1016/0022-2836(73)90018-1. [DOI] [PubMed] [Google Scholar]
- Skurray R. A., Nagaishi H., Clark A. J. Molecular cloning of DNA from F sex factor of Escherichia coli K-12. Proc Natl Acad Sci U S A. 1976 Jan;73(1):64–68. doi: 10.1073/pnas.73.1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor D. E., Levine J. G. Characterization of a plasmid mutation affecting maintenance, transfer and elimination by novobiocin. Mol Gen Genet. 1979 Jul 13;174(2):127–133. doi: 10.1007/BF00268350. [DOI] [PubMed] [Google Scholar]
- Terawaki Y., Takayasu H., Akiba T. Thermosensitive replication of a kanamycin resistance factor. J Bacteriol. 1967 Sep;94(3):687–690. doi: 10.1128/jb.94.3.687-690.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler J., Kline B. C. Mutation and identification of the F plasmid locus determining resistance to acridine orange curing. Plasmid. 1980 Nov;4(3):276–280. doi: 10.1016/0147-619x(80)90066-9. [DOI] [PubMed] [Google Scholar]
- Wehlmann H., Eichenlaub R. Plasmid mini-F encoded proteins. Mol Gen Genet. 1980;180(1):205–211. doi: 10.1007/BF00267371. [DOI] [PubMed] [Google Scholar]
- Wolfson J. S., Hooper D. C., Swartz M. N., McHugh G. L. Antagonism of the B subunit of DNA gyrase eliminates plasmids pBR322 and pMG110 from Escherichia coli. J Bacteriol. 1982 Oct;152(1):338–344. doi: 10.1128/jb.152.1.338-344.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfson J. S., Hooper D. C., Swartz M. N., Swartz M. D., McHugh G. L. Rapid method for screening large numbers of Escherichia coli colonies for production of plasmid-mediated beta-lactamases. Antimicrob Agents Chemother. 1983 Feb;23(2):308–312. doi: 10.1128/aac.23.2.308. [DOI] [PMC free article] [PubMed] [Google Scholar]



