Abstract
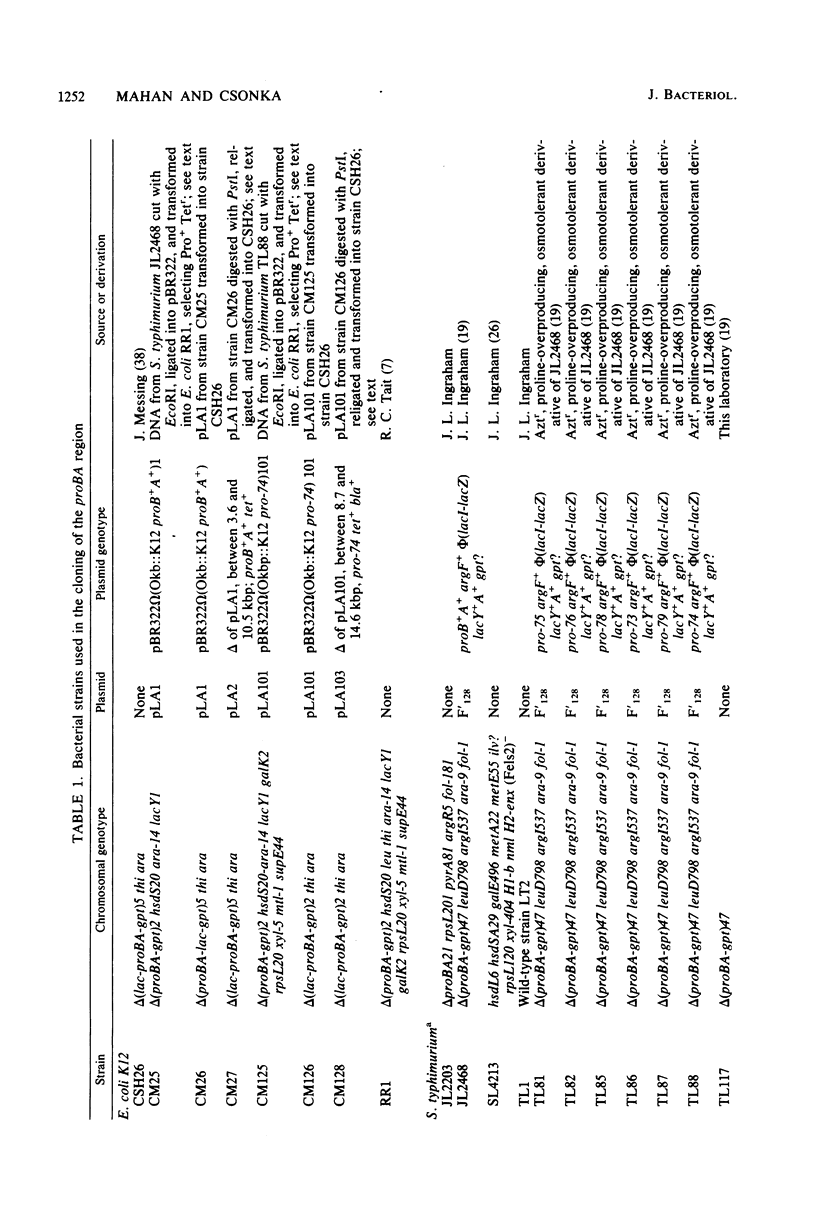
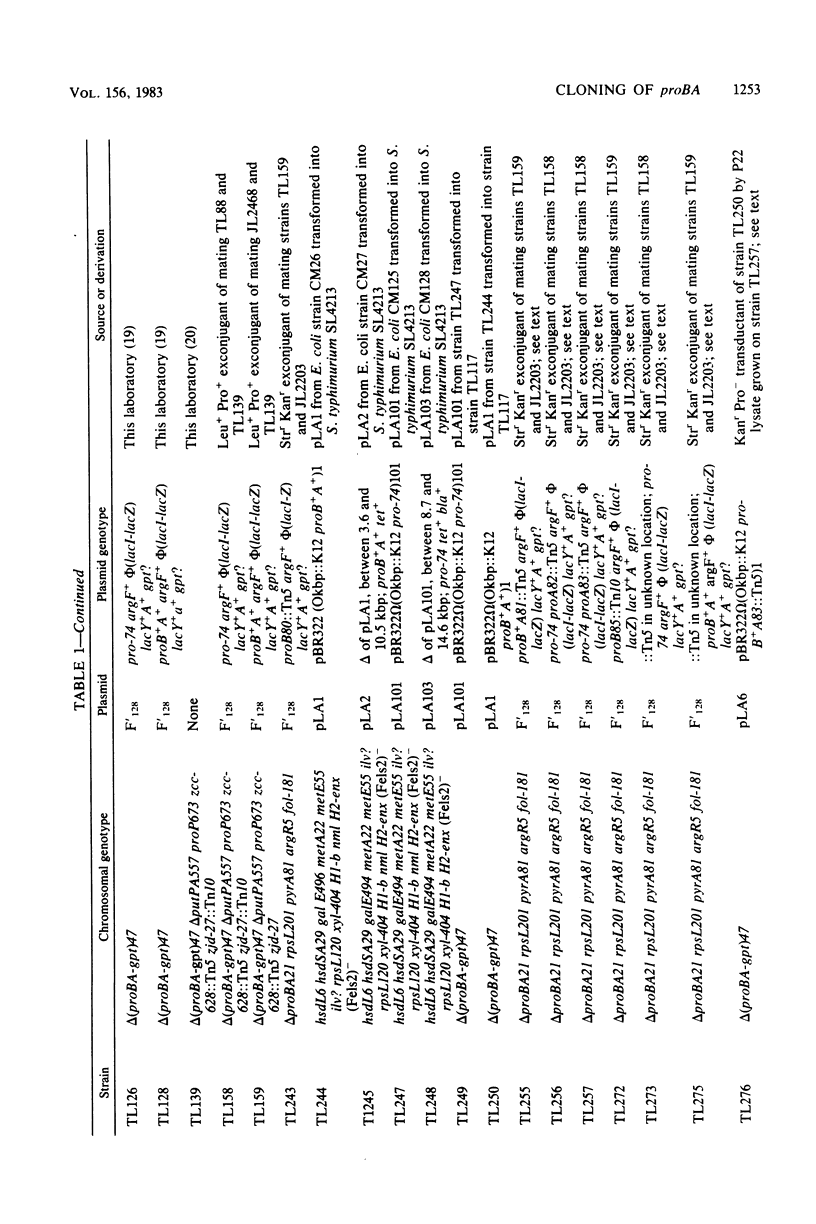
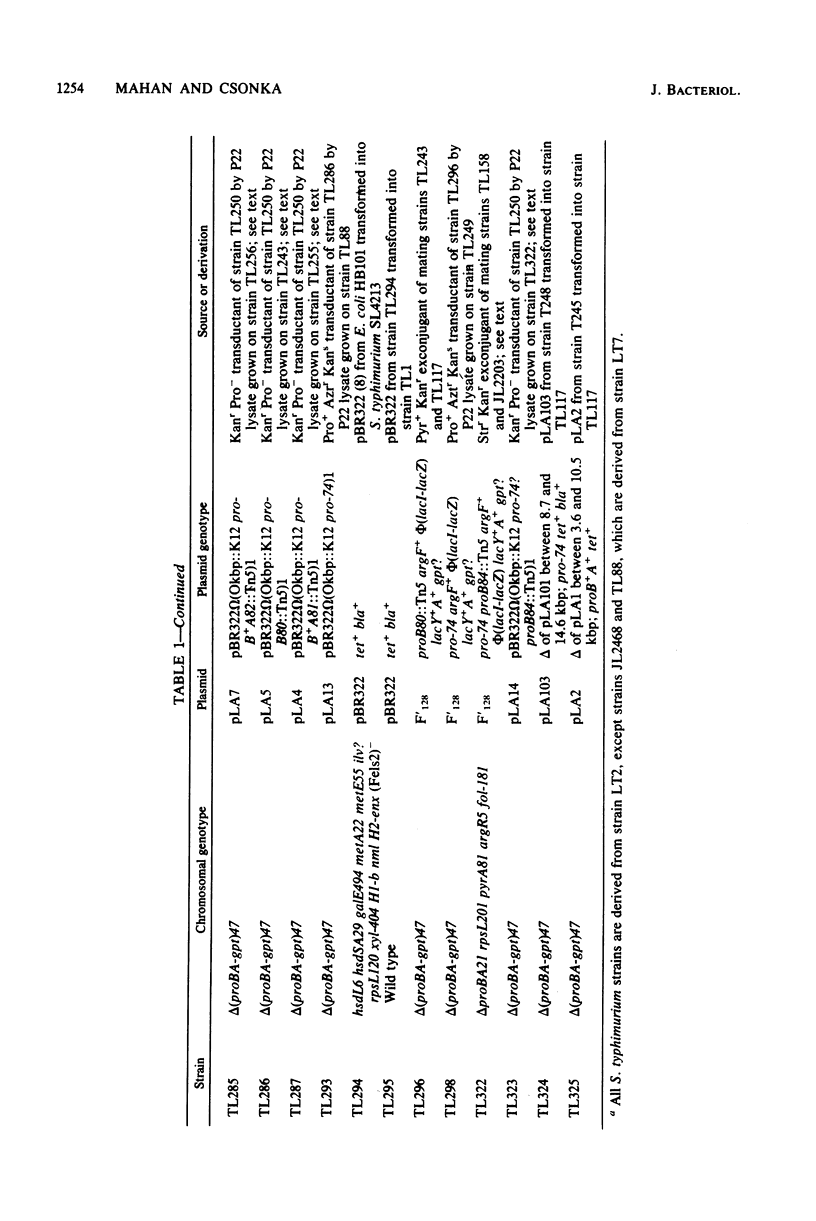
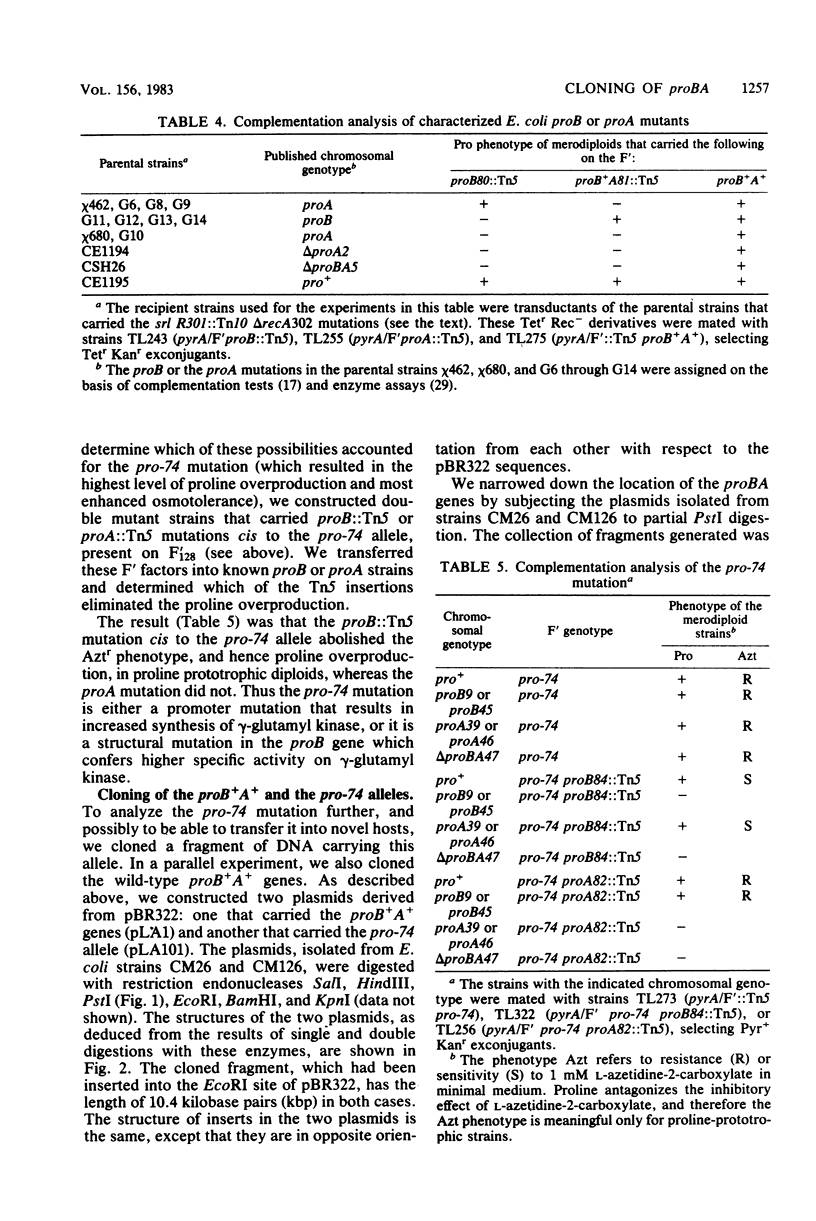
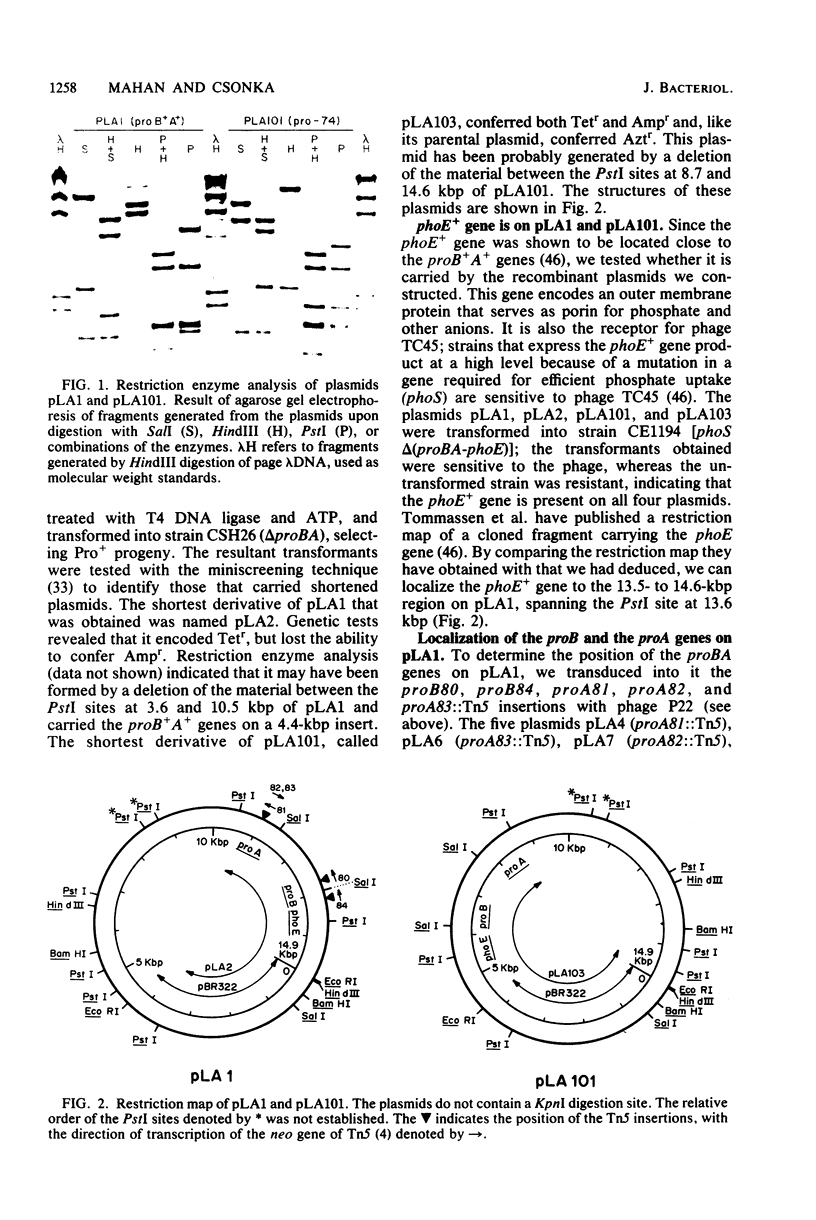
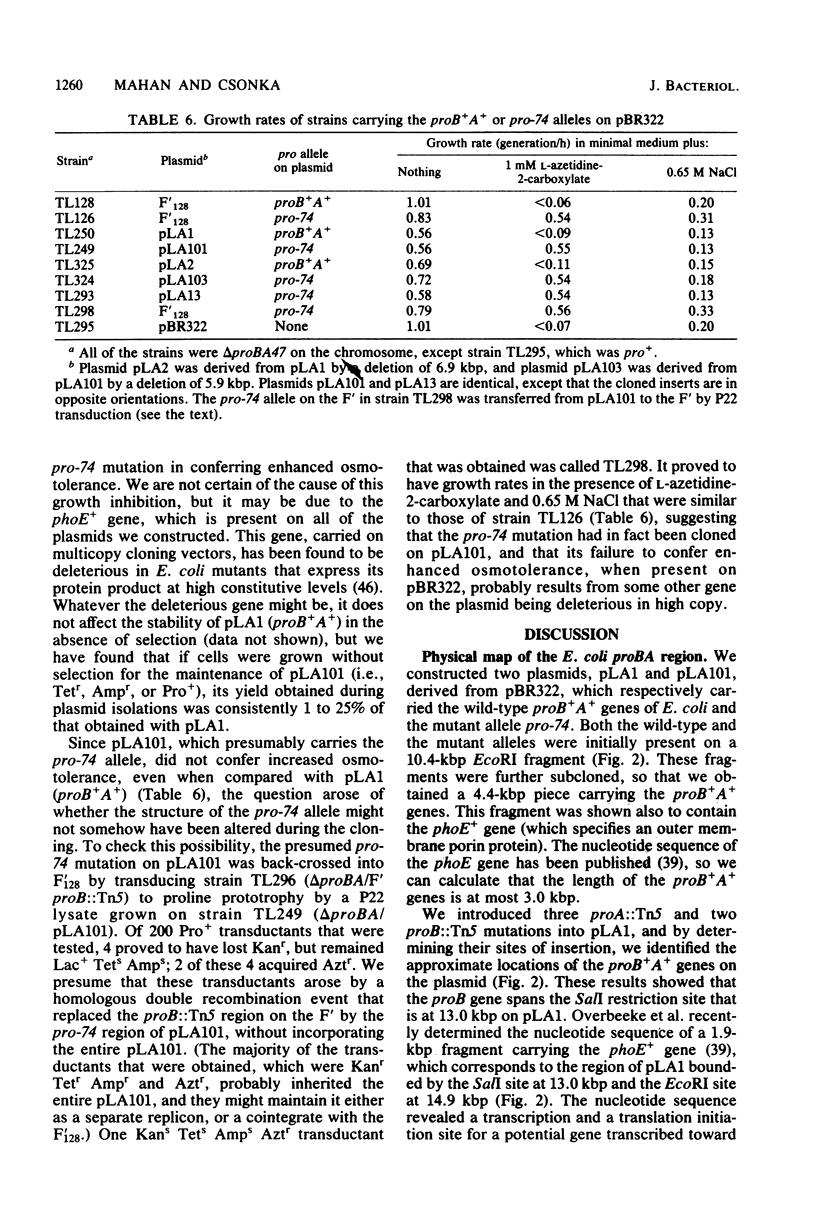
Because of the fact that proline overproduction relieves the inhibitory effects of high external osmotic strength in a number of procaryotes, we wished to clone a mutant allele, pro-74, that confers proline overproduction and enhanced osmotolerance on Salmonella typhimurium and Escherichia coli. Therefore, the pro-74 allele, originally located on an E. coli episome F'128, was cloned into pBR322. In a parallel experiment, the wild type proB+ A+ genes of E. coli were also cloned from F'128 into pBR322. Both the pro-74 and the proB+ A+ alleles were obtained on a 10.4-kilobase-pair fragment that also contained the unrelated phoE gene. Strains carrying either the wild-type proB+ A+ or the pro-74 alleles on pBR322 grew more slowly, both in minimal medium and media of elevated osmotic strength, than strains carrying the same alleles on the low-copy plasmid, F'128, indicating that some gene in the cloned region is deleterious in high copy. We constructed Tn5 insertion mutations in the proB and the proA genes of E. coli, carried on F'128 in S. typhimurium. Using P22 transduction in S. typhimurium, we transferred these proB and proA::Tn5 insertions from F'128 into the cloned proBA genes on pBR322. From the restriction maps of the plasmids thus generated, we determined the approximate locations of the proB and the proA genes. We also performed complementation tests of S. typhimurium and E. coli proB and proA mutants by using the F'128 proB and proA::Tn5 insertions. These tests revealed that the proBA genes of S. typhimurium form an operon, whose direction of transcription is from proB to proA. They also indicated that in S. typhimurium, as in E. coli, the proB+ gene encodes gamma-glutamyl kinase, and the proA+ gene encodes gamma-glutamyl phosphate reductase. Complementation tests also indicated that the pro-74 mutation is either in the proB structural gene, or its promoter-operator.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachmann B. J., Low K. B. Linkage map of Escherichia coli K-12, edition 6. Microbiol Rev. 1980 Mar;44(1):1–56. doi: 10.1128/mr.44.1.1-56.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann B. J. Pedigrees of some mutant strains of Escherichia coli K-12. Bacteriol Rev. 1972 Dec;36(4):525–557. doi: 10.1128/br.36.4.525-557.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baich A. Proline synthesis in Escherichia coli. A proline-inhibitable glutamic acid kinase. Biochim Biophys Acta. 1969 Dec 30;192(3):462–467. doi: 10.1016/0304-4165(69)90395-x. [DOI] [PubMed] [Google Scholar]
- Beck E., Ludwig G., Auerswald E. A., Reiss B., Schaller H. Nucleotide sequence and exact localization of the neomycin phosphotransferase gene from transposon Tn5. Gene. 1982 Oct;19(3):327–336. doi: 10.1016/0378-1119(82)90023-3. [DOI] [PubMed] [Google Scholar]
- Berg C. M., Rossi J. J. Proline excretion and indirect suppression in Escherichia coli and Salmonella typhimurium. J Bacteriol. 1974 Jun;118(3):928–934. doi: 10.1128/jb.118.3.928-934.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blazey D. L., Burns R. O. Transcriptional activity of the transposable element Tn10 in the Salmonella typhimurium ilvGEDA operon. Proc Natl Acad Sci U S A. 1982 Aug;79(16):5011–5015. doi: 10.1073/pnas.79.16.5011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Betlach M. C., Boyer H. W. Construction and characterization of new cloning vehicles. I. Ampicillin-resistant derivatives of the plasmid pMB9. Gene. 1977;2(2):75–93. doi: 10.1016/0378-1119(77)90074-9. [DOI] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Broda P. Modified map positions for lac and the pro markers in Escherichia coli K-12. J Bacteriol. 1974 Feb;117(2):741–746. doi: 10.1128/jb.117.2.741-746.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHEN G. N., RICKENBERG H. V. Concentration spécifique réversible des amino acides chez Escherichia coli. Ann Inst Pasteur (Paris) 1956 Nov;91(5):693–720. [PubMed] [Google Scholar]
- CURTIS S. R., 3rd CHROMOSOMAL ABERRATIONS ASSOCIATED WITH MUTATIONS TO BACTERIOPHAGE RESISTANCE IN ESCHERICHIA COLI. J Bacteriol. 1965 Jan;89:28–40. doi: 10.1128/jb.89.1.28-40.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciampi M. S., Schmid M. B., Roth J. R. Transposon Tn10 provides a promoter for transcription of adjacent sequences. Proc Natl Acad Sci U S A. 1982 Aug;79(16):5016–5020. doi: 10.1073/pnas.79.16.5016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condamine H. Sur la régulation de la production de proline chez E. Coli K 12. Ann Inst Pasteur (Paris) 1971 Feb;120(2):126–143. [PubMed] [Google Scholar]
- Csonka L. N. A third L-proline permease in Salmonella typhimurium which functions in media of elevated osmotic strength. J Bacteriol. 1982 Sep;151(3):1433–1443. doi: 10.1128/jb.151.3.1433-1443.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csonka L. N., Clark A. J. Deletions generated by the transposon Tn10 in the srl recA region of the Escherichia coli K-12 chromosome. Genetics. 1979 Oct;93(2):321–343. doi: 10.1093/genetics/93.2.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csonka L. N. Proline over-production results in enhanced osmotolerance in Salmonella typhimurium. Mol Gen Genet. 1981;182(1):82–86. doi: 10.1007/BF00422771. [DOI] [PubMed] [Google Scholar]
- Deutch A. H., Smith C. J., Rushlow K. E., Kretschmer P. J. Escherichia coli delta 1-pyrroline-5-carboxylate reductase: gene sequence, protein overproduction and purification. Nucleic Acids Res. 1982 Dec 11;10(23):7701–7714. doi: 10.1093/nar/10.23.7701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enomoto M., Stocker B. A. Transduction by phage P1kc in Salmonella typhimurium. Virology. 1974 Aug;60(2):503–514. doi: 10.1016/0042-6822(74)90344-4. [DOI] [PubMed] [Google Scholar]
- Hansen J. B., Olsen R. H. Isolation of large bacterial plasmids and characterization of the P2 incompatibility group plasmids pMG1 and pMG5. J Bacteriol. 1978 Jul;135(1):227–238. doi: 10.1128/jb.135.1.227-238.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayzer D. J., Leisinger T. Proline biosynthesis in Escherichia coli. Stoichiometry and end-product identification of the reaction catalysed by glutamate semialdehyde dehydrogenase. Biochem J. 1981 Aug 1;197(2):269–274. doi: 10.1042/bj1970269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayzer D. J., Leisinger T. The gene-enzyme relationships of proline biosynthesis in Escherichia coli. J Gen Microbiol. 1980 Jun;118(2):287–293. doi: 10.1099/00221287-118-2-287. [DOI] [PubMed] [Google Scholar]
- Hoppe I., Roth J. Specialized transducing phages derived from salmonella phage P22. Genetics. 1974 Apr;76(4):633–654. doi: 10.1093/genetics/76.4.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itikawa H., Demerec M. Salmonella typhimurium proline mutants. J Bacteriol. 1968 Mar;95(3):1189–1190. doi: 10.1128/jb.95.3.1189-1190.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kado C. I., Liu S. T. Rapid procedure for detection and isolation of large and small plasmids. J Bacteriol. 1981 Mar;145(3):1365–1373. doi: 10.1128/jb.145.3.1365-1373.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemper J. Evolution of a new gene substituting for the leuD gene of Salmonella typhimurium: origin and nature of supQ and newD mutations. J Bacteriol. 1974 Dec;120(3):1176–1185. doi: 10.1128/jb.120.3.1176-1185.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Rudulier D., Yang S. S., Csonka L. N. Nitrogen fixation in Klebsiella pneumoniae during osmotic stress. Effect of exogenous proline or a proline overproducing plasmid. Biochim Biophys Acta. 1982 Nov 24;719(2):273–283. doi: 10.1016/0304-4165(82)90099-x. [DOI] [PubMed] [Google Scholar]
- Measures J. C. Role of amino acids in osmoregulation of non-halophilic bacteria. Nature. 1975 Oct 2;257(5525):398–400. doi: 10.1038/257398a0. [DOI] [PubMed] [Google Scholar]
- Miyake T, Demerec M. Proline Mutants of Salmonella Typhimurium. Genetics. 1960 Jun;45(6):755–762. doi: 10.1093/genetics/45.6.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overbeeke N., Bergmans H., van Mansfeld F., Lugtenberg B. Complete nucleotide sequence of phoE, the structural gene for the phosphate limitation inducible outer membrane pore protein of Escherichia coli K12. J Mol Biol. 1983 Feb 5;163(4):513–532. doi: 10.1016/0022-2836(83)90110-9. [DOI] [PubMed] [Google Scholar]
- Roberts L. M., Reeve E. C. Two mutations giving low-level streptomycin resistance in Escherichia coli K 12. Genet Res. 1970 Dec;16(3):359–365. doi: 10.1017/s0016672300002640. [DOI] [PubMed] [Google Scholar]
- Rossi J. J., Vender J., Berg C. M., Coleman W. H. Partial purification and some properties of delta1-pyrroline-5-carboxylate reductase from Escherichia coli. J Bacteriol. 1977 Jan;129(1):108–114. doi: 10.1128/jb.129.1.108-114.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson K. E., Hartman P. E. Linkage map of Salmonella typhimurium, edition V. Microbiol Rev. 1978 Jun;42(2):471–519. doi: 10.1128/mr.42.2.471-519.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmieger H., Backhaus H. Altered cotransduction frequencies exhibited by HT-mutants of Salmonella-phage P22. Mol Gen Genet. 1976 Feb 2;143(3):307–309. doi: 10.1007/BF00269408. [DOI] [PubMed] [Google Scholar]
- Tempest D. W., Meers J. L., Brown C. M. Influence of environment on the content and composition of microbial free amino acid pools. J Gen Microbiol. 1970 Dec;64(2):171–185. doi: 10.1099/00221287-64-2-171. [DOI] [PubMed] [Google Scholar]
- Tommassen J., Overduin P., Lugtenberg B., Bergmans H. Cloning of phoE, the structural gene for the Escherichia coli phosphate limitation-inducible outer membrane pore protein. J Bacteriol. 1982 Feb;149(2):668–672. doi: 10.1128/jb.149.2.668-672.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tristram H., Thurston C. F. Control of proline biosynthesis by proline and proline analogues. Nature. 1966 Oct 1;212(5057):74–75. doi: 10.1038/212074a0. [DOI] [PubMed] [Google Scholar]
- Tully R. E., Hanson A. D., Nelsen C. E. Proline Accumulation in Water-stressed Barley Leaves in Relation to Translocation and the Nitrogen Budget. Plant Physiol. 1979 Mar;63(3):518–523. doi: 10.1104/pp.63.3.518. [DOI] [PMC free article] [PubMed] [Google Scholar]