Abstract
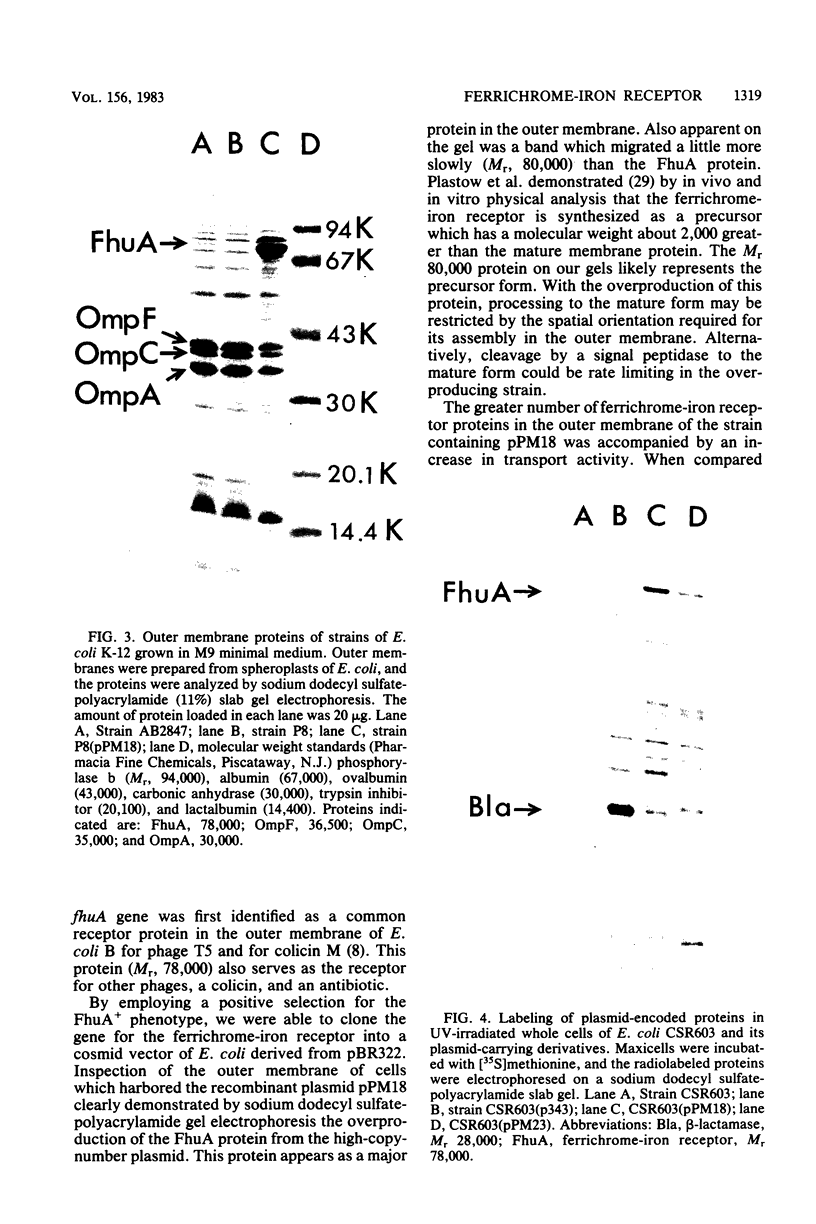
A receptor protein in the outer membrane of Escherichia coli K-12 is required for the binding of ferrichrome-iron at the cell surface and for the transport of iron from this complex into the cell. This protein of Mr 78,000 is the product of the fhuA (previously called tonA) gene, located at 3.5 min on the E. coli chromosome. We cloned the fhuA gene into plasmid p343, a high-copy-number cosmid derived from pBR322. An 8.5-kilobase pair fragment of E. coli chromosomal DNA, generated by hydrolysis with the restriction endonuclease HindIII, was found to have conferred the FhuA+ phenotype to E. coli P8, which lacks the ferrichrome-iron receptor. A partial physical map of this recombinant plasmid pPM18 was established by determining the restriction endonuclease sites for BglII, EcoRI, PstI, PvuII, SmaI, and XhoI. The fhuA gene was localized to a 3.5-kilobase pair fragment of DNA whose extremities were defined by the restriction sites PstI-PvuII. A 7.5-fold enhancement of the rate of transport of iron from the ferrichrome complex was measured for cells which contained pPM18 as compared to wild-type E. coli K-12. Overproduction of the FhuA protein was detected by sodium dodecyl sulfate-polyacrylamide gel electrophoresis of outer membrane proteins of the recombinant plasmid-containing strain. Proteins encoded by the subcloned DNA fragments were identified by [35S]methionine labeling of maxicells of E. coli CSR603, which contained recombinant plasmids; only one polypeptide chain, the presumptive fhuA gene product, was detected.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bindereif A., Braun V., Hantke K. The cloacin receptor of ColV-bearing Escherichia coli is part of the Fe3+-aerobactin transport system. J Bacteriol. 1982 Jun;150(3):1472–1475. doi: 10.1128/jb.150.3.1472-1475.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bindereif A., Neilands J. B. Cloning of the aerobactin-mediated iron assimilation system of plasmid ColV. J Bacteriol. 1983 Feb;153(2):1111–1113. doi: 10.1128/jb.153.2.1111-1113.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Braun V., Burkhardt R., Schneider R., Zimmermann L. Chromosomal genes for ColV plasmid-determined iron(III)-aerobactin transport in Escherichia coli. J Bacteriol. 1982 Aug;151(2):553–559. doi: 10.1128/jb.151.2.553-559.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun V., Hancock R. E., Hantke K., Hartmann A. Functional organization of the outer membrane of escherichia coli: phage and colicin receptors as components of iron uptake systems. J Supramol Struct. 1976;5(1):37–58. doi: 10.1002/jss.400050105. [DOI] [PubMed] [Google Scholar]
- Braun V., Schaller K., Wolff H. A common receptor protein for phage T5 and colicin M in the outer membrane of Escherichia coli B. Biochim Biophys Acta. 1973 Sep 27;323(1):87–97. doi: 10.1016/0005-2736(73)90433-1. [DOI] [PubMed] [Google Scholar]
- Clarke L., Carbon J. A colony bank containing synthetic Col El hybrid plasmids representative of the entire E. coli genome. Cell. 1976 Sep;9(1):91–99. doi: 10.1016/0092-8674(76)90055-6. [DOI] [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. R. Supercoiled circular DNA-protein complex in Escherichia coli: purification and induced conversion to an opern circular DNA form. Proc Natl Acad Sci U S A. 1969 Apr;62(4):1159–1166. doi: 10.1073/pnas.62.4.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulton J. W., Braun V. Protein II influences ferrichrome-iron transport in Escherichia coli K12. J Gen Microbiol. 1979 Jan;110(1):211–220. doi: 10.1099/00221287-110-1-211. [DOI] [PubMed] [Google Scholar]
- Coulton J. W., Naegeli H. U., Braun V. Iron supply of Escherichia coli with polymer-bound ferricrocin. Eur J Biochem. 1979 Aug 15;99(1):39–47. doi: 10.1111/j.1432-1033.1979.tb13228.x. [DOI] [PubMed] [Google Scholar]
- Coulton J. W. The ferrichrome-iron receptor of Escherichia coli K-12. Antigenicity of the fhuA protein. Biochim Biophys Acta. 1982 Jul 16;717(1):154–162. doi: 10.1016/0304-4165(82)90393-2. [DOI] [PubMed] [Google Scholar]
- Covarrubias L., Cervantes L., Covarrubias A., Soberón X., Vichido I., Blanco A., Kupersztoch-Portnoy Y. M., Bolivar F. Construction and characterization of new cloning vehicles. V. Mobilization and coding properties of pBR322 and several deletion derivatives including pBR327 and pBR328. Gene. 1981 Jan-Feb;13(1):25–35. doi: 10.1016/0378-1119(81)90040-8. [DOI] [PubMed] [Google Scholar]
- Hancock R. E., Hantke K., Braun V. Iron transport in Escherichia coli K-12. 2,3-Dihydroxybenzoate-promoted iron uptake. Arch Microbiol. 1977 Sep 28;114(3):231–239. doi: 10.1007/BF00446867. [DOI] [PubMed] [Google Scholar]
- Hancock R. E., Hantke K., Braun V. Iron transport of Escherichia coli K-12: involvement of the colicin B receptor and of a citrate-inducible protein. J Bacteriol. 1976 Sep;127(3):1370–1375. doi: 10.1128/jb.127.3.1370-1375.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hantke K. Regulation of ferric iron transport in Escherichia coli K12: isolation of a constitutive mutant. Mol Gen Genet. 1981;182(2):288–292. doi: 10.1007/BF00269672. [DOI] [PubMed] [Google Scholar]
- Kadner R. J., Heller K., Coulton J. W., Braun V. Genetic control of hydroxamate-mediated iron uptake in Escherichia coli. J Bacteriol. 1980 Jul;143(1):256–264. doi: 10.1128/jb.143.1.256-264.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krone W. J., Oudega B., Stegehuis F., de Graaf F. K. Cloning and expression of the cloacin DF13/aerobactin receptor of Escherichia coli (ColV-K30). J Bacteriol. 1983 Feb;153(2):716–721. doi: 10.1128/jb.153.2.716-721.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird A. J., Ribbons D. W., Woodrow G. C., Young I. G. Bacteriophage Mu-mediated gene transposition and in vitro cloning of the enterochelin gene cluster of Escherichia coli. Gene. 1980 Nov;11(3-4):347–357. doi: 10.1016/0378-1119(80)90074-8. [DOI] [PubMed] [Google Scholar]
- Leong J., Neilands J. B. Mechanisms of siderophore iron transport in enteric bacteria. J Bacteriol. 1976 May;126(2):823–830. doi: 10.1128/jb.126.2.823-830.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luckey M., Pollack J. R., Wayne R., Ames B. N., Neilands J. B. Iron uptake in Salmonella typhimurium: utilization of exogenous siderochromes as iron carriers. J Bacteriol. 1972 Sep;111(3):731–738. doi: 10.1128/jb.111.3.731-738.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugtenberg B., Meijers J., Peters R., van der Hoek P., van Alphen L. Electrophoretic resolution of the "major outer membrane protein" of Escherichia coli K12 into four bands. FEBS Lett. 1975 Oct 15;58(1):254–258. doi: 10.1016/0014-5793(75)80272-9. [DOI] [PubMed] [Google Scholar]
- Mandel M., Higa A. Calcium-dependent bacteriophage DNA infection. J Mol Biol. 1970 Oct 14;53(1):159–162. doi: 10.1016/0022-2836(70)90051-3. [DOI] [PubMed] [Google Scholar]
- Meagher R. B., Tait R. C., Betlach M., Boyer H. W. Protein expression in E. coli minicells by recombinant plasmids. Cell. 1977 Mar;10(3):521–536. doi: 10.1016/0092-8674(77)90039-3. [DOI] [PubMed] [Google Scholar]
- Menichi B., Buu A. Integration of the overproduced bacteriophage T5 receptor protein in the outer membrane of Escherichia coli. J Bacteriol. 1983 Apr;154(1):130–138. doi: 10.1128/jb.154.1.130-138.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plastow G. S., Pratt J. M., Holland I. B. The ferrichrome receptor protein (tonA) of Escherichia coli is synthesised as a precursor in vitro. FEBS Lett. 1981 Aug 31;131(2):262–264. doi: 10.1016/0014-5793(81)80380-8. [DOI] [PubMed] [Google Scholar]
- Radloff R., Bauer W., Vinograd J. A dye-buoyant-density method for the detection and isolation of closed circular duplex DNA: the closed circular DNA in HeLa cells. Proc Natl Acad Sci U S A. 1967 May;57(5):1514–1521. doi: 10.1073/pnas.57.5.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancar A., Hack A. M., Rupp W. D. Simple method for identification of plasmid-coded proteins. J Bacteriol. 1979 Jan;137(1):692–693. doi: 10.1128/jb.137.1.692-693.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda Y., Nishimura A., Nishimura Y., Yamada M., Yasuda S., Suzuki H., Hirota Y. Synthetic ColE1 Plasmids carrying genes for penicillin-binding proteins in Escherichia coli. Plasmid. 1981 Jul;6(1):86–98. doi: 10.1016/0147-619x(81)90056-1. [DOI] [PubMed] [Google Scholar]
- Wagegg W., Braun V. Ferric citrate transport in Escherichia coli requires outer membrane receptor protein fecA. J Bacteriol. 1981 Jan;145(1):156–163. doi: 10.1128/jb.145.1.156-163.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witholt B., Boekhout M., Brock M., Kingma J., Heerikhuizen H. V., Leij L. D. An efficient and reproducible procedure for the formation of spheroplasts from variously grown Escherichia coli. Anal Biochem. 1976 Jul;74(1):160–170. doi: 10.1016/0003-2697(76)90320-1. [DOI] [PubMed] [Google Scholar]




