Abstract
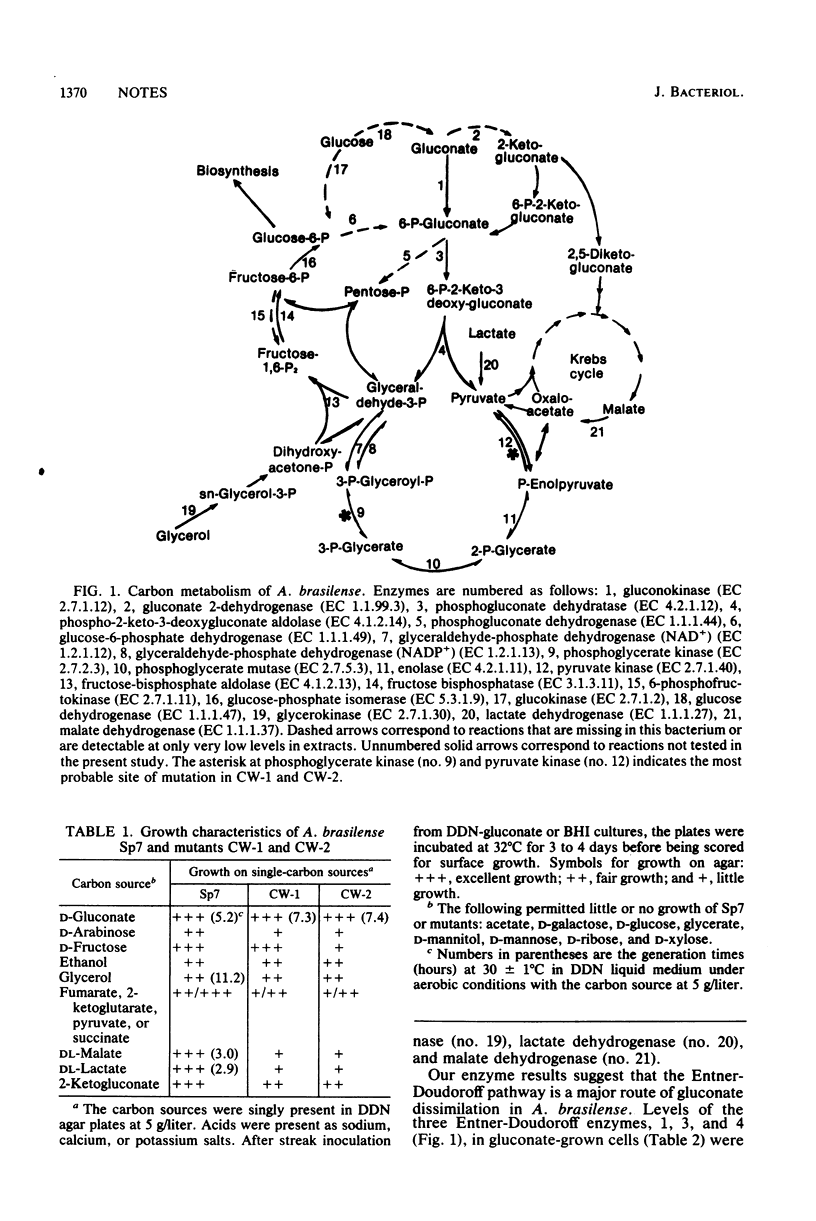
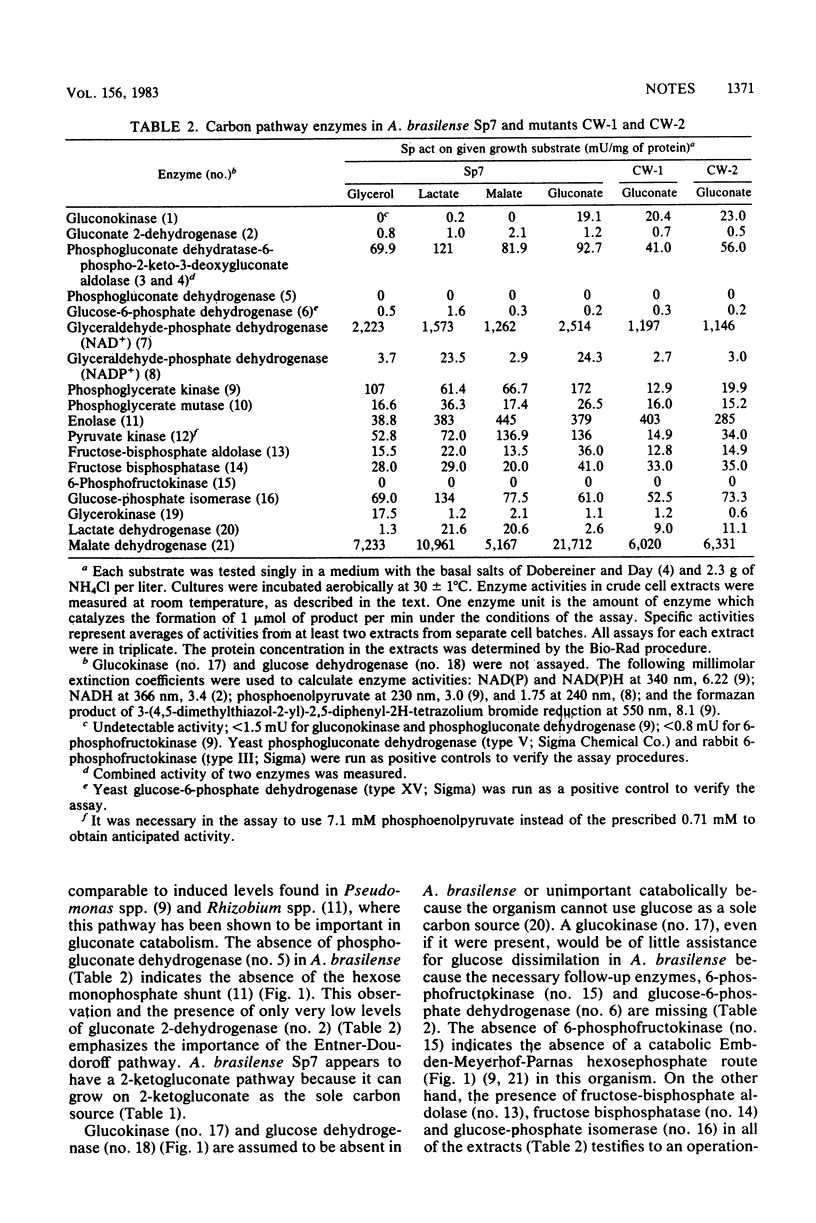
Azospirillum brasilense Sp7 and two mutants were examined for 19 carbon metabolism enzymes. The results indicate that this nitrogen fixer uses the Entner-Doudoroff pathway for gluconate dissimilation, lacks a catabolic but has an anabolic Embden-Meyerhof-Parnas hexosephosphate pathway, has amphibolic triosephosphate enzymes, lacks a hexose monophosphate shunt, and has lactate dehydrogenase, malate dehydrogenase, and glycerokinase. The mutants are severely deficient in phosphoglycerate and pyruvate kinase and also have somewhat reduced levels of other carbon enzymes.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Campbell A. E., Hellebust J. A., Watson S. W. Reductive pentose phosphate cycle in Nitrosocystis oceanus. J Bacteriol. 1966 Mar;91(3):1178–1185. doi: 10.1128/jb.91.3.1178-1185.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale N. L., Beck J. V. Evidence for the Calvin cycle and hexose monophosphate pathway in Thiobacillus ferrooxidans. J Bacteriol. 1967 Oct;94(4):1052–1059. doi: 10.1128/jb.94.4.1052-1059.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grisolia S., Carreras J. Phosphoglycerate mutase from yeast, chicken breast muscle, and kidney (2, 3-PGA-dependent). Methods Enzymol. 1975;42:435–450. doi: 10.1016/0076-6879(75)42149-8. [DOI] [PubMed] [Google Scholar]
- Heath H. E., Gaudy E. T. Relationship between catabolism of glycerol and metabolism of hexosephosphate derivatives by Pseudomonas aeruginosa. J Bacteriol. 1978 Nov;136(2):638–646. doi: 10.1128/jb.136.2.638-646.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irani M., Maitra P. K. Isolation and characterization of Escherichia coli mutants defective in enzymes of glycolysis. Biochem Biophys Res Commun. 1974 Jan;56(1):127–133. doi: 10.1016/s0006-291x(74)80324-4. [DOI] [PubMed] [Google Scholar]
- KOVACHEVICH R., WOOD W. A. Carbohydrate metabolism by Pseudomonas fluorescens. III. Purification and properties of a 6-phosphogluconate dehydrase. J Biol Chem. 1955 Apr;213(2):745–756. [PubMed] [Google Scholar]
- KOVACHEVICH R., WOOD W. A. Carbohydrate metabolism by Pseudomonas fluorescens. IV. Purification and properties of 2-keto-3-deoxy-6-phosphogluconate aldolase. J Biol Chem. 1955 Apr;213(2):757–767. [PubMed] [Google Scholar]
- Keele B. B., Jr, Hamilton P. B., Elkan G. H. Gluconate catabolism in Rhizobium japonicum. J Bacteriol. 1970 Mar;101(3):698–704. doi: 10.1128/jb.101.3.698-704.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam K. B., Marmur J. Isolation and characterization of Saccharomyces cerevisiae glycolytic pathway mutants. J Bacteriol. 1977 May;130(2):746–749. doi: 10.1128/jb.130.2.746-749.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick N. J., Tyler M. E. L-arabinose metabolism in Azospirillum brasiliense. J Bacteriol. 1982 Jan;149(1):364–367. doi: 10.1128/jb.149.1.364-367.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okon Y., Albrecht S. L., Burris R. H. Carbon and ammonia metabolism of Spirillum lipoferum. J Bacteriol. 1976 Nov;128(2):592–597. doi: 10.1128/jb.128.2.592-597.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okon Y., Albrecht S. L., Burris R. H. Factors affecting growth and nitrogen fixation of Spirillum lipoferum. J Bacteriol. 1976 Sep;127(3):1248–1254. doi: 10.1128/jb.127.3.1248-1254.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarrand J. J., Krieg N. R., Döbereiner J. A taxonomic study of the Spirillum lipoferum group, with descriptions of a new genus, Azospirillum gen. nov. and two species, Azospirillum lipoferum (Beijerinck) comb. nov. and Azospirillum brasilense sp. nov. Can J Microbiol. 1978 Aug;24(8):967–980. doi: 10.1139/m78-160. [DOI] [PubMed] [Google Scholar]
- Tiwari N. P., Campbell J. J. Enzymatic control of the metabolic activity of Pseudomonas aeruginosa grown in glucose or succinate media. Biochim Biophys Acta. 1969 Dec 30;192(3):395–401. doi: 10.1016/0304-4165(69)90388-2. [DOI] [PubMed] [Google Scholar]



