Abstract
The apparently chromosomally encoded 3',5"-aminoglycoside phosphotransferase (type III), from the high-level aminoglycoside-resistant Streptococcus pneumoniae BM4200, was compared with homologous enzymes coded for by the plasmids pJH1 and pSH2, originally isolated from Streptococcus faecalis and Staphylococcus aureus, respectively, and also found in a wild strain of S. aureus, BM4600. The enzymes appeared to be indistinguishable, and we conclude that the gene encoding 3',5"-aminoglycoside phosphotransferase (type III) can cross generic barriers within gram-positive cocci.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnes W. M. Plasmid detection and sizing in single colony lysates. Science. 1977 Jan 28;195(4276):393–394. doi: 10.1126/science.318764. [DOI] [PubMed] [Google Scholar]
- Burdett V., Inamine J., Rajagopalan S. Heterogeneity of tetracycline resistance determinants in Streptococcus. J Bacteriol. 1982 Mar;149(3):995–1004. doi: 10.1128/jb.149.3.995-1004.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buu-Hoï A., Horodniceanu T. Conjugative transfer of multiple antibiotic resistance markers in Streptococcus pneumoniae. J Bacteriol. 1980 Jul;143(1):313–320. doi: 10.1128/jb.143.1.313-320.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courvalin P. M., Shaw W. V., Jacob A. E. Plasmid-mediated mechanisms of resistance to aminoglycoside-aminocyclitol antibiotics and to chloramphenicol in group D streptococci. Antimicrob Agents Chemother. 1978 May;13(5):716–725. doi: 10.1128/aac.13.5.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courvalin P., Carlier C., Collatz E. Plasmid-mediated resistance to aminocyclitol antibiotics in group D streptococci. J Bacteriol. 1980 Aug;143(2):541–551. doi: 10.1128/jb.143.2.541-551.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courvalin P., Davies J. Plasmid-medicated aminoglycoside phosphotransferase of broad substrate range that phosphorylates amikacin. Antimicrob Agents Chemother. 1977 Apr;11(4):619–624. doi: 10.1128/aac.11.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courvalin P., Fiandt M. Aminoglycoside-modifying enzymes of Staphylococcus aureus; expression in Escherichia coli. Gene. 1980 May;9(3-4):247–269. doi: 10.1016/0378-1119(90)90326-m. [DOI] [PubMed] [Google Scholar]
- Davies J., Smith D. I. Plasmid-determined resistance to antimicrobial agents. Annu Rev Microbiol. 1978;32:469–518. doi: 10.1146/annurev.mi.32.100178.002345. [DOI] [PubMed] [Google Scholar]
- Eckhardt T. A rapid method for the identification of plasmid desoxyribonucleic acid in bacteria. Plasmid. 1978 Sep;1(4):584–588. doi: 10.1016/0147-619x(78)90016-1. [DOI] [PubMed] [Google Scholar]
- Franke A. E., Clewell D. B. Evidence for a chromosome-borne resistance transposon (Tn916) in Streptococcus faecalis that is capable of "conjugal" transfer in the absence of a conjugative plasmid. J Bacteriol. 1981 Jan;145(1):494–502. doi: 10.1128/jb.145.1.494-502.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas M. J., Dowding J. E. Aminoglycoside-modifying enzymes. Methods Enzymol. 1975;43:611–628. doi: 10.1016/0076-6879(75)43124-x. [DOI] [PubMed] [Google Scholar]
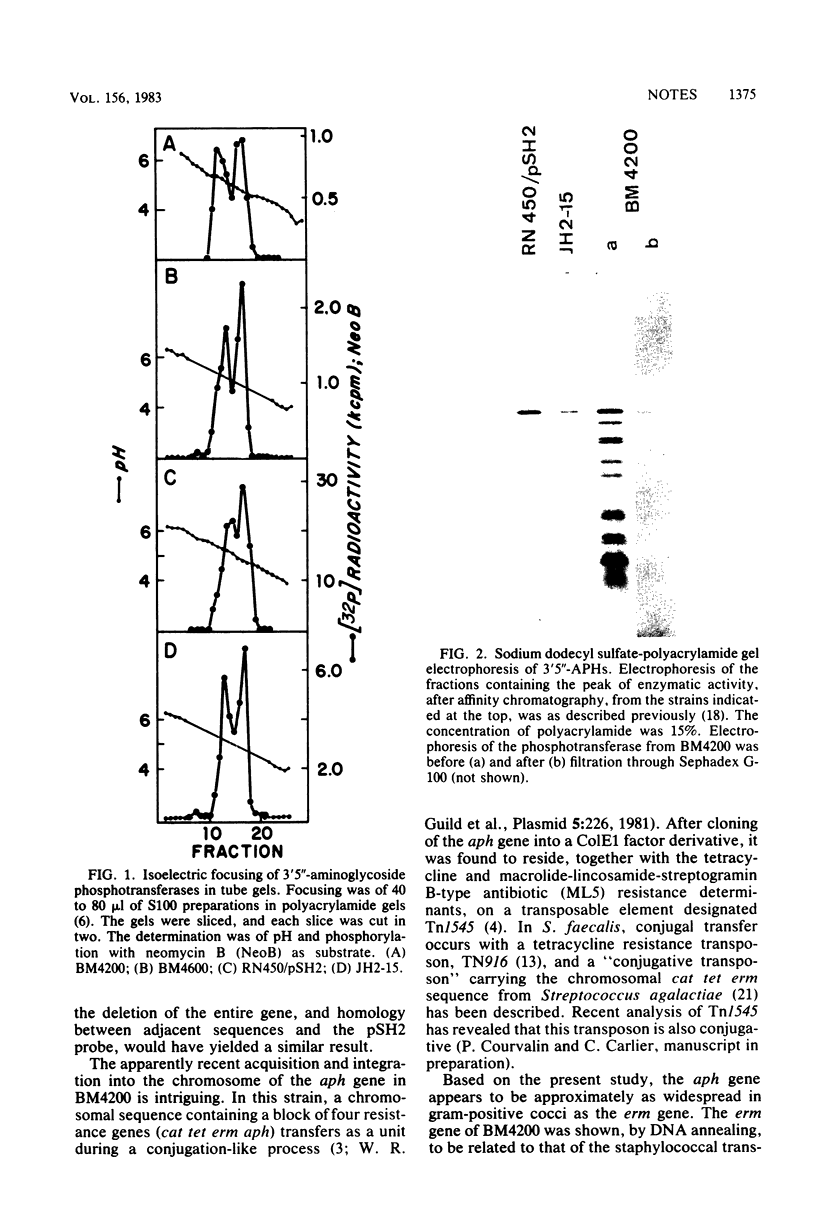
- Horodniceanu T., Buu-Hoï A., Delbos F., Bieth G. High-level aminoglycoside resistance in group A, B, G, D (Streptococcus bovis), and viridans streptococci. Antimicrob Agents Chemother. 1982 Jan;21(1):176–179. doi: 10.1128/aac.21.1.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labigne-Roussel A., Gerbaud G., Courvalin P. Translocation of sequences encoding antibiotic resistance from the chromosome to a receptor plasmid in Salmonella ordonez. Mol Gen Genet. 1981;182(3):390–408. doi: 10.1007/BF00293927. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., Kleid D. G. Nucleotide sequence of the rightward operator of phage lambda. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1184–1188. doi: 10.1073/pnas.72.3.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M. D., Hazum S., Guild W. R. Homology among tet determinants in conjugative elements of streptococci. J Bacteriol. 1981 Oct;148(1):232–240. doi: 10.1128/jb.148.1.232-240.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stiffler P. W., Sweeney H. M., Schneider M., Cohen S. Isolation and characterization of a kanamycin resistance plasmid from Staphylococcus aureus. Antimicrob Agents Chemother. 1974 Oct;6(4):516–520. doi: 10.1128/aac.6.4.516. [DOI] [PMC free article] [PubMed] [Google Scholar]