Abstract
The medial temporal lobe is known to play a role in the processing of olfaction and memory. The specific contribution of the human amygdala to memory for odors has not been addressed, however. The role of this region in memory for odors was assessed in patients with unilateral amygdala damage due to temporal lobectomy (n = 20; 11 left, 9 right), one patient with selective bilateral amygdala damage, and in 20 age-matched normal controls. Fifteen odors were presented, followed 1 h later by an odor–name matching test and an odor–odor recognition test. Signal detection analyses showed that both unilateral groups were impaired in their memory for matching odors with names, these patients were not significantly impaired on odor–odor recognition. Bilateral amygdala damage resulted in severe impairment in both odor–name matching as well as in odor–odor recognition memory. Importantly, none of the patients were impaired on an auditory verbal learning task, suggesting that these findings reflect a specific impairment in olfactory memory, and not merely a more general memory deficit. Taken together, the data provide neuropsychological evidence that the human amygdala is essential for olfactory memory.
Considerable research has illustrated a role for the anteromesial temporal lobes in several aspects of olfactory processing, including odor detection (Rausch and Serafetinides 1975; Eichenbaum et al. 1983), discrimination (Abraham and Mathai 1983), and memory (Rausch et al. 1977; Martinez et al. 1993; Dade et al. 2002). Some authors have suggested a preferential role of the right temporal cortex in olfactory memory (Rausch et al. 1977)— in line with a material-specific advantage of the left hemisphere for verbal memory and the right hemisphere for nonverbal memory (Dobbins et al. 1998; Buchanan et al. 2001). In contrast, Henkin and colleagues described a study in which left temporal excision resulted in greater impairment in olfactory recognition than did right-sided damage (Henkin et al. 1977). However, more recent work has shown that both the right and the left temporal lobes are likely involved in odor memory. Dade et al. (2002) demonstrated the participation of both the right and left temporal lobes in olfactory memory both in a lesion study in patients with temporal lobe damage as well as in a functional neuroimaging experiment using normal participants.
The temporal lobes contain several areas known to be involved in olfactory processing, including the piriform cortex, which is located at the frontotemporal junction, the entorhinal cortex, the periamygdaloid cortex, and anterior cortical nucleus of the amygdala (Eslinger et al. 1982; West and Doty 1995; Savic 2001). The specific role of the human amygdala in olfactory processing has been the focus of several studies in lesion patients (Babinsky et al. 1993; Markowitsch et al. 1994), and using the techniques of functional neuroimaging (Zald and Pardo 1997; Royet et al. 2000; O'Doherty et al. 2001; Gottfried et al. 2002a,b; Anderson et al. 2003) and intracranial stereotactic EEG (Hudry et al. 2001). Babinsky et al. (1993) and Markowitsch et al. (1994) showed impaired odor-paired associate learning in two patients with selective bilateral amygdala damage. Functional imaging studies have shown pronounced amygdala activation during odor processing, specifically more amygdala activity is found in response to high-intensity odorants (Zald and Pardo 1997; Anderson et al. 2003).
This study was designed to assess the role of the amygdaloid complex in two different components of memory for odors. We tested odor memory using two different tasks, a cross-modal odor–name matching test and a unimodal odor–odor recognition task. These two different tasks were chosen to further assess the possibility of separable roles of the left and right amygdalae in odor memory. We tested two specific predictions: (1) If the left amygdala is specialized for verbal–odor associations, as would be predicted by previous work on material specificity following left-sided brain damage, then the group with left amygdala damage would be expected to show the most impairment on odor–name matching, and (2) if the right amygdala plays a greater role in olfactory processing across the board than does the left, then the group with right amygdala damage should be most impaired on both odor–name and odor–odor matching. Additionally, in order to specifically test the role of the amygdala in odor memory, we tested a rare patient with bilateral damage relatively restricted to the amygdala (patient SM046) on these tasks.
RESULTS
Demographics, Neuropsychology, and Neuroanatomy
Participant characteristics including demographics, neuropsychological test performance, and neuroanatomical volumes are shown in Table 1.
Table 1.
Demographic and Neuropsychological Data for All Subjects
| Measure | SM046 | NC | LTL | RTL | Statistic | P |
|---|---|---|---|---|---|---|
| Demographics | ||||||
| Age | 35 | 37 ± 11.2 | 39 ± 9.9 | 38 ± 9.4 | F < 1 | >0.6 |
| Gender | F | 12F/8M | 7F/4M | 4F/5M | χ2 = 1.5 | >0.4 |
| Education | 12 | — | 14 ± 1.8 | 14 ± 1.8 | t < 1 | >0.6 |
| Handedness | R | — | 8R/3L | 8R/1L | χ2 < 1 | >0.5 |
| Years Post-Surgery | — | — | 4.6 ± 5.1 | 6.8 ± 6.2 | t = 0.5 | >0.6 |
| Olfactory Function | ||||||
| UPSIT | 32 | 34.2 ± 3.3 | 31.4 ± 4.5 | 33.0 ± 2.6 | F = 2.4 | 0.1 |
| Neuropsychological Data | ||||||
| AVLT d′ | 2.87 | — | 2.97 ± 0.92 | 3.3 ± 0.6 | t = 1.2 | 0.24 |
| AVLT C | 0.43 | — | 0.06 ± 0.3 | 0.09 ± 0.11 | t < 1 | >0.7 |
| VIQ | 86 | — | 94 ± 9.0 | 100 ± 13.8 | t = 2.0 | 0.062 |
| PIQ | 90 | — | 99 ± 13.7 | 104 ± 16.0 | t < 1 | >0.5 |
| VRT Correct | 5 | — | 7.9 ± 1.7 | 6.8 ± 1.8 | t = 1.1 | 0.3 |
| VRT Errors | 10 | — | 3.3 ± 3.3 | 4.8 ± 3.9 | t < 1 | >0.3 |
| CFT | 13.5 | — | 18 ± 6.2 | 16 ± 6.5 | t < 1 | >0.3 |
| Aphasia | N | — | 9N/2Y | 9N | χ2 = 1.6 | >0.2 |
| WCST Categories | — | — | 5.3 ± 1.3 | 5.1 ± 1.2 | t < 1 | >0.9 |
| COWA | 25 | — | 38 ± 13.2 | 37 ± 10.0 | t < 1 | >0.7 |
| BDI | — | — | 9.0 ± 7.5 | 8.6 ± 8.4 | t < 1 | >0.8 |
| Neuroanatomical Data | ||||||
| Left Amyg Volume | 0 | — | 744 ± 175 | 1908 ± 129 | t = 5.1 | 0.0001 |
| Right Amyg Volume | 0 | — | 1602 ± 97 | 934 ± 168 | t = 3.6 | 0.002 |
| Left HC Volume | 2576 | — | 1398 ± 254 | 3907 ± 218 | t = 7.3 | 0.0001 |
| Right HC Volume | 2893 | — | 4156 ± 154 | 1530 ± 268 | t = 8.9 | 0.0001 |
(NC) Normal control group; (LTL) left temporal lobectomy group; (RTL) right temporal lobectomy group; Education is reported in years; Handedness was assessed with the Edinburgh Handedness Inventory; (BDI) Beck Depression Inventory; (VIQ) WAIS-R Verbal Scale IQ; (PIQ) WAIS-R Performance Scale IQ; (AVLT d′) Auditory-Verbal Learning Test discriminability score; (AVLT C′) Auditory-Verbal Learning Test bias score; (VRT) Visual Retention Test, (Correct) number correct of 10, (Errors) number of errors committed; (CFT) Complex Figure Test, 30 min recall; (Aphasia) subjects classified as N = None, Y = Mild/moderate aphasia; (WCST Categories) Wisconsin Card Sort Test, number of categories achieved; (COWA) Controlled Oral Word Association (no. of words generated for the letters C, F, and L); Right and left amygdala volume as determined from MRI images (in mm3); Right and left hippocampal volume; Years Post-Surgery (time since temporal lobectomy). Statistics were performed comparing temporal lobectomy groups and normal controls (those measures on which F-ratios are reported) or comparing right and left temporal lobectomy groups (those measures on which t-tests are reported). Data from patient SM046 were not included in these analyses. Data are reported as mean ± standard error of the mean.
Odor–Name Matching Test
There were significant group differences in the measures of false-alarm rate [F(2,37) = 5.6, P = 0.007, η2 = 0.23] and in C, the measure of bias, F(2,37) = 3.5, P = 0.041, η2 = 0.16. The measures of hit rate and d' (discriminability) did not show significant overall group differences [F(2,37) = 1.2, P > 0.3, η2 = 0.06; F(2,37) = 1.6, P > 0.2, η2 = 0.08, respectively]. Planned contrasts indicated that the LTL group showed greater rates of false alarms than did the other two groups (vs. NC, P = 0.006; vs. RTL, P = 0.03; see Table 2). The LTL group also showed a more liberal response bias (C) compared with both the NC (P = 0.02) and the RTL group (P = 0.056). This pattern of performance in the LTL group results in a lower d' (see Fig. 1A), indicating lower sensitivity to discriminating old from new items. The RTL group, similarly, shows low discriminability of old from new items, but in contrast to the LTL group, the RTL group shows a response bias more similar to the control group, indicating a more conservative criterion (see Table 2). Due to the low statistical power of comparing across these three groups, data from both temporal lobectomy groups were combined and compared with the normal controls' performance. These analyses indicate that the combined temporal lobectomy group showed a greater false alarm rate, t(38) = 2.3, P < 0.05, a lower d', t(38) = 1.7, P < 0.05, and a lower C, t(38) = 1.7, P = 0.05, compared with the control group. There were no significant group differences in hit rate (t < 1).
Table 2.
Odor–Name Matching Test Results
| Group | Hit rate | False alarm rate | d′ | C |
|---|---|---|---|---|
| NC | 0.37 (0.15) | 0.22 (0.14) | 0.48 (0.54) | 0.61 (0.4) |
| LTL | 0.45 (0.25) | 0.41 (0.2) | 0.15 (0.53) | 0.17 (0.66) |
| RTL | 0.34 (0.13) | 0.25 (0.1) | 0.27 (0.37) | 0.58 (0.33) |
| SM046 | 0.13 (0.04) | 0.15 (0.08) | -0.11 (0.14) | 1.11 (0.29) |
d′ is the standard signal detection measure of recognition accuracy, C is the measure of response bias (Snodgrass and Corwin 1986). Data from SM046 are averaged across two separate testing sessions.
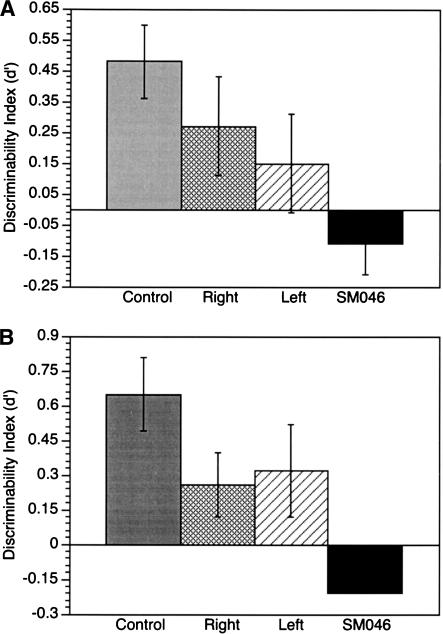
Figure 1.
Discriminability (d') Performance for both Odor–Name Matching and Odor–Odor Matching Tests. Mean (± standard error) of d' across all groups, and including patient SM046. (A) Odor–Name Matching Results. Note that patient SM046 performed this task on two separate occasions; mean values (± standard error) are plotted. (B) Odor–Odor Matching Results. Note that on this test, patient SM046 showed identical performance across both testing sessions, and therefore, no error bars are shown.
Results from Patient SM046 illustrate an inability to distinguish old odorant labels from new ones. She shows a higher false alarm rate (0.15) than hit rate (0.13), resulting in a negative d' discriminability index (–0.11). Figure 1A shows her d' performance in comparison with both unilateral groups and controls. Her performance is also characterized by a conservative response bias (C = 1.11). Across all measures, with the exception of false alarm rate, her performance stands out as the most aberrant from controls.
Odor–Odor Recognition Test
In the yes/no recognition portion of the test, there were no significant group differences in performance. The patterns of performance across groups are, however, similar to those from the odor–name matching test (normal control > left and right temporal lobectomy > SM046; see Fig. 1B). Statistical analyses across all of the four measures (hit rate, false alarm rate, d', and C) show no significant differences, Fs(2,37) < 1.8, Ps > 0.2, η2 < 0.05. Note that the effect sizes for these analyses are small (η2 < 0.05). Planned contrasts revealed no significant pairwise group differences. See Table 3 for means and standard deviations of these data. Due to these small effect sizes comparing across three groups, we compared performance between a combined group of temporal lobectomy patients (right and left) with normal controls. This analysis showed that the combined temporal lobectomy group had lower d' than the normal control group, t(38) = 1.7, P = 0.05. None of the other measures (hit rate, false alarm rate, C) were significantly different between these groups, ts < 1.3, Ps > 0.2.
Table 3.
Odor–Odor Recognition Test Results (Means and Standard Deviations)
| Group | Hit rate | False alarm rate | d′ | C |
|---|---|---|---|---|
| NC | 0.76 (0.15) | 0.55 (0.2) | 0.65 (0.7) | -0.51 (0.53) |
| LTL | 0.73 (0.11) | 0.62 (0.17) | 0.32 (0.47) | -0.49 (0.36) |
| RTL | 0.70 (0.07) | 0.6 (0.16) | 0.25 (0.54) | -0.4 (0.22) |
| SM046 | 0.97 | 0.98 | -0.21 | -1.97 |
d′ is the standard signal detection measure of recognition accuracy, C is the measure of response bias (Snodgrass and Corwin 1986). Data from SM046 are averaged across two separate testing sessions, although on this test, her performance was identical for both sessions, standard deviations are thus not included.
The performance of patient SM046 on the yes/no portion of this test was confounded by the fact that she responded yes to all odorants, whether new or old (identical performance on two separate testing sessions; see Table 3 and Fig. 1B). Thus, she was tested on a follow-up 2 forced-choice odor recognition task (see Materials and Methods for description). Performance on this task was 10 correct of 20, or chance level. This illustrates that even at a shorter time interval (30 min) and using a forced choice test, SM046 is impaired on odor–odor matching recognition memory.
To test for group differences in familiarity ratings, a 3 Group × 2 Stimulus Type (new vs. old) multivariate ANOVA was conducted with Group as a between-subjects factor and Stimulus Type as a within-subjects variable. There was no main effect of Group in familiarity rating [F(2,37) < 1, P > 0.5, η2 = 0.009]. There was a main effect of Stimulus Type, F(2,37) = 19.0, P < 0.0001, η2 = 0.34, indicating increased familiarity ratings for the old odorants compared with ratings for the new odorants. There was a trend toward a Group × Stimulus Type interaction, F(2,37) = 3.1, P = 0.057, η2 = 0.14. A follow-up interaction test comparing the results of the combined temporal lobectomy groups with the control group showed a significant Group × Stimulus Type interaction, F(1,38) = 6.2, P = 0.017, η2 = 0.14. The normal control group rated the old odorants as more familiar than the new odorants [difference score (old familiarity ratings – new familiarity ratings): 1.58 ± 1.3], whereas neither temporal lobectomy group showed this difference (left group, 0.5 ± 1.3; right group, 0.7 ± 1.2). Familiarity ratings for patient SM046 were the same for both the old and the new odors (mean for new odors, 7.76; old odors, 7.87).
Auditory–Verbal Learning and Memory Performance
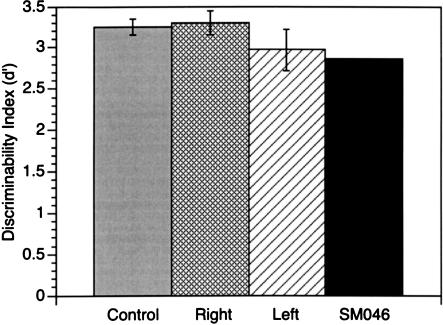
To address the specificity of the above findings to olfactory memory, and to rule out effects of generalized memory deficits, each patient's performance on the Auditory–Verbal Learning Test (AVLT) was analyzed using an identical signal-detection strategy as that used for the olfactory memory tests. Neither temporal lobectomy groups nor patient SM046 showed impairment on this task (11 of 20 unilateral patients showed perfect performance; 15 hits, 0 false alarms). Patient SM046 was similarly unimpaired, recording a performance of 13 hits and 0 false alarms. There was no difference between the right and left temporal lobectomy groups on any measure of performance on the AVLT, ts(18) < 1.2, Ps > 0.2. Figure 2 shows discriminability (d') scores for this test for all patients compared with scores from a normative sample. This pattern of performance on the AVLT indicates that the reduced olfactory memory of those with amygdala damage (both unilateral and bilateral) is not merely due to a global memory impairment.
Figure 2.
Auditory–Verbal Learning Test (AVLT) Performance expressed as discriminability (d') for both unilateral and bilateral amygdala patients and from a normative sample of 21 control subjects drawn from the AVLT normative data. Mean (± standard error) of d' across all groups, and including patient SM046.
Correlations Between Neuroanatomical Volume and Olfactory Memory
Measures of the remaining amygdala and hippocampal volumes were ascertained from all of the patients included in this study (see Allen et al. 2002a,b) for description of anatomical tracing techniques). See Table 1 for mean values of right and left amygdala and hippocampal volumes. These volumes were then used to assess whether the extent of the remaining amygdala or hippocampal volume was predictive of olfactory memory performance using Pearson's correlation coefficient. Results indicated that right amygdala and right hippocampal volumes were positively correlated with false alarm rate in the odor–name task (r = 0.42, 0.46, Ps < 0.05, respectively). Right hippocampal volume was also positively correlated with hit rate in the odor–name task (r = 0.45, P < 0.05). No other measures were correlated with neuroanatomical volume from either the odor–name or the odor–odor task (rs < |0.3|, Ps > 0.1). These associations are perhaps best explained by the high hit rate and false-alarm rate in the odor–name task in the left temporal lobectomy group, such that these patients had larger volumes of both the right amygdala and hippocampus.
DISCUSSION
This experiment replicates previous research showing impaired olfactory memory in patients with unilateral temporal lobectomy (Eskenazi et al. 1986; Dade et al. 2002), and extends these findings by suggesting a specific role for the amygdala in memory for olfactory stimuli.
This study demonstrates relatively selective and partly dissociable impairments in odor memory following damage to temporal lobe structures. Specifically, when asked to match previously presented odorants to a list of verbal descriptors of those odorants, the left temporal lobectomy group and the patient with bilateral amygdala damage (SM046) were impaired.
Similarly, when asked to match previously presented odorants to newly presented odorants, patient SM046 again showed an inability to discriminate previously presented odorants from new odorants even when tested on two different tasks (a yes/no recognition task as well as a two-forced choice recognition task). Patients with temporal lobectomy were less impaired on this task, but their pattern of performance was similar to their pattern on the odor–name task.
In support of our prediction of a lateralized impairment of odor–name matching in the LTL group, this group was most impaired on this task. Analyses drawn from signal-detection theory revealed different response tendencies on the odor–name matching task, with the LTL group showing a liberal response bias and the RTL group responding more conservatively (and more similarly to controls).
Importantly, these deficits in olfactory memory are noted in the absence of a generalized memory deficit in these patients, especially patient SM046 (as determined by performance on the AVLT). These findings thus suggest an olfactory-specific memory deficit following amygdala damage.
Patient SM046 was severely impaired on the performance of both the odor–name matching and odor–odor recognition tasks. On the odor–name matching test, her discriminability was approximately at chance levels during both testing occasions. Previous research with this patient (as well as another patient with bilateral amygdala damage) showed that her performance on a visual-tactile cross-modal matching task is unimpaired (Nahm et al. 1993). This suggests that her inability to match previously presented odorants with their names is not necessarily due to a deficit in cross-modal matching, but may be more specific to the modality of olfaction. Similarly, in the odor–odor recognition task with the yes/no recognition, she showed a complete positive bias, responding yes to all odorants on both testing conditions. Her familiarity ratings during this test were equivalent for the old and the new odorants. A subsequent 2 forced-choice recognition test further illustrated her inability to distinguish old from new odors. Two previous studies have addressed olfactory learning and memory in patients with amygdala damage due to Urbach-Wiethe disease (Babinsky et al. 1993; Markowitsch et al. 1994). Specifically, Markowitsch and colleagues asked two Urbach-Wiethe patients to associate six odors with six abstract figures. Whereas normal controls are able to associate four or five of the odors correctly, both patients were only able to associate one or two odors to the figures. These findings, along with those reported from the current study, suggest that the amygdala is a key structure in olfactory memory processing. Damage to either the right or the left temporal lobe, including the amygdala, resulted in some degree of impairment, specifically in the odor–name matching, but bilateral damage resulted in significant impairment in both measures of olfactory memory. These results further illustrate the olfactory and mnemonic processes that depend critically upon the integrity of the amygdala.
Results from this study are in contrast to previous work suggesting a right hemispheric dominance for olfactory function including odor detection and discrimination (Rausch and Serafetinides 1975; Abraham and Mathai 1983; Martinez et al. 1993). Results from studies on the effects of lateralized temporal lobe damage on odor memory, however, have been mixed, with some work showing a specific impairment following right temporal lobectomy (Rausch et al. 1977), but other studies showing equivalent impairment in left and right sided lobectomy patients (Henkin et al. 1977; Eskenazi et al. 1983, 1986; Dade et al. 2002). Most recently, Dade et al. (2002) have shown that both right and left-sided temporal lobectomy patients are equally impaired in an odor recognition paradigm including three different condtions, (1) after a single odor exposure, (2) after four odor exposures, and (3) after a 24-h delay interval. Interestingly, in a second experiment within the same report using PET imaging, Dade et al. (2002) demonstrated equivalent right and left-sided piriform activity during an odor-recognition task in healthy controls. These findings, along with those of the current investigation, indicate a bilateral involvement of the anteromesial temporal lobe in olfactory memory.
There is extensive literature suggesting a material-specific impairment of memory following lateralized brain damage, in which verbal memory is most impaired following left-sided damage and nonverbal memory-most impaired following right-sided damage (Dobbins et al. 1998; Buchanan et al. 2001; Vanderploeg et al. 2001). Additionally, lateralized processing of odor stimuli have been documented by showing that odor naming is more accurate when stimuli are presented to the left nostril (Herz et al. 1999). This pattern suggests that the primarily ipsilateral projections in the olfactory system influence language-related behavior differentially. On the basis of these findings of lateralization, we predicted that the LTL group would be most impaired in odor–name recognition memory. This prediction was partially supported by the data, as the left temporal lobectomy group showed the poorest performance on this task. Analyses derived from signal-detection theory demonstrated that the LTL group showed a different pattern from the RTL group. Those with left-sided damage showed a higher hit rate and higher false-alarm rate compared with those with right-sided damage, who showed low levels of both hits and false alarms. As both of these two measures (hit rate and false alarm rate) determines the level of discriminability (d'), the left-sided group proved less able to discriminate those odors that had been presented from those that had not been presented. The measure of response bias (C), which serves as an index of conservative versus liberal response tendencies (a higher C indicates a conservative response bias, lower C indicates a liberal response bias), was different between the two unilateral temporal lobectomy groups. The LTL group showed an extremely liberal response bias, whereas the RTL group was quite conservative. It is difficult to determine the nature of these differences in performance on this task between the LTL and RTL groups, but one possibility is that the LTL group's diminished verbal memory resulted in an inability to recall the names of the encoded odorants and resulted in indiscriminant responding on the odor–name matching task.
Performance on the odor–odor recognition test was equivalent for both the LTL and RTL groups and was not statistically different from controls (although analysis comparing the combined temporal lobectomy group with controls showed an impairment in discrimination performance in these patients, suggesting that the 3 group ANOVA lacked statistical power to detect difference among the 3 groups). Previous studies of odor memory have shown brain damage-induced deficits for this type of recognition task both at shorter (Rausch et al. 1977) and longer (Dade et al. 2002) delay intervals than the 1-h delay used in the current study. The recognition task used in the current investigation required only a yes/no response, making this task considerably easier than a four-alternative forced-choice test, for example (Rausch et al. 1977).
These results, along with recent functional neuroimaging studies (Zald and Pardo 1997; Royet et al. 2000; Dade et al. 2002; Gottfried et al. 2002a,b) and animal studies on olfaction (Schoenbaum et al. 1999, 2000; Kilpatrick and Cahill 2003), illustrate the important role that the amygdala plays in olfactory processing. Importantly, the amygdala is continuous with the periamygdaloid cortex, a region of the primary olfactory cortex (Eslinger et al. 1982; West and Doty 1995), and so any damage to the amygdala would most likely impinge upon the primary olfactory cortex. In lesion studies, it is difficult to separate out the individual processes carried out in olfaction by the amygdala alone, but we must focus on the extended amygdala, including the overlying olfactory cortex. Importantly, all of the temporal lobectomy patients had unilateral hippocampal damage in addition to amygdala damage, which would most definitely affect memory for stimuli from any sensory modality. The discrepancy between performance on the odor–name task and performance on the AVLT, however, suggests that whatever components of the MTL that were damaged resulted in a disproportionate affect on olfactory memory compared with auditory–verbal memory. Findings from the current study should be carefully interpreted in light of these anatomical considerations.
There are several possibilities for how the amygdala and continous cortex may influence olfactory memory. (1) The amygdala could be involved in higher-order perceptual processing of olfactory stimuli, making a perceptual representation of the odorant available for memory encoding. This processing would rely on direct input from the olfactory bulb via the piriform and periamygdaloid cortex. Work by Cahill and McGaugh (1990, 1998) and Kilpatrick and Cahill (2003) demonstrates that bilateral inactivation of the basolateral amygdala (BLA) abolishes olfactory learning in rats. Memory for olfaction, therefore, is similarly dependent upon BLA modulation of neural areas such as the hippocampus and caudate (for review, see Cahill and McGaugh 1998). (2) It could trigger emotional physiological responses to an odor stimulus via connections between the amygdala and output systems in the hypothalamus and brainstem (Pitkänen 2000), which could then be used in part to represent aspects of the stimulus for later memory retrieval or to modulate memory as the amygdala is well documented to do (Buchanan and Adolphs 2002). (3) The amygdala could directly affect memory encoding via connections with other neural areas involved in mnemonic processing, such as the hippocampus, thalamus, and ventral striatum (Eslinger et al. 1982; Eichenbaum and Cohen 2001).
Findings from this study, along with previous work in animals, human patients, and healthy controls in functional imaging, converge on the idea that the amygdala (including its connections to primary olfactory cortex) is an integral component in the function of olfactory memory. Whereas unilateral temporal lobe damage including the amygdala affects odor memory processing, bilateral damage specifically to the amygdala, especially, results in a striking inability to remember previously presented odorants. This work demonstrates that the human amygdaloid complex is involved not merely in sensory olfactory processing, but extends to olfactory memory.
MATERIALS AND METHODS
Subjects
A total of 20 subjects with unilateral temporal lobe damage (11 left and 9 right) subsequent to temporal lobectomy for treatment of intractable epilepsy were selected from the Patient Registry of the Division of Cognitive Neuroscience at the University of Iowa. To explore the consequences of bilateral damage specifically to the amygdala, we also included a single rare subject who has complete bilateral amygdala damage, patient SM046. This subject's amygdala damage is a consequence of Urbach-Wiethe disease; her detailed neuroanatomical and neuropsychological profiles have been published previously (Tranel and Hyman 1990; Adolphs et al. 1994). She has complete bilateral damage to the amygdala. Additionally, 20 age-matched normal subjects were recruited through local advertisement. All brain-damaged participants were individually adminstered a 3-h neuropsychological battery that included measures of olfactory function, intellect, anterograde verbal and visual memory, visuoperception, language, and executive functioning (see Table 1 for neuropsychological and demographic data). All subjects gave informed consent to participate in these studies, which were approved by the Human Subjects Committee of the University of Iowa.
Magnetic resonance images were obtained from all patients in a 1.5T General Electric 4096 Plus scanner. The scanning protocol used in this study is identical to that used in (Allen et al. 2002a). All brains were reconstructed in three dimensions in Brainvox (Frank et al. 1997), an interactive family of programs designed to reconstruct, segment, and measure brains from MR acquired images. All regions were traced by hand on contiguous coronal slices of the brain. We included only subjects with single, focal, stable lesions. The volumes of the amygdala and hippocampus were determined bilaterally in both temporal lobectomy groups as well as in patient SM046 (see Table 1 for these data). Although there is variable extent of brain damage among the temporal lobectomy patients, all patients have damage to both the amygdala and hippocampus.
The remaining volumes of the amygdala and hippocampus were traced in both hemispheres of each patient. Whole-brain volumes were also determined. Criteria for the boundaries of both the amygdala and hippocampus were derived from the atlas of Duvernoy (1988). Using a method similar to that of Convit et al. (1999); see also Szabo et al. (2001), pointsets tracing the boundaries of the amygdala and hippocampus were first made in parasagittal and axial planes; these pointsets were then projected to the coronal slices to guide tracing of the ROIs.
Tasks
All subjects were tested individually. Patient SM046 was tested on two separate occasions, and data from both testing sessions have been averaged together; all other subjects were tested once. Only subjects identified as having no basic olfactory perceptual deficits were included in the study. This was determined through the administration of the University of Pennsylvania Smell Identification Test (UPSIT; Doty et al. 1984). This test was administered after completion of the memory tests so as not to influence memory performance due to pre-exposure to the odorants used in the experimental tasks (see below). The UPSIT includes a total of 40 scratch and sniff odorants that are embedded in 10–50 μm urea-formaldehyde polymer microencapsules fixed in folders each containing 10 odorants. On each sheet of the folder, the odorant is affixed to the lower right-hand corner and four multiple choice odor names printed on the sheet. The subject is to mark one of the four alternatives matching the perceived smell. This test has established norms and is able to identify individuals with olfactory deficits (Doty et al. 1984).
Olfactory stimuli for the odor memory tasks consisted of 15 individual odorants from the UPSIT. We modified the original version of the UPSIT by choosing 15 of the original 40 odorants from the full version of the test for use in the encoding phase of the experiment. These 15 odorants were chosen to provide a range of distinctive stimuli, including both pleasant (grape, rose) and unpleasant (natural gas, paint thinner) odorants. Each subject was presented with each odorant after it had been scratched by the experimenter and was allowed to smell it as much as he or she liked for 5 sec (interstimulus interval of 15–20 sec). Importantly, and in contrast to the original version of the UPSIT, subjects were not asked to identify the odorant at the time of encoding, nor were they allowed to see the four multiple choice alternatives typically used in administration of the test. Participants were told that they would be asked about the odors later in a memory test; encoding was not incidental. After the encoding phase, subjects worked on a distractor task consisting of filling out questionnaires for 1 h. At the end of the distractor period, subjects were first presented with the Odor–Name Match Sheet, which consists of a list of 40 odorants, including the 15 that they were exposed to 1 h earlier. The subject was to indicate which odorants had been encountered by circling the names of all odors that could be remembered. The subject then completed the Odor–Odor Recognition test, in which they were presented with all 40 odorants from the UPSIT and asked for two responses, (A) to respond yes or no as to whether they remember smelling that odorant earlier during the testing, and (B) to rate the odor on a 9-point familiarity scale, with 1 being unfamiliar, 5 being somewhat familiar, and 9 being familiar. Importantly, subjects were instructed to rate familiarity on the basis of their experience with the odors during the testing session and not a general sense of familiarity for the odors. The fixed order of task presentation was chosen (as opposed to a counter-balanced order) because the presentation of foil (or new) odors during the odor–odor recognition task would contaminate the odor–name matching task.
For patient SM046, a follow-up 2 forced-choice odor recognition task was constructed (due to her response bias in the odor–odor recognition test; see Results). To circumvent this response bias, we constructed a new test to determine her true odor–odor recognition performance (on a completely separate session, several months after the initial presentation of the odorants). Again using odors drawn from the UPSIT, we presented SM046 with 20 odorants during an encoding session. Thirty minutes later, she was presented with a 2 forced-choice odor recognition test with each of the 20 old odors paired with a new odor that had not been presented previously.
To address general anterograde memory performance, each patient was tested on the Rey Auditory–Verbal Learning (AVLT) test, and results from this test were analyzed using the identical signal detection strategy used for the olfactory memory tests (as described below). Administration of the AVLT was included in each patient's neuropsychological battery. This test involves the auditory presentation of 15 words and an auditory recognition task conducted 30 min later. The recognition task includes the presentation of the 15 target words (those presented earlier) as well as 15 distractor words. Item analysis of this test allows for the determination of hits, false alarms, discriminability, and bias (as described below). Only patients were tested on this task. It is known from normative data that most of the normal subjects perform close to ceiling on the delayed recognition section of the AVLT.
Data Management and Analysis
Data were reduced by computing indices of recognition accuracy for both the Odor–Name Matching test and the Odor–Odor Recognition test. The number of hits (correct recognition of previously presented odorants) and false alarms (incorrect recognition of new odorants) were computed along with indices of recognition accuracy (d') and response bias (C) derived from signal detection theory (Snodgrass and Corwin 1986; Green and Swets 1989). Data were analyzed using a 3-Group (right temporal lobectomy, left temporal lobectomy, normal control) univariate analysis of variance (ANOVA). The associated degrees of freedom and a measure of effect size (η2), are reported for each ANOVA. Additionally, planned pairwise contrasts were conducted for each dependent measure across the three groups.
Acknowledgments
Supported by NINDS Grant P01 NS 19632 and an NRSA to T.W.B. from the National Institute on Aging.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Article and publication are at http://www.learnmem.org/cgi/doi/10.1101/lm.62303.
References
- Abraham, A., and Mathai, K.V. 1983. The effect of right temporal lobe lesions on matching of smells. Neuropsychologia 21: 277–281. [DOI] [PubMed] [Google Scholar]
- Adolphs, R., Tranel, D., Damasio, H., and Damasio, A. 1994. Impaired recognition of emotion in facial expressions following bilateral damage to the human amygdala. Nature 372: 669–672. [DOI] [PubMed] [Google Scholar]
- Allen, J.S., Damasio, H., and Grabowski, T.J. 2002a. Normal neuroanatomical variation in the human brain: An MRI-volumetric study. Am. J. Phys. Anthropol. 118: 341–358. [DOI] [PubMed] [Google Scholar]
- Allen, J.S., Damasio, H., and Tranel, D. 2002b. Hippocampus and temporal lobe volume in anoxia: High-resolution, quantitative MRI and memory correlates. Am. Acad. Neurol. Abst. 58: A265–A266. [Google Scholar]
- Anderson, A.K., Christoff, K., Stappen, I., Panitz, D., Ghahremani, D.G., Glover, G., Garbieli, J.D., and Sobel, N. 2003. Dissociated neural representations of intensity and valence in human olfaction. Nat. Neurosci. 6: 196–202. [DOI] [PubMed] [Google Scholar]
- Babinsky, R., Calabrese, P., Durwen, H.F., Markowitsch, H.J., Brechtelsbauer, D., Heuser, L., Gehlen, W. 1993. The possible contribution of the amygdala to memory. Behav. Neurol. 6: 167–170. [DOI] [PubMed] [Google Scholar]
- Buchanan, T.W. and Adolphs, R. 2002. The role of the human amygdala in emotional modulation of long-term declarative memory. In Emotional cognition: From brain to behavior (eds. S. Moore and M. Oaksford), pp. 9–34. John Benjamins Publishing, Amsterdam.
- Buchanan, T.W., Denburg, N.L., Tranel, D., and Adolphs, R. 2001. Verbal and nonverbal emotional memory following unilateral amygdala damage. Learn Mem. 8: 326–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill, L. and McGaugh, J.L. 1990. Amygdaloid complex lesions differentially affect retention of tasks using appetitive and aversive reinforcement. Behav. Neurosci. 104: 532–543. [DOI] [PubMed] [Google Scholar]
- Cahill, L. and McGaugh, J.L. 1998. Mechanisms of emotional arousal and lasting declarative memory. Trends Neurosci. 21: 294–299. [DOI] [PubMed] [Google Scholar]
- Convit, A., McHugh, P., Wolf, D.T., de Leon, M.J., Bobinski, M., De Santi, S., Roche, A., and Tsui, W. 1999. MRI volume of the amygdala: A reliable method allowing separation from the hippocampal formation. Psychiatry Res. 90: 113–123. [DOI] [PubMed] [Google Scholar]
- Dade, L.A., Zatorre, R.J., and Jones-Gotman, M. 2002. Olfactory learning: Convergent findings from lesion and brain imaging studies in humans. Brain 125: 86–101. [DOI] [PubMed] [Google Scholar]
- Dobbins, I.G., Kroll, N.E., Tulving, E., Knight, R.T., and Gazzaniga, M.S. 1998. Unilateral medial temporal lobe memory impairment: Type deficit, function deficit, or both? Neuropsychologia 36: 115–127. [DOI] [PubMed] [Google Scholar]
- Doty, R.L., Shaman, P., and Dann, M. 1984. Development of the University of Pennsylvania Smell Identification Test: A standardized microencapsulated test of olfactory function. Physiol. Behav. 32: 489–502. [DOI] [PubMed] [Google Scholar]
- Duvernoy, H.M. 1988. The human hippocampus: An atlas of applied anatomy. Springer-Verlag, New York.
- Eichenbaum, H. and Cohen, N.J. 2001. From conditioning to conscious recollection: Memory systems of the brain. Oxford University Press, Oxford, UK.
- Eichenbaum, H., Morton, T.H., Potter, H., and Corkin, S. 1983. Selective olfactory deficits in case H.M. Brain 106: 459–472. [DOI] [PubMed] [Google Scholar]
- Eskenazi, B., Cain, W.S., Novelly, R.A., and Friend, K.B. 1983. Olfactory functioning in temporal lobectomy patients. Neuropsychologia 21: 365–374. [DOI] [PubMed] [Google Scholar]
- Eskenazi, B., Cain, W.S., Novelly, R.A., and Mattson, R. 1986. Odor perception in temporal lobe epilepsy patients with and without temporal lobectomy. Neuropsychologia 24: 553–562. [DOI] [PubMed] [Google Scholar]
- Eslinger, P.J., Damasio, A.R., and Van Hoesen, G.W. 1982. Olfactory dysfunction in man: Anatomical and behavioral aspects. Brain Cogn. 1: 259–285. [DOI] [PubMed] [Google Scholar]
- Frank, R.J., Damasio, H., and Grabowski, T.J. 1997. Brainvox: An interactive, multimodal visualization and analysis system for neuroanatomical imaging. Neuroimage 5: 13–30. [DOI] [PubMed] [Google Scholar]
- Gottfried, J.A., Deichmann, R., Winston, J.S., and Dolan, R.J. 2002a. Functional heterogeneity in human olfactory cortex: An event-related functional magnetic resonance imaging study. J. Neurosci. 22: 10819–10828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottfried, J.A., O'Doherty, J., and Dolan, R.J. 2002b. Appetitive and aversive olfactory learning in humans studied using event-related functional magnetic resonance imaging. J. Neurosci. 22: 10829–10837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green, D.M. and Swets, J.A. 1989. Signal detection theory and psychophysics. Peninsula Publishing, Los Altos, CA.
- Henkin, R.I., Comiter, H., Fedio, P., and O'Doherty, D. 1977. Defects in taste and smell recognition following temporal lobectomy. Trans. Am. Neurol. Assoc. 102: 146–150. [PubMed] [Google Scholar]
- Herz, R.S., McCall, C., and Cahill, L. 1999. Hemispheric lateralization in the processing of odor pleasantness versus odor names. Chem. Senses 24: 691–695. [DOI] [PubMed] [Google Scholar]
- Hudry, J., Ryvlin, P., Royet, J.P., and Mauguiere, F. 2001. Odorants elicit evoked potentials in the human amygdala. Cereb. Cortex 11: 619–627. [DOI] [PubMed] [Google Scholar]
- Kilpatrick, L. and Cahill, L. 2003. Modulation of memory consolidation for olfactory learning by reversible inactivation of the basolateral amygdala. Behav. Neurosci. 117: 184–188. [DOI] [PubMed] [Google Scholar]
- Markowitsch, H.J., Calabrese, P., Wurker, M., Durwen, H.F., Kessler, J., Babinsky, R., Brechtelsbauer, D., Heuser, L., and Gehlen, W. 1994. The amygdala's contribution to memory—A study on two patients with Urbach-Wiethe disease. Neuroreport 5: 1349–1352. [PubMed] [Google Scholar]
- Martinez, B.A., Cain, W.S., de Wijk, R.A., Spencer, D.D., Novelly, R.A., and Sass, K.J. 1993. Olfactory functioning before and after temporal lobe resection for intractable seizures. Neuropsychology 7: 351–363. [Google Scholar]
- Nahm, F.K.D., Tranel, D., Damasio, H., and Damasio, A.R. 1993. Cross-modal associations and the human amygdala. Neuropsychologia 31: 727–744. [DOI] [PubMed] [Google Scholar]
- O'Doherty, J., Rolls, E.T., Francis, S., Bowtell, R., and McGlone, F. 2001. Representation of pleasant and aversive taste in the human brain. J. Neurophysiol. 85: 1315–1321. [DOI] [PubMed] [Google Scholar]
- Pitkänen, A. 2000. Connectivity of the rat amygdaloid complex. In The amygdala: A functional analysis (ed. J.P. Aggleton). Oxford University Press, Oxford, UK.
- Rausch, R. and Serafetinides, E.A. 1975. Specific alterations of olfactory function in humans with temporal lobe lesions. Nature 255: 557&ndash558. [DOI] [PubMed] [Google Scholar]
- Rausch, R., Serafetinides, E.A., and Crandall, P.H. 1977. Olfactory memory in patients with anterior temporal lobectomy. Cortex 13: 445–452. [DOI] [PubMed] [Google Scholar]
- Royet, J.P., Zald, D., Versace, R., Costes, N., Lavenne, F., Koenig, O., and Gervaisk, R. 2000. Emotional responses to pleasant and unpleasant olfactory, visual, and auditory stimuli: A positron emission tomography study. J. Neurosci. 20: 7752–7759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savic, I. 2001. Processing of odorous signals in humans. Brain Res. Bull. 54: 307–312. [DOI] [PubMed] [Google Scholar]
- Schoenbaum, G., Chiba, A.A., and Gallagher, M. 1999. Neural encoding in orbitofrontal cortex and basolateral amygdala during olfactory discrimination learning. J. Neurosci. 19: 1876–1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenbaum, G., Chiba, A.A., and Gallagher, M. 2000. Changes in functional connectivity in orbitofrontal cortex and basolateral amygdala during learning and reversal training. J. Neurosci. 20: 5179–5189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snodgrass, J.G. and Corwin, J. 1986. Pragmatics of measuring recognition memory: Applications to dementia and amnesia. J. Exper. Psychol.: General 117: 34–50. [DOI] [PubMed] [Google Scholar]
- Szabo, C.A., Xiong, J., Lancaster, J.L., Rainey, L., and Fox, P. 2001. Amygdalar and hippocampal volumetry in control participants: Differences regarding handedness. Am. J. Neuroradiol. 22: 1342–1345. [PMC free article] [PubMed] [Google Scholar]
- Tranel, D. and Hyman, B.T. 1990. Neuropsychological correlates of bilateral amygdala damage. Arch. Neurol. 47: 349–355. [DOI] [PubMed] [Google Scholar]
- Vanderploeg, R.D., Curtiss, G., Schinka, J.A., and Lanham Jr., R.A. 2001. Material-specific memory in traumatic brain injury: Differential effects during acquisition, recall, and retention. Neuropsychology 15: 174–184. [PubMed] [Google Scholar]
- West, S.E. and Doty, R.L. 1995. Influence of epilepsy and temporal lobe resection on olfactory function. Epilepsia 36: 531–542. [DOI] [PubMed] [Google Scholar]
- Zald, D.H. and Pardo, J.V. 1997. Emotion, olfaction, and the human amygdala: Amygdala activation during aversive olfactory stimulation. Proc. Natl. Acad. Sci. 94: 4119–4124. [DOI] [PMC free article] [PubMed] [Google Scholar]