Abstract
The metazoan parasitic blood flukes, Schistosoma spp., infect over 200 million people worldwide and cause extensive human morbidity and mortality. Research strategies for development of anti-schistosomal agents are impeded by the organism’s complex molluscan–mammalian life cycle, which limits experimental approaches and availability of material. We derived long-term continuously proliferative cultures of Schistosoma mansoni sporocysts capable of generating cercariae in vitro. Cultured organisms retained the ability to parasitize the host, and they exhibited developmental regulation of candidate stage-specific genes in the host-free culture system. Evidence for expression of a reverse transcriptase also was found in the cultured organisms, pointing to this activity as a possible mechanistic contributor to the dynamic relationship between the parasite and its hosts. Continuous in vitro propagation of the asexual sporocyst stage allows isolation of clonally derived parasite populations and provides a means to study schistosomal molecular genetics, metabolism, and evasion of host defenses.
Schistosoma mansoni, Schistosoma haematobium, and Schistosoma japonicum create widespread disease in tropical developing countries (1). These parasites thwart attempts to produce practical and effective vaccines, and pharmacological approaches are problematic (2). Schistosome eggs are laid by females paired with males and residing in the vasculature of parasitized vertebrate hosts. To be successfully infective, eggs must penetrate the intestinal wall and pass in feces to fresh water. From these released eggs hatch free-swimming miracidia, the first larval form. Successful miracidia encounter and penetrate the appropriate species of snail and develop into sporocysts, the second larval from. In the following weeks, these primary sporocysts asexually generate first-generation and second-generation daughter sporocysts in the snail. From the later-generation sporocysts develop the third larval form, free-swimming cercaria. To be successfully infective in the human or other vertebrate host, cercaria must encounter and penetrate the skin of the host and undergo further changes to form schistosomula. These move through several further developmental stages, ultimately leading to sexual pairing in the veins of the parasitized host and egg production.
Approaches to elucidate the mechanisms schistosomes employ to escape host immunity, as well as molecular genetic and pharmacological investigations, have been limited by the lack of an in vitro system for the continuous proliferative culture of the parasite in the absence of the host. Recently, Yoshino and colleagues (3, 4) have reported significant success in limited culture of the intramolluscan stages of S. mansoni and S. japonicum, but these techniques do not allow extended culture of proliferating sporocysts.
Here we describe long-term cultures of S. mansoni in vitro and the development from the snail-infective miracidial stage, through generations of mother and daughter sporocysts, to the human-infective cercarial stage. Our procedures exploit the Bge cell line (ATCC CRL 1494) (5, 6), established from cells of embryonic Biomphalaria glabrata, the molluscan host for S. mansoni. Sporocysts from long-term cultures successfully parasitized snails and spontaneously produced cercariae in vitro at low frequency.
The cultured sporocysts also expressed enzymatic reverse transcriptase activity, raising questions regarding the role of this polymerase in several biological phenomena in schistosomes, including transposition, host mimicry, dynamic genetic variability, and development of drug resistance. This culture system allows indefinite propagation of parasitic trematodes without the requirement of the complicated, multihost life cycle, and it provides a greatly simplified experimental system for the study of these organisms.
MATERIALS AND METHODS
Sporocyst Preparation.
S. mansoni, PR1 strain, has been maintained continuously by us since 1975. Since 1992, hamsters have served as the definitive host in place of Swiss Webster mice. The molluscan intermediate host was M-line Biomphalaria glabrata (7). For the initiation of in vitro cultures, miracidia were transformed into primary (mother) sporocysts (the first intramolluscan stage) by overnight culture in medium F (8) containing 20 μg/ml gentamicin and 1% bovine albumin. Shed ciliated plates were washed from the mother sporocysts.
Bge Cell Preparation.
Bge cells were cultured in a previously described medium formulation (5), with the addition of phenol red (0.2%) (3) as pH indicator. The cells were passaged by treating with 0.02% trypsin in Chernin’s balanced salt solution (9) for 5 min. In preparation for coculture with sporocysts, Bge cultures were seeded at ≈2.0 × 105 cells per well of a 24-well plate, or at ≈8.0 × 105 cells per T-25 flask at least 1 day before addition of sporocysts.
Coculture Preparation and Maintenance.
Cocultures were maintained at 26°C in sporocyst medium, a mixture of medium F, DME/F12 (Life Technologies), and Bge medium (1:1:2) with the addition of 5% Serum Plus (JRH Biosciences), 5% fetal bovine serum (HyClone), 0.001% 2-mercaptoethanol (Life Technologies), 0.05% chemically defined lipid concentrate (Life Technologies), and 20 μg/ml gentamicin. Both synxenic (mixed) and membrane-separated culture systems were successfully initiated. In the latter, 0.45-μm transparent membrane inserts (Becton Dickinson) maintained sporocysts above Bge cells in 1 ml of sporocyst medium.
Unless otherwise indicated, the cocultures were initiated with approximately 2 × 105 Bge cells and about 200 sporocysts per well in a 24-well plate. Media were replaced at intervals of 2–7 days. Six to eight weeks after initiation, established cocultures were passaged and seeded onto freshly harvested Bge cells, and then passaged approximately once each month.
Axenic Culture Preparation and Maintenance.
Axenic sporocyst cultures were held in hermetically closed plastic containers (CBS Scientific) in a nitrogen atmosphere maintained by blowing N2 gas into the chambers. Cultures were maintained in sporocyst medium, either fresh or conditioned 16–20 hr on exponentially growing Bge cells or on synxenic cocultures. Conditioned medium was passed through 0.22-μm filters (Gelman) and either used immediately or stored at 4°C for up to 1 week.
Determination of the Infective Potential of Sporocysts Grown in Vitro.
Naive Biomphalaria glabrata, M-line, were prepared for surgery by cleaning the shell with 70% ethanol, holding in a 2% aqueous antibiotic/antimycotic (Sigma) bath for 45 min, and again wiping with 70% ethanol. With sterile instruments, a hole was made in the shell overlying the digestive (mid-gut) gland. By using an elongated silane-treated Pasteur pipette, established cocultures containing about 3 sporocysts were deposited below the mantle tissue, avoiding puncture of the digestive gland. Petroleum jelly was wiped into the hole to reduce leakage of injected material or hemolymph. After 16–20 hr in sterile artificial spring water (10), surviving snails were placed in larger aquaria for long-term culture. The success of the infections was determined by counting shed cercariae 5–8 weeks later, as described (8).
Reverse Transcription–PCR (RT-PCR).
Total RNA was isolated by using the GlassMAX RNA Microisolation Spin Cartridge System (Life Technologies). The concentration of RNA in the samples was determined spectrophotometrically and by comparing the staining of samples after fractionation on denaturing agarose gels. One microgram of total RNA was treated with amplification grade DNase I (Life Technologies) before use as template. Reverse transcription, using 1 μg total RNA, was carried out with the Reverse Transcription System kit (A3500) from Promega.
PCR primers for the calcium-binding protein gene were 5′-GCTGGTTCTATTGTTCTG and 5′-CAAGGAGTTCATCAGTAT. The sequence spans a 91-bp intron of the calcium-binding protein gene; predicted RT-PCR product is 233 bp. Primers used for the protease were 5′-AGACCAATCGCACAAACA and 5′-TCAAATATTGGAGCGTAC. The sequence spans a 589-bp intron from the protease gene; predicted RT-PCR product is 451 bp. Details of gene sequences and the biological roles of these proteins have been published (11–16). One-tenth part of the reverse transcription mixture was used for PCR. The temperature cycling program employed 28 cycles with an annealing and extension time of 30 sec. Annealing temperature was 55°C. PCR products were analyzed on Tris/borate/EDTA polyacrylamide gels after staining with Syber-Green (Molecular Probes).
Enzyme Assays.
For assay of alkaline phosphatase, sporocyst samples were washed with Chernin’s balanced salt solution and fixed with 3% glutaraldehyde in the salt solution for 30 min at room temperature. Samples were then stained for endogenous alkaline phosphatase by using a detection kit from Sigma. For assay of reverse transcriptase, ≈10,000 washed sporocysts (3–4 days after initiation of culture) were extracted with 200 μl of lysis buffer (50 mM Tris⋅HCl/8 mM KCl/2.5 mM DTT/0.75 mM EDTA/0.05% Triton X-100, pH 7.8). The extract was centrifuged and the supernatant (40 μl) was assayed by using a reverse transcriptase assay kit from Boehringer Mannheim. The assay uses digoxigenin and biotin-labeled nucleotides and a sandwich ELISA that relates DNA synthesis by reverse transcriptase to a colorimetric peroxidase reaction. Results are reported as OD units at 405 nm corrected for OD in control wells treated identically except no digoxigenin-labeled nucleotides were added.
RESULTS
Continuously Proliferative in Vitro Cultures of S. mansoni.
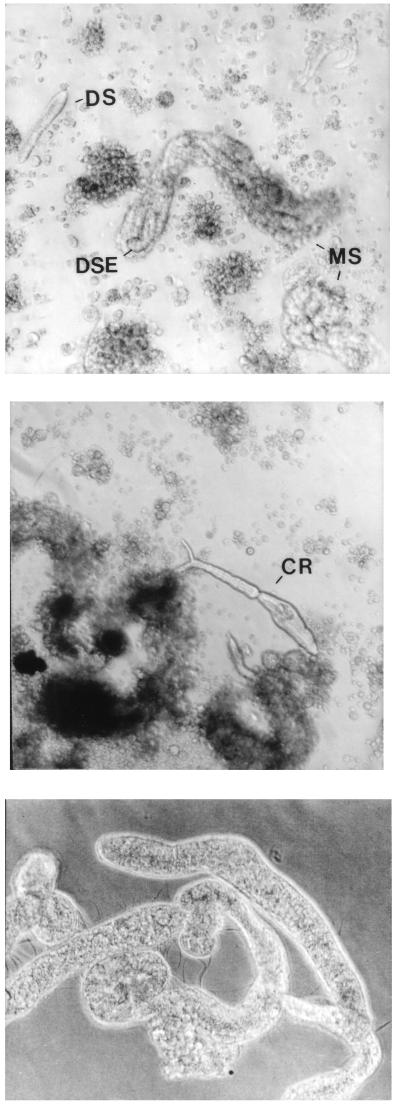
Sporocysts produced by transformation of miracidia obtained from parasitized rodents, when placed in culture with the Bge (Biomphalaria glabrata embryo) cell line (3, 5, 6), became completely covered with Bge cells within 7 days and were seen to contain daughter sporocysts after 14 days (Fig. 1). Sporocyst movements were somewhat limited due to the constraints of encapsulation by the adherent Bge cells. Second-generation (daughter) sporocysts appeared 2–5 weeks after initiation of these cocultures and were also encapsulated by Bge cells within 3 days after birth. Both mother and daughter sporocysts increased in length and width 2- to 3-fold in 1 week.
Figure 1.
Proliferating sporocysts and cercaria produced in vitro. (Top) Daughter sporocyst (DS) and developing daughter sporocyst embryos (DSE) inside a mother sporocyst (MS), cocultured with Bge cells. (Middle) Cercaria produced from proliferating daughter sporocyst culture maintained for 8 months in vitro. (Bottom) Sequestered sporocysts separated from Bge cells by a permeable membrane. Cultures were initiated by transformation of parasitized rodent-derived miracidia and maintained as described in the text. (×150.)
Sporocyst development was influenced by both Bge cell numbers and sporocyst numbers in the culture vessels (Table 1). These results were consistent with a previous report of dependence of larval growth and survival on both Bge and parasite density in vitro (4). Cocultures with less than 10 sporocysts usually did not embryonate. Cocultures with 10–40 sporocysts produced fewer than 20 daughter sporocysts but did not continue to propagate after a second generation. Cocultures with fewer than 50 sporocysts had increased gestation times up to 5 weeks. Cocultures with more than 50 sporocysts had a normal gestation time of about 3 weeks and continued as established coculture systems, yielding new daughter generations on a continuous basis. Gestation times were increased and number of daughter sporocysts were reduced if fewer than 5.0 × 105 Bge cells were seeded in each well. These sporocysts were not encapsulated by the Bge cells for 2 weeks, and daughter sporocysts did not appear until 35 days.
Table 1.
Proliferation of daughter sporocysts in vitro
| Culture condition | Time to appearance, weeks | Production in vitro |
|---|---|---|
| Bge, 105 cells/well | 3 | Continuous |
| Bge, 1.5 × 104 cells/well | 5 | Continuous |
| <10 sporocysts/well | — | 0 |
| 10–40 sporocysts/well | 5 | 20* |
| >40 sporocysts/well | 3 | Continuous |
| Without Bge | —† | 0 |
| Sequestered sporocysts‡ | 4 | Continuous |
| After in vitro–in vivo passage | 4 | Continuous |
Unless otherwise indicated, cultures were initiated at 105 Bge cells per well and >50 sporocysts per well. Cultures from in vitro–in vivo passage were derived from miracidia collected from a hamster previously infected with cercariae derived from injection of snails with sporocysts from established cultures. Embryogenesis was evident when embryos were seen moving within the bodies of their mothers. Times are approximate.
No daughter sporocysts.
Sporocysts survived 4 weeks.
Sporocysts separated from Bge cells by a permeable membrane.
It is difficult to determine how many daughter sporocysts arise from a given mother sporocyst because birth occurs repeatedly and daughter sporocysts cannot be definitively ascribed to a given mother sporocyst. Within a given well there is, on average, a 2- to 3-fold increase of the sporocyst mass per month. Cultures were initiated in February 1997, and subcultures diluted and passaged from this primary culture have continued to reproduce. More than 30 subsequent independently initiated primary cultures consistently yielded multigenerational daughter sporocysts.
Essential to indefinite proliferation was the addition of medium F (8) to the culture medium; if medium F was not included in the basal nutrient mix, cultures could be initiated and maintained for periods of time similar to those in previous reports (3, 4), but continuous sporocyst proliferation could not be maintained. The importance to indefinite proliferation in this system of other medium components (e.g., Bge medium, DME, lipid supplement) has not yet been systematically examined, but it might be expected that constituents of these and other medium components are necessary, but not sufficient, for continuous propagation of the cultures.
Elimination of direct interaction of Bge cells with sporocysts was achieved by culture of sporocysts on membranes over the cells. The tegument was well defined with numerous tegumental processes, indicative of vigorous good health. Subtegumental cells were bright and germinal balls were observed. The sporocysts in the membrane-separate cultures also formed out-pocketings. Daughter sporocysts developed throughout the body of the mother sporocysts, exiting through the tegument at numerous sites. Individual mother sporocysts gave rise to 10–20 daughter sporocysts in multiple births over 2 weeks. Developing embryos in the daughter sporocysts appeared within 1 week of birth, and a third sporocyst generation appeared approximately 1 week later. Birth cycles among mother and daughter sporocysts were not synchronized, and both mother sporocysts and daughter sporocysts in the cultures showed evidence of developing embryos over the same time period.
Proliferating cultures in the absence of Bge cells could be obtained if sporocysts were maintained at high density in Bge-conditioned medium and held under reduced oxygen (Fig. 1). Growth and development were similar to those of sporocysts grown with Bge cells, but daughter sporocyst proliferation was somewhat reduced. If conditioned medium was not added or reduced oxygen conditions were not maintained, sporocyst deterioration progressed over the first 14 days of culture, as evidenced by vacuolization, granularity, and tegument rupture.
Infective Potential of Sporocysts Grown in Vitro.
Snails were injected below the mantle tissue overlaying the digestive gland with sporocysts from established 6-month-old cocultures. Five to 8 weeks later, cercariae were obtained from approximately 10% of the injected snails and used to infect hamsters. This success rate is likely a reflection of the difficulty of the injection procedure, rather than an indication of the health of the sporocysts derived in vitro, because similar rates are obtained in direct snail-to-snail transfers of sporocysts. Eight weeks later, eggs were collected from the liver of an infected hamster, and miracidia were hatched, transformed in vitro to sporocysts, and cocultured with Bge cells. Subsequently long-term proliferating cocultures were derived (Table 1).
Whereas most individuals born in vitro were sporocysts, cercariae have appeared sporadically in more than 15 independent synxenic cultures of different ages (Fig. 1). The majority of these appeared normal and exhibited the classic light-stimulated twitch. Attempts to demonstrate infective potential of cercariae derived in vitro were unsuccessful, but these results are of questionable significance because of difficulty obtaining sufficient numbers of cultured cercariae; mice infected with similarly low numbers of snail-derived cercariae also did not develop infections. Cercariae survived in culture approximately 1 week. Attempts to induce controlled cercarial differentiation by addition of potential differentiation-inducing chemicals, host-derived additives, or environmental changes have been unsuccessful to date.
Developmental Regulation of Stage-Specific Genes in the Host-Free Culture System.
As an early marker of cercarial development, we examined a previously identified serine protease that is localized in cells of the acetabular gland regions, the first identifiable cercarial-specific cells appearing in the germinal areas of daughter sporocysts (11–15). This protease is a significant immune target of the parasitized host, and it has been suggested to aid the parasite in mammalian host invasion and protection from the host immune system (11, 14, 15).
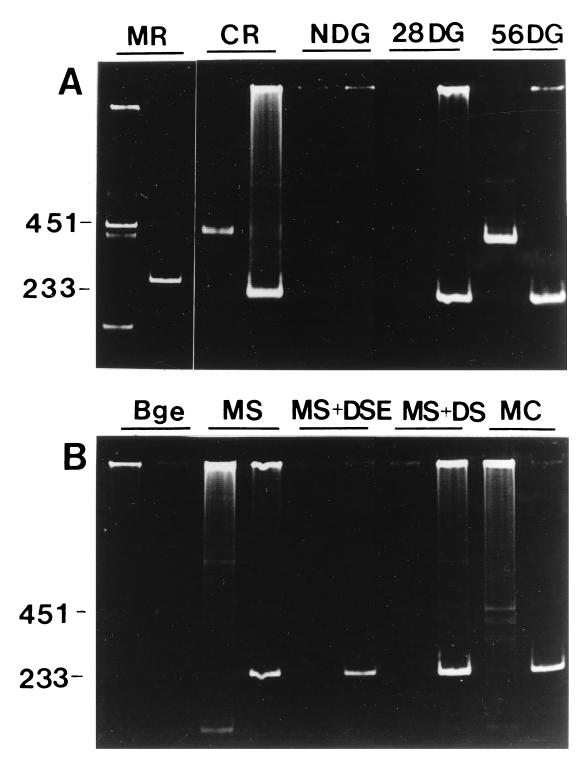
Using an RT-PCR assay for protease mRNA, we detected message in snail digestive glands 56 days after infection and in emergent cercariae. Assays to more precisely determine the time course of induction of protease mRNA expression relative to the beginning of cercarial shedding have not yet been performed. The mRNA was not detected in infected snail digestive glands 4 weeks after infection or in uninfected digestive gland (Fig. 2). Unexpectedly, protease mRNA was observed in freshly cultured miracidia; the signal declined sharply after 2 days in culture and did not reappear during the development of mother sporocyst and daughter sporocysts in vitro. In some advanced cultures, including cultures that produced mature cercariae, faint RT-PCR signals for protease mRNA were detectable.
Figure 2.
RT-PCR assay of mRNA expression of cercarial-marker serine protease and the calcium-binding protein gene. (A) MR, miracidia; CR, cercariae; NDG, non-infected digestive gland of snail; 28DG, digestive gland 28 days after infection, containing sporocysts; 56DG, digestive gland 56 days after infection, containing cercariae. For each sample, the left lane shows RT-PCR for protease, and the right lane shows RT-PCR for the calcium-binding protein gene. (B) Bge, established embryonic snail cell culture; MS, mother sporocysts after 2 days of culture (freshly transformed); MS+DSE, in vitro culture of mother sporocysts containing developing sporocyst embryos; MS+DS, culture containing mother sporocysts and emergent daughter sporocysts; MC, coculture of Bge and sporocysts in which cercarial differentiation had been detected.
In our hands, snails 4 weeks after infection were not sufficiently near patency to anticipate the presence of mature cercariae in the sporocysts, so our failure to detect protease mRNA at this stage of the infective cycle was expected. Variation among and within laboratories regarding the precise point at which cercariae appear in sporocysts of infected snails might be expected because of differences in substrain of snail or parasite, water temperature, nutritional state and overall health of the snails, as well as differences in the precise infection protocol.
RT-PCR was also employed to examine mRNA expression of a putatively cercarial-specific calcium-binding protein of unknown function (16). Surprisingly, mRNA for the calcium-binding protein gene was detected in miracidia, infected digestive glands, and snail-derived cercariae. The signal was not detected in material from noninfected snail digestive glands (Fig. 2). In vitro, the calcium-binding protein gene mRNA was detected in all cultures containing schistosome material, and not in Bge cells.
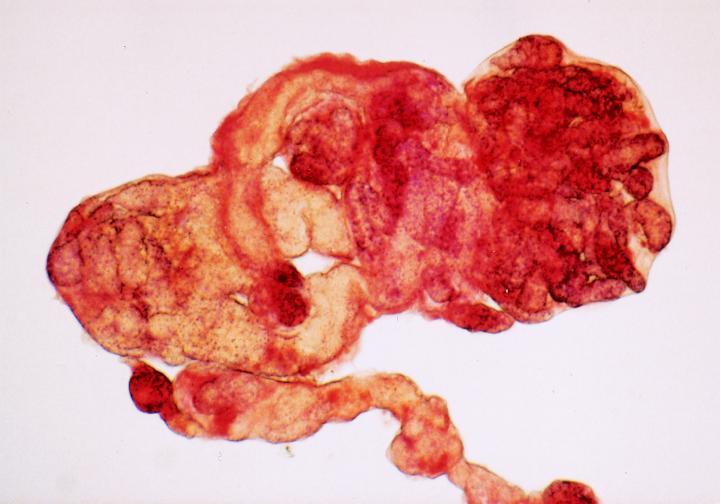
We also examined expression of alkaline phosphatase, a phosphatidylinositol-anchored surface membrane enzyme recognized as a marker of embryonal stem cells and primordial germ cells in vertebrates (17, 18). The protein is expressed in metabolically active stages of S. mansoni development and in germ cells of developing miracidia in vivo (19, 20). In vitro, the enzyme was strikingly localized to developing daughter sporocysts within the mother sporocyst (Fig. 3).
Figure 3.
Localization of alkaline phosphatase in developing daughter sporocysts. Sporocyst samples were washed with Chernin’s balanced salt solution and fixed with 3% glutaraldehyde in the salt solution for 30 min at room temperature. Samples were then stained for endogenous alkaline phosphatase by using a kit from Sigma. (×400.)
Reverse Transcriptase in S. mansoni Sporocysts.
During the course of development of the RT-PCR assays discussed above, we encountered unexpected amplification products that led us to speculate that sporocysts might express an active reverse transcriptase. Direct assay of reverse transcriptase confirmed this (Table 2). The activity from approximately 104 sporocysts was of the same order of magnitude as from 106 mouse embryo cells genetically altered as retroviral packing cells to express reverse transcriptase (84psiAM, ATTC CRR 8859). Experiments in which sporocyst extract was mixed with control retroviral reverse transcriptase showed a powerful inhibitory activity of the sporocyst extract on the control retroviral reverse transcriptase, suggesting that the reverse transcriptase activity detected in sporocyst extracts might be considerably higher if it could be assayed in the absence of the endogenous inhibitor (Table 2).
Table 2.
Reverse transcriptase (RT) activity in cultured sporocysts
| Exp. | Sample | RT activity, OD405 |
|---|---|---|
| A | Lysis buffer (no extract) | 0.065 |
| Sporocyst extract | 0.121 | |
| Viral RT control | 0.907 | |
| B | Lysis buffer (no extract) | 0.076 |
| RT control | 0.750 | |
| Viral RT control + sporocyst extract | 0.156 | |
| Extract from mouse cells expressing RT | 0.188 |
Experiments were carried out as described in the text. Positive reverse transcriptase control contained 1 ng of purified viral enzyme. Extract volume added to reverse transcriptase control in Exp. B was 20 μl. Mouse cells expressing reverse transcriptase were genetically altered retroviral packing cells (83psiAM, ATTC CLR 8859).
RT-PCR using primers derived from reverse transcriptase-related sequences identified in schistosome cDNA libraries (21, 22) identified an amplification product of the appropriate size when RNA from in vitro-cultured sporocysts and primers derived from schistosome cDNA sequences related to Caenorhabditis elegans reverse transcriptase cDNA were used (not shown). No signal was identified when these primers were used in RT-PCR with RNA from Bge cells.
DISCUSSION
Effective strategies for the development of drugs and vaccines against schistosomes require an understanding of the basic metabolic pathways of these metazoan parasites, and elucidating the mechanisms they employ to escape host immunity. However, the involvement of a large community of scientists in this endeavor has been impeded by the expense and expertise required to maintain the complex life cycle. The need to maintain colonies of host species has been circumvented with the protistan parasites Plasmodium (malaria) and Trypanosoma (sleeping sickness) by the development of in vitro culture systems. For most metazoan parasites, including helminths, continuous in vitro propagation has not been achieved.
Our success builds on earlier attempts by ourselves and others to develop various types of continuous cultures derived from Schistosoma spp. (3–6, 11–20, 23–27). Prior to this report, culture of intramolluscan stages were maximally successful over periods from 7 weeks (S. mansoni) to 5 months (S. japonicum) (3, 4). These investigations critically relied on the use of Bge cells in coculture and significantly extended the longevity and development of sporocysts in culture. Success reported here in continuous proliferation derives, at least partially, from identification of media more optimal for proliferation and implementation of methods to compensate for the high sensitivity of the cultures to oxidative damage.
Our work also addressed several enduring questions regarding trematode development. We observed daughter sporocysts produced from a single mother sporocyst over a period of several days, exiting by penetration of the maternal body wall. This observation supports the current model of multi-exiting cercariae as the means by which the infective form is freed from the sporocyst in vivo. In addition, the simplified environment under which continuous daughter sporocyst production occurred in vitro will allow addressing the issue of host-derived neuroendocrine involvement in parasite development. Although Bge-derived factors appear to be important for parasite development, the presence of a functional snail neuroendocrine system is not essential. In vitro culture studies with schistosome species parasitic to birds similarly indicate little, if any, neuroendocrine influence on sporocyst growth and development (28). The beneficial influence of Bge cells on schistosome development may be due to a classical peptide growth factor. Alternatively, Bge cells may release to the medium some critical metabolic intermediate(s) which the schistosome is incapable of synthesizing, or they may remove from the medium an inhibitory component.
It appears that the sporocysts grown in vitro are highly sensitive to oxidative stress; axenically grown sporocysts die unless held in hypobaric oxygen. Interestingly, it has been suggested that the means by which resistant individuals of the host snail kill sporocysts include the production of reactive oxygen species (29). Despite this sensitivity, sporocyst growth and embryogenesis could continue under normoxic conditions (air) when 2-mercaptoethanol and Bge cells were both present. We presume that mercaptoethanol is functioning in this system as a direct reducing agent, although other possibilities (e.g., metal chelation) exist. Bge cells also may promote growth by scavenging free radicals generated by means of oxygen; however, we have been unable to replace Bge cells with antioxidants such as vitamin E or vitamin C.
Using our in vitro system, we found both the schistosomal protease and calcium-binding protein genes were expressed at developmental stages not previously recognized, and we identified the vertebrate germinal cell marker alkaline phosphatase in developing sporocysts. Host influences on expression of the calcium-binding protein gene have been proposed (16), and our results showing expression patterns in host-free culture that are distinct from those found in parasites in intact hosts may support this idea.
Miracidial expression of the protease suggests that this enzyme may play roles in the intramolluscan phase of the life cycle similar to those proposed in the mammalian phase (11, 14, 15). The RT-PCR results suggest that the protease mRNA can be used as a marker for cercarial development in our cultures, and RT-PCR for the calcium-binding protein mRNA can be used to assess presence of the parasite or parasite cells in cocultures.
Our detection of reverse transcriptase activity in sporocysts is supported by identification of expressed sequence tags related to reverse transcriptase of other species that have been found in cDNA libraries derived from adult worm and egg stages of the schistosome life cycle (21, 22). Reverse transcriptase-related sequences are commonly found in genomes of higher organisms, possibly reflecting historical retroviral infections and/or transposon activity. However, these sequences seldom can be demonstrated to code for functional protein. Our identification of cell-derived, enzymatically functional reverse transcriptase in schistosome cultures points to a possible mechanism by which schistosomes might manipulate their genomes, including possibly incorporating into the parasite genome host-derived nucleic acid sequences.
S. mansoni, S. haematobium, and S. japonicum thwart attempts to produce practical and effective vaccines. The drug praziquantel constitutes the only functional therapy for infected individuals, and its use is not widespread because of expense, logistical impracticality, and emergence of parasite resistance, and because individual humans once cleared of the parasite are susceptible to reinfection. The recent work of Cousteau et al. (4) with S. japonicum, extending the earlier work of Yoshino and Laursen (3), suggests that it may be possible to adapt the approach we have described here to yield sporocysts (and eventually cercariae) of S. japonicum as well. The third important human schistosome (S. haematobium) parasitizes snails (Bulinus spp.) that are close relatives of Biomphilaria glabrata, so we expect that it also may thrive in coculture with Bge cells.
The in vitro system we describe will yield sporocysts in the absence of the molluscan host on a continuing basis. While the circumstances that optimally induce cercarial production remain to be determined, the continuous in vitro production of infective sporocysts will facilitate access to this human parasite by a greater number of investigators, expediting research on vaccine development, pharmacology, and the means to transfect this parasite with genes that will help elucidate its biology.
Acknowledgments
This paper is dedicated to Amber, who could have been a coauthor, but chose a different way. The work was supported by National Institutes of Health Grants RR12063 and AI16137 and by the United Nations Development Program/World Bank/World Health Organization Program for Research and Training in Tropical Diseases. Some schistosome life stages or materials for this work were supplied by Dr. F. A. Lewis through National Institute of Allergy and Infectious Diseases Contract NO1-A1-55270 to the Biomedical Research Institute, Rockville, MD.
ABBREVIATION
- RT-PCR
reverse transcription–PCR
References
- 1.Berquist N W, Colley D G. Parasitol Today. 1998;14:99–104. doi: 10.1016/s0169-4758(97)01207-6. [DOI] [PubMed] [Google Scholar]
- 2.Doenhoff M J. Parasitol Today. 1998;14:105–109. doi: 10.1016/s0169-4758(97)01204-0. [DOI] [PubMed] [Google Scholar]
- 3.Yoshino T P, Laursen J., Jr Parasitology. 1995;81:714–722. [PubMed] [Google Scholar]
- 4.Coustau C, Ataev G, Jourdane T, Yoshino T P. Exp Parasitol. 1997;87:77–87. doi: 10.1006/expr.1997.4184. [DOI] [PubMed] [Google Scholar]
- 5.Hansen E L. In: Invertebrate Tissue Culture. Maramorosch K, editor. New York: Academic; 1976. pp. 75–99. [Google Scholar]
- 6.Bayne C J. In: Invertebrate Tissue Culture. Maramorosch K, editor. New York: Academic; 1976. pp. 61–74. [Google Scholar]
- 7.Richards C S, Merritt J W., Jr Am J Trop Med Hyg. 1972;21:425–443. doi: 10.4269/ajtmh.1972.21.425. [DOI] [PubMed] [Google Scholar]
- 8.Stibbs H, Owczarzak A, Bayne C, DeWan P. J Invert Pathol. 1979;33:159–170. doi: 10.1016/0022-2011(79)90149-6. [DOI] [PubMed] [Google Scholar]
- 9.Chernin E. J Parasitol. 1963;49:353–364. [PubMed] [Google Scholar]
- 10.Ulmer M J. In: Experiments and Techniques in Parasitology. MacInnis A, Voge M, editors. New York: Freeman; 1970. pp. 143–144. [Google Scholar]
- 11.Fishelson Z, Amiri P, Friend D S, Marikovsky M, Petitt M, Newport G, McKerrow J H. Exp Parasitol. 1992;75:87–98. doi: 10.1016/0014-4894(92)90124-s. [DOI] [PubMed] [Google Scholar]
- 12.Ghendler Y, Parizade M, Arnon R, McKerrow J H, Fishelson Z. Exp Parasitol. 1996;83:73–82. doi: 10.1006/expr.1996.0051. [DOI] [PubMed] [Google Scholar]
- 13.Price H P, Doenhoff M J, Sayers J R. Parasitology. 1997;114:447–453. doi: 10.1017/s0031182096008657. [DOI] [PubMed] [Google Scholar]
- 14.Pierrot C, Godin C, Liu J L, Capron A, Khalife J. Parasitology. 1996;113:519–526. doi: 10.1017/s0031182000067561. [DOI] [PubMed] [Google Scholar]
- 15.Darani H, Curtis R, McNeice C, Price H, Sayers J, Doenhoff M J. Parasitology. 1997;115:237–247. doi: 10.1017/s0031182097001303. [DOI] [PubMed] [Google Scholar]
- 16.Ram D, Romano B, Schechter I. Parasitology. 1994;108:289–300. doi: 10.1017/s0031182000076137. [DOI] [PubMed] [Google Scholar]
- 17.Robertson E J, editor. Teratocarcinomas and Embryonic Stem Cells; A Practical Approach. Oxford: IRL; 1987. [Google Scholar]
- 18.Sun L, Bradford S, Ghosh C, Collodi P, Barnes D. J Marine Biotech. 1995;3:211–215. [PubMed] [Google Scholar]
- 19.Giboda M, Zdarska Z. Folia Parasitol. 1994;41:55–58. [PubMed] [Google Scholar]
- 20.Payares G, McLaren D, Evans W, Smithers S. Parasitology. 1985;91:93–99. doi: 10.1017/s0031182000056535. [DOI] [PubMed] [Google Scholar]
- 21.Franco G R, Adams M D, Soares M B, Simpson A J, Venter J C, Pena S D. Gene. 1995;152:141–147. doi: 10.1016/0378-1119(94)00747-g. [DOI] [PubMed] [Google Scholar]
- 22.Neto E D, Harrop R, Correa-Oliveira R, Wilson R A, Pena S D, Simpson A J. Gene. 1997;186:135–142. doi: 10.1016/s0378-1119(96)00699-3. [DOI] [PubMed] [Google Scholar]
- 23.Clemens L E, Basch P F. J Parasitol. 1989;75:417–442. [PubMed] [Google Scholar]
- 24.Weller T H, Wheeldon S K. Am J Trop Med Hyg. 1982;31:335–348. doi: 10.4269/ajtmh.1982.31.335. [DOI] [PubMed] [Google Scholar]
- 25.Hobbs D, Fryer S, Duimstra J, Hedstrom O, Brodie A, Menino J, Bayne C, Barnes D. J Parasitol. 1994;79:913–921. [PubMed] [Google Scholar]
- 26.Bayne C J, Menino J S, Hobbs D J, Barnes D W. J Parasitol. 1995;80:29–35. [PubMed] [Google Scholar]
- 27.Bayne C, Barnes D. Cytotechnology. 1997;23:205–210. doi: 10.1023/A:1007924022900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mellink J J, van den Bovenkamp W. Z Parasitenkd. 1985;71:337–351. doi: 10.1007/BF00928336. [DOI] [PubMed] [Google Scholar]
- 29.Dikkeboom R, van der Knaap W P, van den Bovenkamp W, Tijnagel J M, Bayne C J. Dev Comp Immunol. 1988;12:509–520. doi: 10.1016/0145-305x(88)90068-7. [DOI] [PubMed] [Google Scholar]