Abstract
This study reviews the role of the serotonin 5-HT2A receptor in learning as measured by the acquisition of the rabbit's classically conditioning nictitating membrane response, a component of the eyeblink response. Agonists at the 5-HT2A receptor including LSD (d-lysergic acid diethylamide) enhanced associative learning at doses that produce cognitive effects in humans. Some antagonists such as BOL (d-bromolysergic acid diethylamide), LY53,857, and ketanserin acted as neutral antagonists in that they had no effect on learning, whereas others (MDL11,939, ritanserin, and mianserin) acted as inverse agonists in that they retarded learning through an action at the 5-HT2A receptor. These results were placed in the context of what is known concerning the anatomical distribution and electrophysiological effects of 5-HT2A receptor activation in frontal cortex and hippocampus, as well as the role of cortical 5-HT2A receptors in schizophrenia. It was concluded that the 5-HT2A receptor demonstrates constitutive activity, and that variations in this activity can produce profound alterations in cognitive states.
The past few decades have brought an increasing awareness of serotonin's role in behavior. The development of drugs acting on the serotonergic system of brain that allow for the treatment of depression, anxiety, appetite regulation, and post-traumatic stress disorders has focused a great deal of attention on the role of serotonin in processes involving emotional states. Commensurate with our increasing understanding of the role of serotonin in behavioral processes has been the identification of at least seven serotonin (5-hydroxytryptamine; 5-HT) receptor sub-types. More recently, investigators have focused on the role of serotonin in cognitive functions, including learning and memory (Harvey 1996; Barnes and Sharp 1999; Meneses 1999, 2002; Williams et al. 2002) and in the deficits in attention and associative processes seen in schizophrenia (Meltzer 1999). Serotonin receptor subtypes that have been demonstrated to occur in brain regions capable of playing a role in learning and memory include the 5-HT1, 5-HT2, 5-HT3, 5-HT4, 5-HT6, and 5-HT7 class of receptors (Barnes and Sharp 1999; Meneses 2002). Table 1 summarizes studies that have examined the effects of serotonin agonists on learning. It can be seen that agonists at the 5-HT1A receptor subtype had either no effect (n = 2) or impaired learning (n = 3), whereas both 5-HT2A/2C and 5-HT4 receptor agonists primarily improved learning. The majority of serotonin antagonists are reported to have no effect on learning (Table 2). Thus, all of the 5-HT1A (n = 4) and 5-HT3 (n = 4) antagonists that have been examined were found to have no effect on learning. However, two of the six 5-HT2A/2C antagonists impaired learning, as did one of the two 5-HT4 antagonists.
Table 1.
Effect of Agonists at Serotonin Receptor (5-HT) Subtypes on Learning
| Drug | Receptor | Task | Species | Effect | Reference |
|---|---|---|---|---|---|
| Lisuride | 5-HT1A | EBR | Rabbit | No Effect | Welsh et al. (1998a) |
| 8-OH-DPAT | 5-HT1A | EBR | Rabbit | No Effect | Welsh et al. (1998a) |
| 8-OH-DPAT | 5-HT1A | CAR | Rat | Impaired | Meneses and Hong (1994) |
| Buspirone | 5-HT1A | CAR | Rat | Impaired | Alhaider et al. (1993) |
| Tandospirone | 5-HT1A | VM | Human | Impaired | Yasuno et al. (2003) |
| TFMPP | 5-HT2A/2C | CAR | Rat | Enhanced | Alhaider et al. (1993) |
| Quipazine | 5-HT2A/2C | CAR | Rat | Enhanced | Alhaider et al. (1993) |
| RS67333 | 5-HT4 | SPL | Rat | No Effect | Fontana et al. (1997) |
| RS67333 | 5-HT4 | OD | Rat | Enhanced | Marchetti et al. (2000) |
| RS17017 | 5-HT4 | MTS | Macaque | Enhanced | Terry et al. (1998) |
| SL65.0155 | 5-HT4 | ORT | Rat | Enhanced | Moser et al. (2002) |
(ACC) Aversive classical conditioning; (ASL) autoshaping learning; (CAR) conditioned avoidance response; (EBR) associative conditioning of the eyeblink response; (MTS) matching to sample; (OD) olfactory discrimination; (ORT) object recognition task; (PWL) perceptual word list; (SPL) spatial learning; (VD) visual discrimination; (VM) verbal memory; (VSL) visuo-spatial learning; (8-OH-DPAT) 8-hydroxy-2-[di-n-propylamino]tetralin; (TFMPP) 1-[m-trifluoromethylphenyl]piperazine.
Table 2.
Effect of Serotonin Receptor Antagonists on Learning
| Drug | Receptor | Task | Species | Effect | References |
|---|---|---|---|---|---|
| WAY100135 | 5-HT1A | SPL | Rat | No Effect | Carli et al. (1995) |
| WAY100635 | 5-HT1A | VSL | Marmoset | No Effect | Harder et al. (1996) |
| WAY100635 | 5-HT1A | SPL | Rat | No Effect | Carli et al. (1997a) |
| Spiperone | 5-HT1A/2A | CAR | Rat | No Effect | Alhaider et al. (1993) |
| Pindolol | 5-HT1A/1B | CAR | Rat | No Effect | Alhaider et al. (1993) |
| Ketanserin | 5-HT2A | CAR | Rat | No Effect | Alhaider et al. (1993) |
| Mianserin | 5-HT2A/2C | CAR | Rat | No Effect | Alhaider et al. (1993) |
| Cinanserin | 5-HT2A/2C | VD | Rat | No Effect | Kobayashi et al. (1995) |
| Metergoline | 5-HT2A/2C | PWL | Human | No Effect | Vitiello et al. (1997) |
| Cyproheptadine | 5-HT2A/2C | CAR | Rat | Impaired | Ma and Yu (1993) |
| Cyproheptadine | 5-HT2A/2C | CAR | Rat | Impaired | Titov et al. (1983) |
| Ritanserin | 5-HT2A/2C | ACC | Human | Impaired | Hensman et al. (1991) |
| MDL72,222 | 5-HT3 | CAR | Rat | No Effect | Alhaider et al. (1993) |
| WAY100289 | 5-HT3 | SPL | Rat | No Effect | Hodges et al. (1995) |
| Ondansetron | 5-HT3 | ORT | Marmoset | No Effect | Barnes et al. (1990) |
| Ondansetron | 5-HT3 | SPL | Rat | No Effect | Carli et al. (1997b) |
| DAU6215 | 5-HT3 | SPL | Rat | No Effect | Pitsikas et al. (1994) |
| SDZ205557 | 5-HT4 | ORT | Rat | No Effect | Moser et al. (2002) |
| RS67532 | 5-HT4 | OD | Rat | Impaired | Marchetti et al. (2000) |
See Table 1 for abbreviations.
Although the data cited above indicate that activation of serotonin receptors or their blockade can produce alterations in learning, these data do not provide the consistent outcomes that would allow for definite conclusions concerning the precise role of the various receptor subtypes in learning. However, this is not surprising, as different behavioral paradigms invoke different forms of learning that are mediated by differently distributed neuronal networks. In this regard, it is important to note that, even within a particular paradigm, alterations in the precise manner by which stimuli are presented can produce systematic differences in the brain regions undergoing learning-dependent changes. For example, it is well known that contextual fear conditioning requires hippocampal mediation, whereas signaled fear conditioning does not (Phillips and LeDoux 1994). Consequently, activation of serotonin receptors in different brain regions would be expected to have different effects, depending on the behavioral paradigm used, as well as on the precise role of the different classes of serotonin receptors in mediating activity within particular neuronal networks. On the basis of these considerations, our laboratory chose to use a single behavioral paradigm (classical eyeblink conditioning) and to restrict our studies to an examination of the role of the serotonin 5-HT2A receptor in learning. This chapter details the results of studies providing evidence that the serotonin 5-HT2A receptor demonstrates intrinsic activity that determines the rate of associative learning.
Serotonin 5-HT2A receptors in frontal cortex and hippocampus modulate local circuitry. In contrast to the limited experimental data on other serotonin receptor subtypes, a great deal of systematic data has been collected to indicate an important role for 5-HT2A receptors in modulating neuronal circuitry in medial prefrontal cortex and hippocampus. Both of these brain areas are known to be involved importantly in associative learning across a number of species and learning paradigms, including the classically conditioned eyeblink response in rabbits (Buchanan and Powell 1982; Port et al. 1985; Solomon et al. 1986; Kronforst-Collins and Disterhoft 1998; Weible et al. 2000) and humans (Clark and Squire 1998). The 5-HT2A receptors are located in both the medial prefrontal cortex and hippocampus of the rat (Pazos et al. 1985), rabbit (Aloyo and Harvey 2000), primate (Jakab and Goldman-Rakic 1998), and human (Hoyer et al. 1986; López-Giménez, et al. 1998; Barnes and Sharp 1999). The 5-HT2A receptors found in the rabbit frontal cortex are pharmacologically similar to human receptors (Aloyo and Harvey 2000). Electrophysiological studies in the rat (Sheldon and Aghajanian 1991; Marek and Aghajanian 1994) and primate (Williams et al. 2002) indicate that 5-HT2A receptors can modulate cortical neuronal excitability, and thus, may be expected to play an important role in modulating learning. The 5-HT2A receptors have been shown to be located on both the dendrites of cortical pyramidal cells as well as on interneurons (Jakab and Goldman-Rakic 1998), and mediate excitation in both neuronal types (Tanaka and North 1993; Aghajanian and Marek 1997). Thus, activation of 5-HT2A receptors in cortex can produce both a direct excitation and a feed-forward inhibition of cortical pyramidal cells. In addition, the location of 5-HT2A receptors in cortex and hippocampus on cholinergic (Quirion et al. 1985) and glutamatergic (Aghajanian and Marek 2000; Lambe et al. 2000; Hasuo et al. 2002) axon terminals can serve to regulate the release of these transmitters. On the basis of studies that have used intrahippocampal injections of drugs that alter glutamatergic and cholinergic transmission during trace conditioning of the rabbit's eyeblink response (Thompson et al. 1992; Weiss et al. 2000), the increased release of acetylcholine and glutamate in hippocampus would also be expected to enhance learning. Thus, activation of 5-HT2A receptor would be expected to increase learning through post-synaptic actions on cortical pyramidal cells (Williams et al. 2002) as well as through heteroceptors located on presynaptic terminals of cortical cholinergic and glutamatergic neurons.
Classical Conditioning of the Rabbit's Nictitating Membrane Response: A Model of Associative Learning
Classical conditioning of the rabbit's nictitating membrane response (a component of the eyeblink) was chosen for our studies because it has been acknowledged to provide a reliable measure of associative learning and to exhibit all of the associative processes observed in humans (Gormezano et al. 1983). Consequently, it has been used widely to model a variety of clinical states characterized by deficits in associative processes, including schizophrenia (Sears et al. 2000), Alzheimer's dementia (Woodruff-Pak 2001), autism, and obsessive-compulsive disorder (Steinmetz et al. 2001). Eyeblink conditioning has also provided an excellent model for examining the neuronal circuitry underlying associative learning (e.g., Thompson 1986; Harvey and Welsh 1996; Steinmetz 2000), the receptor systems that play a major role in the acquisition and retention of associative learning (e.g., Harvey 1987, 1996; Schindler and Harvey 1990; Romano and Harvey 1992), and the molecular cascade that is responsible for the alterations in synaptic efficacy that are required for the permanent storage of learned associations (Geinisman et al. 2001; Zhen et al. 2001). The wide use of this behavioral paradigm is also due to the fact that alterations in the acquisition of conditioned responses (CRs) can be further analyzed in terms of alterations in nonassociative processes such as sensitization and pseudoconditioning, as well as alterations in performance factors.
Classical eyeblink conditioning can be carried out by use of either delay or trace-conditioning procedures. In delay conditioning, the conditioned stimulus (CS) is presented for a given period of time and then its offset occurs simultaneously with onset of the unconditioned stimulus (US). The time between CS onset and US onset is referred to as the CS–US interval. One can use either short-delay procedures (e.g., a 200-msec CS–US interval) or long-delay intervals (e.g., 800–1500-msec CS–US intervals). In trace conditioning, the CS is presented for a fixed period of time (e.g., 100 msec). Offset of the CS is followed by a trace period (e.g., 500 msec), during which time no stimuli are presented. The US is then presented at the end of the trace period. Of special relevance for the studies described here, medial prefrontal cortex and hippocampus areas having a high density of 5-HT2A receptors are critically involved in acquisition of the rabbit's eyeblink response during trace-conditioning procedures (Solomon et al. 1986; Gibbs and Powell 1991; McEchron and Disterhoft 1997; Kronforst-Collins and Disterhoft 1998; Romano 1999; Beylin et al. 2001) or during delay eyeblink conditioning using long CS–US intervals (Beylin et al. 2001). These brain regions are also important for trace eyeblink conditioning in the human (Clark and Squire 1998; LaBar and Disterhoft 1998). For these reasons, we chose to use this behavioral paradigm to explore the role of serotonin 5-HT2A receptors in associative learning, and the results of these studies are summarized below.
Serotonin 5-HT2A Receptor Agonists Enhance Associative Learning
Agonists at the 5-HT2A receptor such as d-lysergic acid diethylamide (LSD), 2,5-dimethoxy-4-methylamphetamine (DOM), methylenedioxyamphetamine (MDA), and methylenedioxymethamphetamine (MDMA) enhance acquisition of the rabbit's eyeblink response (Table 3). The enhancement of associative learning produced by these agonists was most likely due to increased activity at the 5-HT2A receptor in frontal cortex and hippocampus, because, as noted above, those brain regions have been demonstrated to mediate eyeblink conditioning in rabbits and humans. These findings are complementary with experiments in monkeys that have indicated that activation of 5-HT2A receptors facilitates mnemonic processes occurring in prefrontal pyramidal cells that participate in spatial working memory (Williams et al. 2002). More importantly, the enhancement of eyeblink conditioning produced by 5-HT2A receptor agonists occurred in the range of doses that are known to produce alterations in associative and other cognitive processes in humans (see Table 4). Consistent with our findings, it has been reported that the 5-HT2A/2C receptor agonists quipazine and TFMPP (1-[m-trifluoromethylphenyl]piperazine) enhanced acquisition of the conditioned avoidance response in the rat (see Table 1; Alhaider et al. 1993). However, it is not clear whether the enhancement of learning produced by 5-HT2A agonists in our studies might not also be produced by agonists acting at other serotonin receptor subtypes. So far, only the 5-HT1A receptor agonists 8-OH-DPAT and lisuride have been examined, and they had no effect on acquisition of the eyeblink response (see Table 1; Welsh et al. 1998a). However, investigators using other measures of learning have reported that 5-HT1A receptor agonists impair learning. Thus, as noted in Table 1, 8-OH-DPAT (Meneses and Hong 1994) and buspirone (Alhaider et al. 1993) retarded acquisition of the conditioned avoidance response in the rat and tandospirone retarded acquisition of a verbal memory task in humans (Yasuno et al. 2003). As mentioned in the introduction, it will be important in future research to determine how activation of particular serotonin receptors produces differential effects depending on the learning task used.
Table 3.
Effects of 5-HT2 Agonists and Antagonists on Associative Learning in the Rabbit as Measured by the Classically Conditioned Nictitating Membrane Response, a Component of the Eyeblink
|
Serotonin receptor agonists that enhanced associative learning
|
||||||
| Drug | 5-HT receptor | Effective doses (μmole/kg) | Reference | |||
| LSD | 2A/2C | 0.001-0.100, i.v. | Gimpl et al. (1979) | |||
| LSD | 2A/2C | 0.080-0.197, i.v. | Siegel and Freedman (1988) | |||
| DOM | 2A/2C | 0.3-3.0, i.v. | Harvey et al. (1982) | |||
| MDA | 2A/2C | 1.0-10, s.c. | Romano et al. (1991) | |||
| MDMA | 2A/2C | 4.1-16.5, s.c. | Romano and Harvey (1994) | |||
| Serotonin receptor antagonists with no effect on learning | ||||||
| Drug | 5-HT receptor | Dose range (μmole/kg) | Reference | |||
| BOL | 2A/2C | 0.003-0.30, i.v. | Harvey et al. (1982) | |||
| BOL | 2A/2C | 0.06-5.8, s.c. | Romano et al. (2000) | |||
| LY53,857 | 2A/2C | 0.067-6.7, s.c. | Welsh et al. (1998a) | |||
| Ketanserin | 2A | 1.0-10, s.c. | Harvey et al. (1999) | |||
| Serotonin receptor antagonists that impair associative learning | ||||||
| Drug | 5-HT receptor | Effective doses (μmole/kg) | Reference | |||
| Ritanserin | 2A/2C | 0.067-6.7, s.c. | Welsh et al. (1998a,b) | |||
| Mianserin | 2A/2C | 0.1-10.0, s.c. | Romano et al. (1991) | |||
| MDL11,939 | 2A | 0.067-6.7, s.c. | Welsh et al. (1998a,b) | |||
| Pizotifen | 2A/2C | 8.4-33.8, s.c. | Ginn and Powell (1986) | |||
(LSD) d-lysergic acid diethylamide; (DOM) 2,5-dimethoxy-4-methylamphetamine; (MDA) methylenedioxyamphetamine; (MDMA) methylenedioxymethamphetamine; (BOL) d-bromolysergic acid diethylamide.
Table 4.
Comparison of the Efficacy of 5-HT2 Agonists on CR Acquisition and Cognitive Effects in Humans
| Drug | Rabbit ED 50a(μg/kg) | Human doseb(μg/kg) |
|---|---|---|
| LSD | 0.8c | 1.0h |
| DOM | 52d | 50i |
| MDMA | 500e | 250j |
| MDA | 800f | 1000k |
| BOL | No effectd,g | No effecth |
See Table 3 for abbreviations.
Dose of drug required to produce a half maximal enhancement of learning.
Lowest dose required to obtain reliable hallucinations in humans.
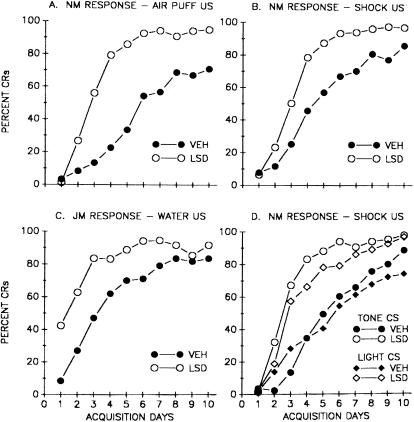
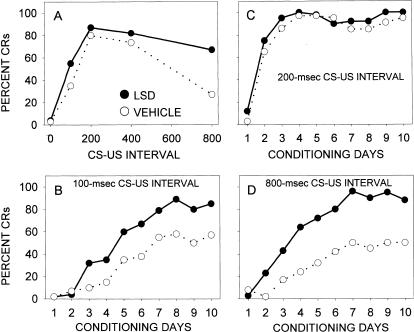
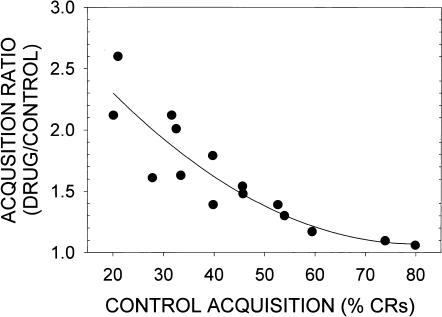
As shown in Figure 1, the increases in learning produced by the prototypic agonist LSD was not dependent on the modality of the CS and US or on the use of defensive eyeblink conditioning. Thus, LSD increased learning when a tone CS and air puff US was used (Fig. 1A), when a tone CS and shock US was used (Fig. 1B), when a classically conditioned appetitive jaw movement response was employed using a tone CS and water as the US (Fig. 1C), and when tone or light CSs were paired with a shock US (Fig. 1D). However, the effects of LSD on learning were critically dependent on the CS–US interval used. It is well known that the rate of acquisition of CRs during eyeblink conditioning is a function of the CS–US interval (Gormezano et al. 1983). As shown in Figure 2A, control animals characteristically demonstrate low rates of CR acquisition at short CS–US intervals, for example, 100 msec, maximum rates of acquisition at CS–US intervals of 200–400 msec, followed by low rates of acquisition at longer CS–US intervals. LSD significantly enhanced CR acquisition at short (100 msec) and long (800 msec) CS–US intervals, but failed to do so at the optimum intervals of 200 and 400 msec (Fig. 2A; Harvey et al. 1988). This is further illustrated by acquisition curves in Figure 2, B, C, and D. At the 200-msec CS–US interval, both LSD-injected and vehicle control groups acquired CRs rapidly and at the same rate (Fig. 2C). In contrast, LSD produced a robust enhancement of CR acquisition at CS–US intervals of 100 msec (Fig. 2B) or 800 msec (Fig. 2D) that generated low rates of acquisition in vehicle controls. In other studies, enhancement of eyeblink CRs was seen during trace-conditioning procedures using CS–US intervals >1000 msec (Siegel and Freedman 1988). The factors that determine the ability of LSD to enhance learning may not be due to the CS–US interval being used as such, but rather to the rate of learning generated at different CS–US intervals. Thus, on the basis of a number of experiments, it was found that LSD failed to enhance learning during procedures that generated high rates of CR acquisition in controls, regardless of the CS–US interval used, but did enhance learning during procedures that generated lower rates of CR acquisition in controls (Fig. 3). Because rate of learning is an index of task difficulty, it is possible that activation of the 5-HT2A receptor had a proportionately greater effect as increases in task difficulty placed greater demands on attentional and associative processes.
Figure 1.
Enhancement of associative learning produced by the 5-HT2A agonist LSD under different experimental conditions. The ordinate presents the mean percent conditioned responses (CRs) during each of 10 conditioning days derived from a minimum of 10 animals per group. (A,B,C,D) The dose of LSD was 13 μg/kg (0.030 μmole/kg) administered intravenously into the marginal ear vein of the rabbit. Conditioning of the NM response was based on a delay procedure using an 800-msec CS, whose offset occurred simultaneously with the onset of a 100-msec unconditioned stimulus (US). (A) Acquisition of the conditioned nictitating membrane (NM) response (a component of the eyeblink) during tone CS and corneal airpuff US conditioning (Schindler et al. 1985a); (B) acquisition of the NM response during tone CS and paraorbital shock US conditioning (Gimpl et al. 1979); (C) Acquisition of the appetitive jaw movement (JM) response during pairings of tone CS and water US in water-deprived rabbits. Jaw movements are produced during the ingestion of water, and during conditioning, these movements begin to be initiated to the tone CS prior to water presentation (Gormezano et al. 1980); (D) acquisition of the NM response to tone or light CSs paired with a shock US. The intensity of the tone CS had been adjusted so as to support the same rate of conditioning as the light CS (Schindler et al. 1985b).
Figure 2.
Enhancement of associative learning produced by the 5-HT2A agonist LSD (13 μg/kg) at different CS–US intervals. Acquisition of the NM response during the pairing of a tone CS and paraorbital shock US. A trace procedure was employed using a 100-msec tone and shock US. Thus, the 0-msec CS–US interval indicates the simultaneous presentation of the stimuli and the 100 msec CS–US interval represents a delay procedure (0 trace). The 200-, 400-, and 800-msec CS–US intervals represent trace conditioning of 100, 300, and 500 msec, respectively. Data are taken from Harvey et al. (1988).
Figure 3.
The relationship between the mean percentage of CRs generated by various NM conditioning procedures in control animals (abscissa) and the degree of enhancement of learning produced by LSD (13 μg/kg). The effects of LSD are expressed as an acquisition ratio, calculated as the mean percentage of CRs for animals injected with LSD divided by the mean percentage of CRs for their respective controls. Data were taken from Gimpl et al. (1979), Harvey et al. (1982, 1988), Schindler et al. (1985a,b), and Welsh et al. (1998a,b).
Control experiments using the explicitly unpaired presentations of CS and US indicated that the enhancement of CR acquisition produced by LSD, DOM, MDA, and MDMA was due to an enhancement of associative learning (Harvey et al. 1982; Romano et al. 1991; Romano and Harvey 1994). For example, baseline responding in control animals was low (1%–3%), as was responding to the unpaired tone stimulus (2%–8%). LSD had no effect on these measures of nonassociative responding during either defensive eyeblink or appetitive jaw movement conditioning procedures. The amphetamine derivatives DOM, MDA, and MDMA produced only small (4%) increases in base-line responding. MDA and MDMA also did not increase responding to the CS. However, DOM did produce a small, but significant, increase in responding to the CS, the mean values being 1.6% for controls and 11% for animals injected with the highest dose of DOM (3 μmole/kg). Lower doses of DOM had no effect on responding to the CS during the unpaired procedures, although these doses (0.3 and 1.0 μmole/kg) did enhance the rate of learning. Thus, the enhancement of CR acquisition produced by the 5-HT2 agonists listed in Table 3 represented an increase in associative learning and not to such nonassociative factors as sensitization, pseudoconditioning, or increases in base-line rates of responding.
Effect of Serotonin 5-HT2A Antagonists on Associative Learning
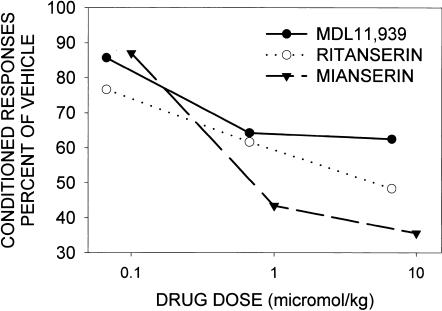
As indicated in Table 3, some antagonists at the 5-HT2A receptor, for example, d-bromolysergic acid diethylamide (BOL), LY53,857 and ketanserin, had no significant effect on associative learning and could, therefore, be classified as neutral antagonists. Surprisingly, some antagonists were not neutral, rather, they retarded learning in the rabbit (Table 3). As shown in Figure 4, MDL11,939, a highly selective 5-HT2A antagonist, as well as the nonselective 5-HT2A/2C antagonists ritanserin and mianserin produced a dose-dependent reduction in CR acquisition (Welsh et al. 1998a,b; Romano et al. 2000). In a separate study, the 5-HT2A/2C antagonist pizotifen was also found to retard the acquisition of the rabbit's eyeblink response (Table 3; Ginn and Powell 1986). Explicitly unpaired CS–US procedures indicated that the retardation of CR acquisition produced by these drugs was due to a decrease in associative learning (Welsh et al. 1998b). In agreement with results in the rabbit, ritanserin also retarded classical aversive conditioning in humans (Table 2; Hensman et al. 1991). Another nonselective 5-HT2A/2C antagonist cyproheptadine impaired acquisition of the conditioned avoidance response in the rat (Table 2; Titov et al. 1983; Ma and Yu 1993). Also in agreement with our results, ketanserin was reported to have no effect on acquisition of the conditioned avoidance response in the rat; however, in contrast to our findings, these investigators also reported no effect of mianserin (see Table 2; Alhaider et al. 1993). Again, it is not yet clear how differences in outcome may be related to the learning task used or other factors.
Figure 4.
Retardant effect of MDL11,939, ritanserin, and mianserin on acquisition of NM response as a function of dose. Drugs were injected subcutaneously 1 h prior to each conditioning session. Conditioning used the pairing of a tone CS with an air puff US. Drug effects are expressed as the percentage of vehicle controls. Data are taken from Welsh et al. (1998b) and Romano et al. (2000).
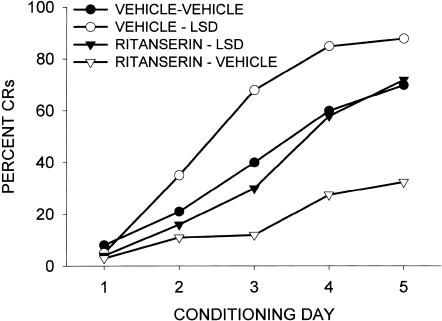
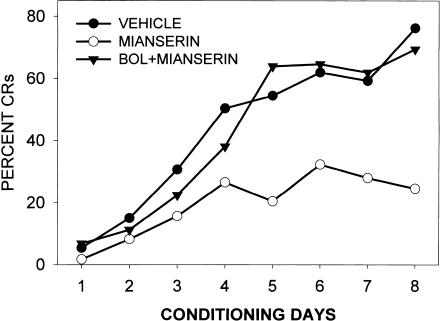
A number of control experiments were carried out to determine the basis for the retardation of learning produced by MDL11,939, ritanserin, and mianserin in the rabbit. It was possible that these drugs were not retarding learning through an action at the 5-HT2A receptor, but rather through actions at other serotonergic or nonserotonergic receptors. First, we examined whether ritanserin was acting as an antagonist at the serotonin 5-HT2A receptor by examining its ability to block the effects of the 5-HT2A receptor agonist LSD. Figure 5 illustrates the fact that a dose of ritanserin producing a retardation of CR acquisition was also able to block the enhancement of CRs produced by LSD. However, whereas these results could be due to the actions of ritanserin at the 5-HT2A receptor (a true pharmacological antagonism), they may still have been due to a physiological antagonism mediated through actions at other receptors. Therefore, we examined whether the neutral 5-HT2A receptor antagonist BOL could block the retardation of CR acquisition produced by mianserin. As is illustrated in Figure 6, the large retardation of learning produced by mianserin was completely reversed by the neutral antagonist BOL. Thus, the retardation of associative learning produced by mianserin was due to an action at the 5-HT2A receptor.
Figure 5.
Antagonism by ritanserin (1 μmole/kg) of the enhancement of CR acquisition produced by LSD (0.030 μmole/kg). Ritanserin was injected subcutaneously 60 min prior and LSD intravenously 20 min prior to each acquisition session. Acquisition of the NM response was measured during the pairing of a tone CS and air puff US. Data are taken from Welsh et al. (1998a).
Figure 6.
Antagonism by BOL (5.8 μmole/kg) of the retardant effects of mianserin (10 μmole/kg) on acquisition of the NM response. Mianserin was injected 1 h and BOL 20 min prior to each conditioning session by use of the pairing of a tone CS and air puff US. All injections were subcutaneous. Data are taken from Romano et al. (2000).
The ability of the antagonists MDL11,939, ritanserin, and mianserin to retard learning, to block the effects of agonists, and to have their own effects blocked by neutral antagonists, meets the criterion for establishing them as inverse agonists (Kenakin 1996; Strange 2002). Our identification of drugs as agonists, neutral antagonists, and inverse agonists on the basis of their effects on associative learning is supported by in vitro studies of 5-HT2A and 5-HT2C receptors in transfected cell lines. In such systems, LSD increases PI hydrolysis, an effect that is blocked by antagonists. Moreover, the antagonists fall into two classes. For example, as in the in vivo studies, mianserin decreased PI hydrolysis, indicating that it was an inverse agonist, whereas BOL was identified as a neutral antagonist, in that it had no effect on basal PI hydrolysis by itself, but blocked both the effects of the agonist LSD and the inverse agonist mianserin (Barker et al. 1994; Westphal and Sanders-Bush 1994; Egan et al. 1998). Similar studies have not been possible in native tissue due to the low levels of constitutive activity. However, the effects of 5-HT2A agonists, neutral antagonists, and inverse agonists on associative learning not only provide the first in vivo demonstration of inverse agonism at the 5-HT2A receptor, but also suggest that there is a functionally high level of constitutive (intrinsic) activity at that receptor that regulates the rate of associative learning.
Implications of Inverse Agonism for Associative Learning and Psychopathology
The existence of constitutive activity at a receptor not only allows for the occurrence of both agonist and inverse agonist actions of drugs, but also raises the possibility that alterations in constitutive activity produced by genetic and/or environmental events could have profound effects on behavior. There are recent data indicating that there is a decrease in 5-HT2A receptor density (Aurora and Meltzer 1991) and in 5-HT2A receptor mRNA expression in frontal cortex of schizophrenic patients (Burnet et al. 1996; Hernandez and Sokolov 2000), and that neuroleptic treatment elevates the level of 5-HT2A mRNA in schizophrenics to or above that of controls (Hernandez and Sokolov 2000). Additional studies have led to the conclusion that the antipsychotic actions of most drugs are due to their inverse agonist actions at serotonin 5-HT2A receptors (Meltzer 1999; Weiner et al. 2001) due to the ability of inverse agonists to upregulate 5-HT2A receptors in frontal cortex (Milligan and Bond 1997; Leurs et al. 1998; Hernandez and Sokolov 2000; Strange 2002). These proposals are supported by the finding that chronic administration of the inverse agonist MDL11,939 produces an up-regulation of 5-HT2A receptors in the frontal cortex of the rabbit with no change in the density of 5-HT2C receptors (Aloyo et al. 2001). Moreover, the up-regulation of the 5-HT2A receptor was accompanied by increased responsiveness to the behavioral effects of 5-HT2A receptor agonists (Aloyo et al. 2001). In conclusion, the data cited in this review indicate that the serotonin 5-HT2A receptor is involved importantly in learning, and that alterations in this receptor can lead to abnormalities in cognitive functions in both humans and experimental animals.
Acknowledgments
The author thanks his colleagues Anthony G. Romano and Vincent J. Aloyo for the many discussions that informed the writing of this review. Supported by NIHGrant MH16841-36 from the National Institutes of Health.
Article and publication are at http://www.learnmem.org/cgi/doi/10.1101/lm.60803.
References
- Abramson, H.A., Jarvik, M.E., Kaufman, M.R., Kornetsky, C., Levine, A., and Wagner, M. 1955. Lysergic acid diethylamide (LSD-25). I. Physiological and perceptual responses. J. Psychol. 39: 3–60. [Google Scholar]
- Aghajanian, G.K. and Marek, G.J. 1997. Serotonin induces excitatory postsynaptic potentials in apical dendrites of neocortical pyramidal cells. Neuropharmacology 36: 589–599. [DOI] [PubMed] [Google Scholar]
- Aghajanian, G.K. and Marek, G.J. 2000. Serotonin model of schizophrenia: Emerging role of glutamate mechanisms. Brain Res. Brain Res. Rev. 31: 302–312. [DOI] [PubMed] [Google Scholar]
- Alhaider, A.A., Ageel, A.M., and Ginawi, O.T. 1993. The quipazine- and TFMPP-increased conditioned avoidance response in rats: Role of 5-HT1C/5-HT2 receptors. Neuropharmacology 32: 1427–1432. [DOI] [PubMed] [Google Scholar]
- Aloyo, V.J. and Harvey, J.A. 2000. Antagonist binding at 5-HT2A and 5-HT2C receptors in the rabbit: High correlation with the profile for the human receptors. Eur. J. Pharmacol. 406: 163–169. [DOI] [PubMed] [Google Scholar]
- Aloyo, V.J., Dave, K.D., Rahman, T., and Harvey, J.A. 2001. Selective and divergent regulation of cortical 5-HT2A receptors in the rabbit. J. Pharmacol. Exp. Ther. 299: 1066–1072. [PubMed] [Google Scholar]
- Aurora, R.C. and Meltzer, H.Y. 1991. Serotonin2 (5-HT2) receptor binding in the frontal cortex of schizophrenic patients. J. Neural. Transm. 85: 19–29. [DOI] [PubMed] [Google Scholar]
- Barker, E.L., Westphal, R.S., Schmidt, D., and Sanders-Bush, E. 1994. Constitutively active 5-hydroxytryptamine 2C (5-HT2C) receptors reveal novel inverse agonist activity of receptor ligands. J. Biol. Chem. 269: 11687–11690. [PubMed] [Google Scholar]
- Barnes, J.M., Costall, B., Coughlan, J., Domeney, A.M., Gerrard, P.A., Kelly, M.E., Naylor, R.J., Onaivi, E.S., Tomkins, D.M., and Tyers, M.B. 1990. The effects of ondansetron, a 5-HT3 receptor antagonist, on cognition in rodents and primates. Pharmacol. Biochem. Behav. 35: 955–962. [DOI] [PubMed] [Google Scholar]
- Barnes, N.M. and Sharp, T. 1999. A review of central 5-HT receptors and their function. Neuropharmacology 38: 1083–1152. [DOI] [PubMed] [Google Scholar]
- Beylin, A.V., Gandhi, C.C., Wood, G.E., Talk, A.C., Matzel, L.D., and Shors, T.J. 2001. The role of the hippocampus in trace conditioning: Temporal discontinuity or task difficulty? Neurobiol. Learn. Mem. 76: 447–461. [DOI] [PubMed] [Google Scholar]
- Braun, U., Shulgin, A.T., and Braun, G. 1980. Centrally active n-substituted analogs of 3,4-methylenedioxyphenylisopropylamine (3,4-methylenedioxyamphetamine). J. Pharmaceut. Sci. 69: 192–195. [DOI] [PubMed] [Google Scholar]
- Brawley, P. and Duffield, J.C. 1972. The pharmacology of hallucinogens. Pharmacol. Rev. 24: 31–66. [PubMed] [Google Scholar]
- Buchanan, S.L. and Powell, D.A. 1982. Cingulate cortex: Its role in Pavlovian conditioning. J. Comp. Physiol. Psychol. 96: 755–774. [DOI] [PubMed] [Google Scholar]
- Burnet, P.W.J., Eastwood, S.L., and Harrison, P.J. 1996. 5-HT1A and 5-HT2A receptor mRNAs and binding sites are differentially altered in schizophrenia. Neuropsychopharmacology 15: 442–455. [DOI] [PubMed] [Google Scholar]
- Carli, M., Luschi, R., and Samanin, R. 1995. (S)-WAY 100135, a 5-HT1A receptor antagonist, prevents the impairment of spatial learning caused by intrahippocampal scopolamine. Eur. J. Pharmacol. 283: 133–139. [DOI] [PubMed] [Google Scholar]
- Carli, M., Bonalumi, P., and Samanin, R. 1997a. WAY 100635, a 5-HT1A antagonist, prevents the impairment of spatial learning caused by intrahippocampal administration of scopolamine or 7-chlorokynurenic acid. Brain Res. 774: 167–174. [DOI] [PubMed] [Google Scholar]
- Carli, M., Luschi, R., and Samanin, R. 1997b. Dose-related impairment of spatial learning by intrahippocampal scopolamine: Antagonism by ondansetron, a 5-HT3 receptor antagonist. Behav. Brain Res. 82: 185–194. [DOI] [PubMed] [Google Scholar]
- Clark, R.E. and Squire, L.R. 1998. Classical conditioning and brain systems: The role of awareness. Science 280: 77–81. [DOI] [PubMed] [Google Scholar]
- Cole, J.C. and Sumnall, H.R. 2003. Altered states: The clinical effects of ecstasy. Pharmacol. Ther. 98: 35–58. [DOI] [PubMed] [Google Scholar]
- Egan, C.T., Herrick-Davis, K., and Teitler, M. 1998. Creation of a constitutively activated state of the 5-hydroxytryptamine2A receptor by site-directed mutagenesis: Inverse agonist activity of antipsychotic drugs. J. Pharmacol. Exp. Ther. 286: 85–90. [PubMed] [Google Scholar]
- Fontana, D.J., Daniels, S.E., Wong, E.H., Clark, R.D., and Eglen, R.M. 1997. The effects of novel, selective 5-hydroxytryptamine (5-HT)4 receptor ligands in rat spatial navigation. Neuropharmacology 36: 689–696. [DOI] [PubMed] [Google Scholar]
- Geinisman, Y., Berry, R.W., Disterhoft, J.F., Power, J.M., and Van der Zee, E.A. 2001. Associative learning elicits the formation of multiple-synapse boutons. J. Neurosci. 21: 5568–5573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs, C.M. and Powell, D.A. 1991. Single-unit activity in the dorsomedial prefrontal cortex during the expression of discriminative bradycardia in rabbits. Behav. Brain Res. 43: 79–92. [DOI] [PubMed] [Google Scholar]
- Gimpl, M.P., Gormezano, I., and Harvey, J.A. 1979. Effects of LSD on learning as measured by classical conditioning of the rabbit nictitating membrane response. J. Pharmacol. Exp. Ther. 208: 330–334. [PubMed] [Google Scholar]
- Ginn, S.R. and Powell, D.A. 1986. Pizotifen attenutates classical eyeblink and heart rate conditioning in rabbits. Physiol. Psychol. 14: 36–41. [Google Scholar]
- Gormezano, I., Harvey, J.A., and Aycock, E. 1980. Sensory and associative effects of LSD on classical appetitive conditioning of the rabbit jaw movement response. Psychopharmacology 70: 137–143. [DOI] [PubMed] [Google Scholar]
- Gormezano, I., Kehoe, E.J., and Marshall, B. 1983. Twenty years of classical conditioning research with the rabbit. In Progress in psychobiology andphysiological psychology (ed. J.M. Sprague and A.N. Epstein), Vol. 10, pp. 197–275. Academic Press, New York. [Google Scholar]
- Harder, J.A., Maclean, C.J., Alder, J.T., Francis, P.T., and Ridley, R.M. 1996. The 5-HT1A antagonist, Way100635, ameliorates the cognitive impairment induced by fornix transaction in the marmoset. Psychopharmacology 127: 245–254. [PubMed] [Google Scholar]
- Harvey, J.A. 1987. Effects of drugs on associative learning. In Psychopharmacology, the thirdgeneration of progress. (ed. H. Meltzer), pp. 1485–1491. Raven Press, New York.
- Harvey, J.A. 1996. Serotonergic regulation of associative learning. Behav. Brain Res. 73: 47–50. [DOI] [PubMed] [Google Scholar]
- Harvey, J.A. and Welsh, J.P. 1996. Learning and performance: A critical review of the role of the cerebellum in instrumental and classical conditioning. In The acquisition of motor behavior in vertebrates. (eds. J.R. Bloedel, T.J. Ebner, and S.P. Wise), pp. 115–141. MIT press, Cambridge, MA.
- Harvey, J.A., Gormezano, I., and Cool V.A. 1982. Effects of d-lysergic acid diethylamide, d-2-bromolysergic acid diethylamide, dl-2,5-dimethoxy-4-methylamphetamine and d-amphetamine on classical conditioning of the rabbit nictitating membrane response. J. Pharmacol. Exp. Ther. 221: 289–294. [PubMed] [Google Scholar]
- Harvey, J.A., Gormezano, I., Cool-Hauser, V.A., and Schindler, C.W. 1988. Effects of LSD on classical conditioning as a function of CS–UCS interval: Relationship to reflex facilitation. Pharmacol. Biochem. Behav. 30: 433–441. [DOI] [PubMed] [Google Scholar]
- Harvey, J.A., Welsh, S.E., Hood, H., and Romano, A.G. 1999. Effects of 5-HT2 receptor antagonists on a cranial nerve reflex in the rabbit: Evidence for inverse agonism. Psychopharmacology 141: 162–168. [DOI] [PubMed] [Google Scholar]
- Hasuo, H., Matsuoka, T., and Akasu, T. 2002. Activation of presynaptic 5-hydroxytryptamine 2A receptors facilitates excitatory synaptic transmission via protein kinase C in the dorsolateral septal nucleus. J. Neurosci. 22: 7509–7517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensman, R., Guimaraes, F.S., Wang, M., and Deakin, J.F. 1991. Effects of ritanserin on aversive classical conditioning in humans. Psychopharmacology 104: 220–224. [DOI] [PubMed] [Google Scholar]
- Hernandez, I. and Sokolov, B.P. 2000. Abnormalities in 5-HT2A receptor mRNA expression in frontal cortex of chronic elderly schizophrenics with varying histories of neuroleptic treatment. J. Neurosci. Res. 59: 218–225. [PubMed] [Google Scholar]
- Hodges, H., Sowinski, P., Sinden, J.D., Netto, C.A., and Fletcher, A. 1995. The selective 5-HT3 receptor antagonist, WAY 100289, enhances spatial memory in rats with ibotenate lesions of the forebrain cholinergic projection system. Psychopharmacology 117: 318–332. [DOI] [PubMed] [Google Scholar]
- Hoyer, D., Pazos, A., Probst, A., and Palacios, J.M. 1986. Serotonin receptors in the human brain: II. Characterization and autoradiographic localization of 5-HT1C and 5-HT2 recognition sites. Brain Res. 376: 97–107. [DOI] [PubMed] [Google Scholar]
- Jakab, R.L. and Goldman-Rakic, P.S. 1998. 5-Hydroxytryptamine2A serotonin receptors in the primate cerebral cortex: Possible site of action of hallucinogenic and antipsychotic drugs in pyramidal cell apical dendrites. Proc. Natl. Acad. Sci. 95: 735–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenakin, T. 1996. The classification of seven transmembrane receptors in recombinant expression systems. Pharmacol. Rev. 48: 413–463. [PubMed] [Google Scholar]
- Kobayashi, M., Ohno, M., Yamamoto, T., and Watanabe, S. 1995. Concurrent blockade of β-adrenergic and muscarinic receptors disrupts working memory but not reference memory in rats. Physiol. Behav. 58: 307–314. [DOI] [PubMed] [Google Scholar]
- Kronforst-Collins, M.A. and Disterhoft, J.F. 1998. Lesions of the caudal area of rabbit medial prefrontal cortex impair trace eyeblink conditioning. Neurobiol. Learn. Mem. 69: 147–162. [DOI] [PubMed] [Google Scholar]
- LaBar, K.S. and Disterhoft, J.F. 1998. Conditioning, awareness, and the hippocampus. Hippocampus 8: 620–626. [DOI] [PubMed] [Google Scholar]
- Lambe, E.K., Goldman-Rakic, P.S., and Aghajanian, G.K. 2000. Serotonin induces EPSCs preferentially in layer V pyramidal neurons of the frontal cortex in the rat. Cereb. Cortex 10: 974–980. [DOI] [PubMed] [Google Scholar]
- Leurs, R., Smit, M.J., Alewijnse, A.E., and Timmerman, H. 1998. Agonist independent regulation of constitutively active G-protein coupled receptors. Trends Biochem. Sci. 23: 418–422. [DOI] [PubMed] [Google Scholar]
- López-Giménez, J.F., Vilaró, M.T., Palacios, J.M., and Mengod, G. 1998. [3H]MDL100,907 labels 5-HT2A receptors selectively in primate brain. Neuropharmacology 37: 1147–1158. [DOI] [PubMed] [Google Scholar]
- Ma, T.C. and Yu, Q.H. 1993. Effect of 20 (S)-ginsenoside-Rg2 and cyproheptadine on two-way active avoidance learning and memory in rats. Arzneimittel Forschung—Drug Res. 43: 1049–1052. [PubMed] [Google Scholar]
- Marchetti, E., Dumuis, A., Bockaert, J., Soumireu-Mourat, B., and Roman, F.S. 2000. Differential modulation of the 5-HT(4) receptor agonists and antagonist on rat learning and memory. Neuropharmacology 39: 2017–2027. [DOI] [PubMed] [Google Scholar]
- Marek, G.J. and Aghajanian, G.K. 1994. Excitation of interneurons in piriform cortex by 5-hydroxytryptamine: Blockade by MDL100,907, a highly selective 5-HT2A receptor antagonist. Eur. J. Pharmacol. 259: 137–141. [DOI] [PubMed] [Google Scholar]
- Marquardt, G.M., DiStefano, V., and Ling, L.L. 1978. Pharmacological effects of (±)-, (S)-, and (R)-MDA. In Psychopharmacology of hallucinogens. (eds. R.C. Stillman and R.E. Willette), pp. 84–104. Pergamen Press, New York.
- McEchron, M.D. and Disterhoft, J.F. 1997. Sequence of single neuron changes in CA1 hippocampus of rabbits during acquisition of trace eyeblink conditioned responses. J. Neurophysiol. 78: 1030–1044. [DOI] [PubMed] [Google Scholar]
- Meltzer, H.Y. 1999. The role of serotonin in antipsychotic drug action. Neuropsychopharmacology 21: 106S–115S. [DOI] [PubMed] [Google Scholar]
- Meneses, A. 1999. 5-HT system and cognition. Neurosci. Biobehav. Rev. 23: 1111–1125. [DOI] [PubMed] [Google Scholar]
- Meneses, A. 2002. Tianeptine: 5-HT uptake sites and 5-HT(1–7) receptors modulate memory formation in an autoshaping Pavlovian/instrumental task. Neurosci. Biobehav. Rev. 26: 309–319. [DOI] [PubMed] [Google Scholar]
- Meneses, A. and Hong, E. 1994. Modification of 8-OH-DPAT effects on learning by manipulation of the assay condition. Behav. Neural Biol. 61: 29–35. [DOI] [PubMed] [Google Scholar]
- Milligan, G. and Bond, R.A. 1997. Inverse agonism and the regulation of receptor number. Trends Pharmacol. Sci. 18: 468–474. [DOI] [PubMed] [Google Scholar]
- Moser, P.C., Bergis, O.E., Jegham, S., Lochead, A., Duconseille, E., Terranova, J.P., Caille, D., Berque-Bestel, I., Lezoualc'h, F., Fischmeister, R., et al. 2002. SL65.0155, a novel 5-hydroxytryptamine(4) receptor partial agonist with potent cognition-enhancing properties. J. Pharmacol. Exp. Ther. 302: 731–741. [DOI] [PubMed] [Google Scholar]
- Pazos, A., Cortes, R., and Palacios, J.M. 1985. Quantitative autoradiographic mapping of serotonin receptors in the rat brain. II. Serotonin-2 receptors. Brain Res. 346: 231–249. [DOI] [PubMed] [Google Scholar]
- Phillips, R.G. and LeDoux, J.E. 1994. Lesions of the dorsal hippocampal formation interfere with background but not foreground contextual fear conditioning. Learn. Mem. 1: 34–44. [PubMed] [Google Scholar]
- Pitsikas, N., Brambilla, A., and Borsini, F. 1994. Effect of DAU 6215, a novel 5-HT3 receptor antagonist, on scopolamine-induced amnesia in the rat in a spatial learning task. Pharmacol. Biochem. Behav. 47: 95–99. [DOI] [PubMed] [Google Scholar]
- Port, R.L., Mikhail, A.A., and Patterson, M.M. 1985. Differential effects of hippocampectomy on classically conditioned rabbit nictitating membrane response related to interstimulus interval. Behav. Neurosci. 99: 200–208. [DOI] [PubMed] [Google Scholar]
- Quirion, R., Richard, J., and Dam, T.V. 1985. Evidence for the existence of serotonin type-2 receptors on cholinergic terminals in rat cortex. Brain Res. 333: 345–349. [DOI] [PubMed] [Google Scholar]
- Romano, A.G. 1999. Variations in CS associability and multiple unit hippocampal activity in the rabbit. Behav. Brain Res. 103: 163–173. [DOI] [PubMed] [Google Scholar]
- Romano, A.G. and Harvey, J.A. 1992. Drug effects on sensorimotor integration in a model system. In Drugs of abuse andneurobiology. (ed. R.R. Watson), pp. 23–37. CRC Press, Boca Raton, FL.
- Romano, A.G. and Harvey, J.A. 1994. MDMA enhances associative and nonassociative learning in the rabbit. Pharmacol. Biochem. Behav. 47: 289–293. [DOI] [PubMed] [Google Scholar]
- Romano, A.G., Bormann, N.M., and Harvey, J.A. 1991. A unique enhancement of associative learning produced by methylenedioxyamphetamine. Behav. Pharmacol. 2: 225–231. [PubMed] [Google Scholar]
- Romano, A.G., Hood, H., and Harvey, J.A. 2000. Dissociable effects of the 5-HT2 antagonist mianserin on associative learning and performance in the rabbit. Pharmacol. Biochem. Behav. 67: 103–110. [DOI] [PubMed] [Google Scholar]
- Rothlin, E. 1957. Pharmacology of lysergic acid diethylamide and some of its related compounds. J. Pharm. Pharmacol. 9: 569–587. [DOI] [PubMed] [Google Scholar]
- Schindler, C.W. and Harvey, J.A. 1990. The use of classical conditioning procedures in behavioral pharmacology. In Contemporary research in behavioral pharmacology, drug development research. (eds. S.I. Dworkin, S.T. Higgins, and W.K. Bickel), Vol. 20, pp. 169–187. Wiley-Liss, Inc., New York. [Google Scholar]
- Schindler, C.W., Gormezano, I., and Harvey, J.A. 1985a. Effects of morphine and LSD on the classically conditioned nictitating membrane response. Pharmacol. Biochem. Behav. 22: 41–46. [DOI] [PubMed] [Google Scholar]
- Schindler, C.W., Gormezano, I., and Harvey, J.A. 1985b. Effects of drugs on classical conditioning. In Behavioral pharmacology: The current status. (eds. L.S. Seiden and R. Balster), pp. 55–71. Alan R. Liss, New York.
- Sears, L.L., Andreasen, N.C., and O'Leary, D.S. 2000. Cerebellar functional abnormalities in schizophrenia are suggested by classical eyeblink conditioning. Biol. Psychiatry 48: 204–209. [DOI] [PubMed] [Google Scholar]
- Sheldon, P.W. and Aghajanian, G.K. 1991. Excitatory responses to serotonin (5-HT) in neurons of the rat piriform cortex: Evidence for mediation by 5-HT1C receptors in pyramidal cells and 5-HT2 receptors in interneurons. Synapse 9: 208–218. [DOI] [PubMed] [Google Scholar]
- Shulgin, A.T. 1978. Psychotomimetic drugs: Structure-activity relationships. In Handbook of psychopharmacology. Vol. 11, Stimulants (eds. L.L. Iversen, S.D. Iversen, and S.H. Snyder), pp. 243–333. Plenum Press, New York. [Google Scholar]
- Siegel, S. and Freedman, D.X. 1988. Effects of LSD-25 on classical trace conditioning. Pharmacol. Biochem. Behav. 30: 427–431. [DOI] [PubMed] [Google Scholar]
- Snyder, S.H., Faillace, L., and Hollister, L. 1967. 2,5-Dimethoxy-4-methylamphetamine (STP): A new hallucinogenic drug. Science 158: 669–670. [DOI] [PubMed] [Google Scholar]
- Solomon, P.R., Vander Schaaf, E.R., Norbe, A.C., Weisz, D.J., and Thompson, R.F. 1986. Hippocampus and trace conditioning of the rabbit's nictitating membrane response. Behav. Neurosci. 100: 729–744. [DOI] [PubMed] [Google Scholar]
- Steinmetz, J.E. 2000. Brain substrates of classical eyeblink conditioning: A highly localized but also distributed system. Behav. Brain Res. 110: 13–24. [DOI] [PubMed] [Google Scholar]
- Steinmetz, J.E., Tracy, J.A., and Green, J.T. 2001. Classical eyeblink conditioning: Clinical models and applications. Integr. Physiol. Behav. Sci. 36: 220–238. [DOI] [PubMed] [Google Scholar]
- Strange, P.G. 2002. Mechanisms of inverse agonism at G-protein-coupled receptors. Trends Pharmacol. Sci. 23: 89–95. [DOI] [PubMed] [Google Scholar]
- Tanaka, E. and North, R.A. 1993. Actions of 5-hydroxytryptamine on neurons of the rat cingulate cortex. J. Neurophysiol. 69: 1749–1757. [DOI] [PubMed] [Google Scholar]
- Terry, A.V., Buccafusco, J.L., Jackson, W.J., Prendergast, M.A., Fontana, D.J., Wong, E.H., Bonhaus, D.W., Weller, P., and Eglen, R.M. 1998. Enhanced delayed matching performance in younger and older macaques administered the 5-HT4 receptor agonist RS17017. Psychopharmacology 135: 407–415. [DOI] [PubMed] [Google Scholar]
- Thompson, R.F. 1986. The neurobiology of learning and memory. Science 223: 941–947. [DOI] [PubMed] [Google Scholar]
- Thompson, L.T., Moskal, J.R., and Disterhoft, J.F. 1992. Hippocampus-dependent learning facilitated by a monoclonal antibody or D-cycloserine. Nature 359: 638–641. [DOI] [PubMed] [Google Scholar]
- Titov, S.A., Shamakina, I.I., and Ashmarin, I.P. 1983. Effect of lysyl-vasopressin and vasotocin on a disorder of the conditioned avoidance reaction by a serotonin receptor blockader. Biulleten Eksperimentalnoi Biologii i Meditsiny 95: 31–33. [PubMed] [Google Scholar]
- Vitiello, B., Martin, A., Hill, J., Mack, C., Molchan, S., Martinez, R., Murphy, D.L., and Sunderland, T. 1997. Cognitive and behavioral effects of cholinergic, dopaminergic, and serotonergic blockade in humans. Neuropsychopharmacology 16: 15–24. [DOI] [PubMed] [Google Scholar]
- Weible, A.P., McEchron, M.D., and Disterhoft, J.F. 2000. Cortical involvement in acquisition and extinction of trace eyeblink conditioning. Behav. Neurosci. 114: 1058–1067. [DOI] [PubMed] [Google Scholar]
- Weiner, D.M., Burstein, E.S., Nash, N., Croston, G.E., Currier, E.A., Vanover, K.E., Harvey, S.C., Donohue, E., Hansen, H.C., Andersson, C.M., et al. 2001. 5-Hydroxytryptamine2A receptor inverse agonists as antipsychotics. J. Pharmacol. Exp. Ther. 299: 268–276. [PubMed] [Google Scholar]
- Weiss, C., Preston, A.R., Oh, M.M., Schwarz, R.D., Welty, D., and Disterhoft, J.F. 2000. The M1 muscarinic agonist CI-1017 facilitates trace eyeblink conditioning in aging rabbits and increases the excitability of CA1 pyramidal neurons. J. Neurosci. 20: 783–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh, S.E., Kachelries, W.J., Romano, A.G., Simansky, K.J., and Harvey, J.A. 1998a. Effects of LSD, ritanserin, 8-OH-DPAT and lisuride on classical conditioning in the rabbit. Pharmacol. Biochem. Behav. 59: 469–475. [DOI] [PubMed] [Google Scholar]
- Welsh, S.E., Romano, A.G., and Harvey, J.A. 1998b. Effects of serotonin 5-HT2A/2C antagonists on associative learning in the rabbit. Psychopharmacology 137: 157–163. [DOI] [PubMed] [Google Scholar]
- Westphal, R.S. and Sanders-Bush, E. 1994. Reciprocal binding properties of 5-hydroxytryptamine type 2C receptor agonists and inverse agonists. Mol. Pharmacol. 46: 937–942. [PubMed] [Google Scholar]
- Williams, G.V., Rao, S.G., and Goldman-Rakic, P.S. 2002. The physiological role of 5-HT2A receptors in working memory. J. Neurosci. 22: 2843–2854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff-Pak, D.S. 2001. Eyeblink classical conditioning differentiates normal aging from Alzheimer's disease. Integr. Physiol. Behav. Sci. 36: 87–108. [DOI] [PubMed] [Google Scholar]
- Yasuno, F., Suhara, T., Nakayama, T., Ichimiya, T., Okubo, Y., Takano, A., Ando, T., Inoue, M., Maeda, J., and Suzuki, K. 2003. Inhibitory effect of hippocampal 5-HT1A receptors on human explicit memory. Am. J. Psychiatry 160: 334–340. [DOI] [PubMed] [Google Scholar]
- Zhen, X., Du, W., Romano, A.G., Friedman, E., and Harvey, J.A. 2001. The p38 mitogen-activated protein kinase is involved in associative learning in rabbits. J. Neurosci. 21: 5513–5519. [DOI] [PMC free article] [PubMed] [Google Scholar]