Abstract
The 5-HT3 receptor for serotonin is expressed within limbic structures and is known to modulate neurotransmitter release, suggesting that this receptor may influence learning and memory. Perturbations in serotonergic neurotransmission lead to changes in the ability to attend, learn, and remember. To examine the role of 5-HT3 receptors in learning, memory, and attention, 5-HT3 receptor overexpressing (5-HT3-OE) transgenic mice and their wild-type littermates (WT) were tested in Pavlovian contextual and cued fear conditioning, fear extinction, and latent inhibition (LI) paradigms. Prepulse inhibition (PPI) was assessed to reveal changes in sensorimotor gating. Additionally, anxious behaviors, shock sensitivity, and reactions to novel stimuli were evaluated. 5-HT3-OE mice displayed enhanced contextual conditioning, whereas cued conditioning remained the same as that of WT mice. 5-HT3-OE mice did not differ from WT in extinction rates to either the context or cue. LI was enhanced for 5-HT3-OE mice compared to WT. PPI remained unchanged. No differences in sensitivity to footshock or startle were found. However, 5-HT3-OE mice demonstrated heightened exploratory behavior in response to novel environmental stimuli and decreased anxiety as measured in the elevated plus-maze. Results indicate that overexpression of the 5-HT3 receptor in mouse forebrain results in enhanced hippocampal-dependent learning and attention. Enhanced inspective behavior in response to novelty may contribute to the observed improvements in learning, memory, and attention due to 5-HT3 receptor overexpression.
Of the seven classes of serotonin receptors, the 5-HT3 receptor is the only ionotropic receptor found in vertebrates (Derkach et al. 1989; Maricq et al. 1991). Only two subunits, 3A and 3B, are currently known (Hope et al. 1993; Werner et al. 1994; Davies et al. 1999; Dubin 1999). Of these, only the 3A subunit is expressed within the rodent brain, where it can form a homomeric receptor (Morales and Wang 2002; but see Monk et al 2001). Sharing up to 30% sequence homology with the nicotinic acetylcholine receptor, the 5-HT3 receptor belongs to the superfamily of ligand-gated ion channels (Ortells and Lunt 1995). Ligand binding results in channel opening and allows for the influx of Na+ and Ca2+ (McMahon and Kauer 1997; Nayak et al. 1999).
The 5-HT3 receptor is primarily localized presynaptically as a heteroreceptor (MacDermott et al. 1999; Khakh and Henderson 2000; but see van Hooft and Vijverberg 2000). This localization allows for the modulation of release of neurotransmitters such as dopamine (Campbell et al. 1996; Allan et al 2001), norepinephrine (Matsumoto et al. 1995), acetylcholine (Consolo et al. 1994; Giovannini et al. 1998), and μ-amino-butyric acid (GABA; McMahon and Kauer 1997). Work by Morales and Bloom (1997) showed that a large portion of 5-HT3 receptor-positive neurons in the forebrain are interneurons.
5-HT3 receptor mRNA and binding sites are found in abundance in the rodent forebrain, namely the entorhinal cortex, piriform cortex, amygdala, and hippocampus (Kilpatrick et al. 1987; Barnes et al. 1990a; Tecott et al. 1993). As these structures, particularly the hippocampus and amygdala, are involved in learning and memory (Maren 2001), the 5-HT3 receptor has been postulated to play a role in these processes.
Indeed, data strongly suggest that antagonism of the 5-HT3 receptor can improve learning and memory in associative tasks (Costall and Naylor 1992; Hong and Meneses 1996; Roychoudhury and Kulkarni 1997). Work by Costall's group demonstrated that administration of a 5-HT3 receptor antagonist, ondansetron, could improve cognition in both rodent and primate in terms of basal and muscarinic antagonist (scopolamine)-induced impairments in performance (Barnes et al. 1990b). However, basal performance of the rat in the more difficult spatial t-maze was not enhanced by ondansetron, whereas basal performance in a simpler portion of this task was (Barnes et al. 1990b). Further, Carli et al. (1997) reported that blockade of 5-HT3 receptors prevented the deficit caused by scopolamine, but failed to change basal performance in a spatial discrimination task. Additional work by Costall's group (Bratt et al. 1994) revealed that in a more complex stone maze task based on the t-maze, ondansetron failed to reverse a scopolamine-induced deficit and, in fact, further impaired performance in this hippocampal-dependent task at one dose tested. Taken together, these results indicate that the ability of ondansetron to reverse learning deficits may depend on the neurotransmitter system being manipulated as well as the task employed.
The role of the 5-HT3 receptor in learning and memory paradigms which require the hippocampus remains unclear. To address this problem, we assessed hippocampal-dependent and -independent learning in 5-HT3 overexpressing transgenic mice (5-HT3-OE). There is a clear increase in 5-HT3 receptor expression within the hippocampus and amygdala as well as other areas of the forebrain in 5-HT3-OE mice (Engel et al. 1998). The CaMKIIα promoter was used in the construction of the transgene to limit expression spatially, to the forebrain, and temporally, after birth (Engel et al. 1998). Data from electrophysiological and binding studies indicate that transgenic 5-HT3 receptors are functionally indistinguishable from endogenous receptors and suggest that the transgenic receptors form functional ion channels (Engel et al. 1998; Sung et al. 2000). Overexpression has been observed in both interneurons and principal neurons of 5-HT3-OE mice (M. Morales, pers. comm.).
Lesion studies have shown that, whereas the amygdala is necessary for both contextual and cued fear conditioning, only contextual fear conditioning requires the hippocampal formation (Kim and Fanselow 1992; Phillips and LeDoux 1992). Therefore, the impact of 5-HT3 receptor transgene expression in these two areas can be assessed. The present study is the first to address this receptor using fear conditioning. Here we report the impact of 5-HT3 receptor overexpression on learning, memory, and attention, as well as related behaviors such as sensorimotor gating, anxiety, and response to novelty.
RESULTS
5-HT3-OE Mice Have Enhanced Contextual Fear Conditioning
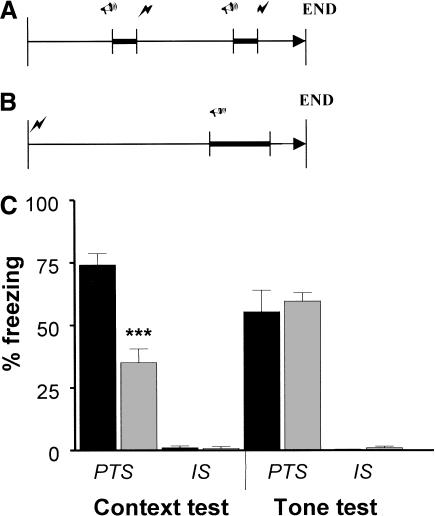
Because a gender difference was noted only in the pain sensitivity paradigm, data from all other experiments were collapsed across gender. Figure 1 presents the fear conditioning paradigms and results for mice trained with a paired tone-shock (PTS) and mice trained with an immediate shock unpaired from the tone (IS). The IS training has been shown to be a valuable control for exposure to the footshock and tone while producing a deficit in associative learning as reflected by reduced levels of freezing relative to PTS-trained animals (Lattal and Abel 2001). In the context test, a two-way ANOVA revealed a significant main effect of genotype, F(1,28) = 31.2, P < 0.001 and a significant main effect of training condition, F(1,28) = 244.6, P < 0.001. A significant interaction between genotype and training condition was also found in the context test, F(1,28) = 31.2, P < 0.001, reflecting the enhanced contextual conditioning seen for the 5-HT3-OE PTS group relative to WT PTS, which was not evident in the IS group. However, the effect of genotype was not significant for the tone test, whereas a significant effect of training condition was found, F(1,32) = 122.5, P < 0.001. Behavior in the altered context (data not shown) was not significantly different between genotypes, F(1,32) = 0.104 or training, F(1,32) = 0.384, indicating that a lack of difference in freezing to the tone for both IS and PTS groups was not likely due to a difference in freezing within the altered context.
Figure 1.
5-HT3-OE mice have enhanced contextual fear conditioning. (A) PTS training paradigm. The animal is inserted into the testing context. At 90 sec, a tone comes on and terminates after 30 sec with a 2-sec footshock. Ninety sec later, the tone begins again and after 30 sec coterminates with the footshock again. Thirty sec after the last footshock the animal is removed from the conditioning context. (B) IS training paradigm. The animal is inserted into the testing context and immediately given a 2-sec footshock. At 180 sec, a tone comes on and terminates after 60 sec. Thirty sec later, the animal is removed from the conditioning context. (C) Fear conditioning results. Data are mean ± SEM. Black bars, 5-HT3-OE. Gray bars, WT. Left, % freezing context test. Right, % freezing tone test. ***: P < 0.001 between PTS context groups.
Ondansetron Blocks the Enhancement in Contextual Fear Conditioning
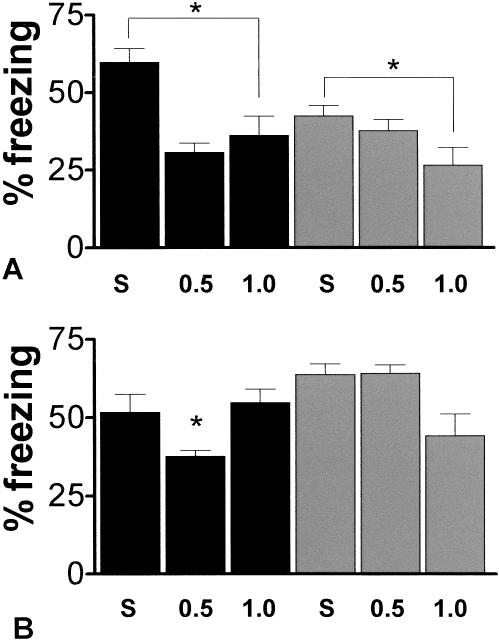
Figure 2 shows the effects of saline, 0.5 and 1.0 mg/kg i.p. ondansetron on fear conditioning in the context and tone tests. In the context test, no significant main effects were evident, but a post-hoc Tukey HSD test found a significant difference (P = 0.05) between the saline and 1.0 mg/kg ondansetron treatments. Results suggest that antagonism of the 5-HT3 receptor impairs hippocampal-dependent contextual fear conditioning in both 5-HT3-OE and WT mice. Furthermore, both doses of ondansetron were able to reduce the freezing response of 5-HT3-OE mice in the context test to that of WT saline-treated levels. No main effects were found in the tone test, but a significant interaction of genotype and dose, F(2,42) = 4.094, P = 0.024 was revealed, reflecting an impairment in fear conditioning to the tone for the 5-HT3-OE 0.5 mg/kg ondansetron group. A similar impairment in conditioning to the tone is noted in the WT 1.0 mg/kg ondansetron group. Although it may be possible that the impairment in freezing to the tone for the 5-HT3-OE 0.5 mg/kg group, which is amygdala-dependent, influenced the conditioning to the context, which also depends on the amygdala, the continued reduction in fear conditioning to the context at the 1.0 mg/kg dose in the absence of an apparent amygdala-dependent impairment might indicate that the reversal of the learning enhancement in 5-HT3-OE mice administered ondansetron is due to hippocampal-dependent processes.
Figure 2.
Ondansetron impairs fear conditioning. Data are mean ± SEM. Black bars, 5-HT3-OE; gray bars WT. (A) % freezing context test. *: P = 0.05 between the saline and 1.0 mg/kg ondansetron groups. (B) % freezing tone test. *: P = 0.024 for a genotype × dose interaction.
Extinction Is Not Different Between WT and 5-HT3-OE Mice
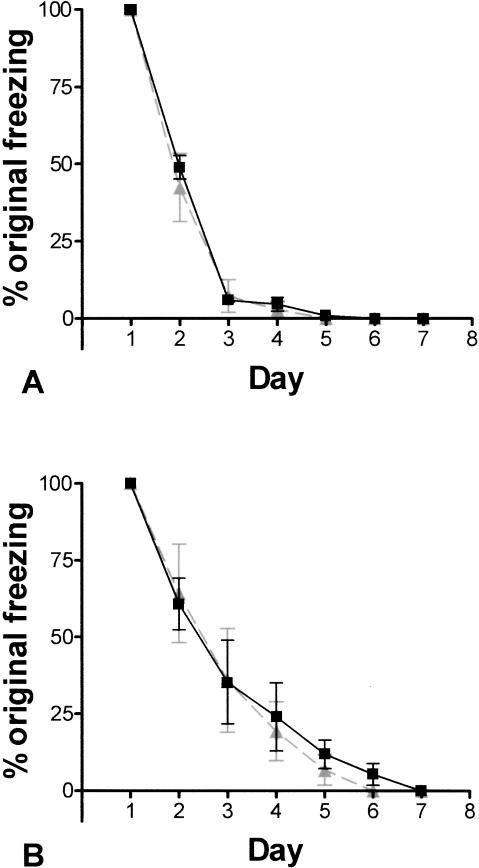
Figure 3 illustrates the extinction rates to the context and tone for 5-HT3-OE and WT mice. 5-HT3-OE mice did not extinguish to the context or the tone at a faster rate than did WT mice, as there was no significant effect of genotype using a two-way ANOVA. A significant effect of day was found for both context and tone tests, F(6,70) = 234.8, P < 0.001 and F(6,70) = 35.2, P < 0.001, respectively. The rates of extinction indicate that 5-HT3-OE mice do not have enhanced contextual extinction compared to WT mice, despite the enhanced contextual conditioning seen with fear conditioning.
Figure 3.
5-HT3-OE mice and WT mice do not differ in extinction. Data are mean ± SEM. Black boxes, 5-HT3-OE; gray boxes, WT. (A) % freezing context test. (B) % freezing tone test.
5-HT3-OE Mice Are Less Anxious in the Elevated Plus-Maze
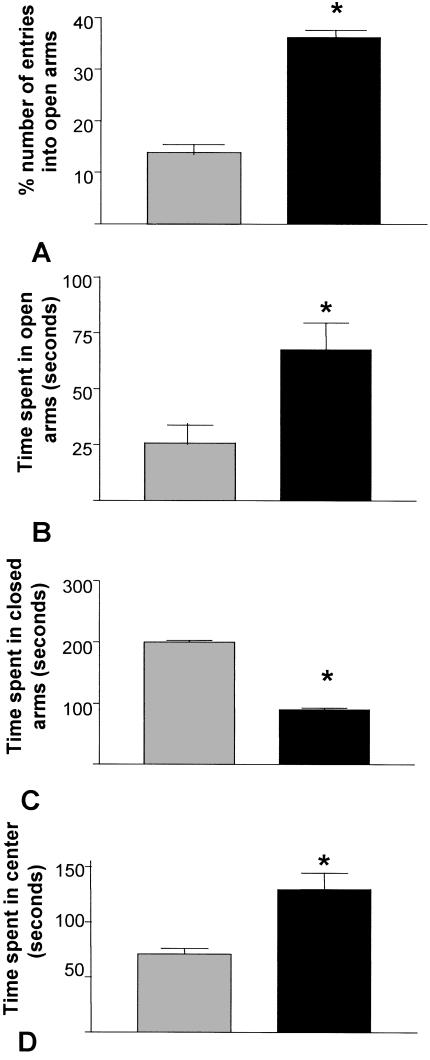
Figure 4 illustrates that 5-HT3-OE mice are less anxious in the elevated plus-maze as reflected by the increased number of entries into the open arms of the maze, increased time spent in the open arms, and decreased time spent in the closed arms. Time spent in the open center area was also increased relative to WT mice, indicating a decrease in anxious behaviors observed for 5-HT3-OE mice. All differences were significant (P < 0.02) using unpaired t-tests. Although entries into open arms were increased for 5-HT3-OE mice relative to WT, the concomitant decrease in entries into the closed arms would seem consistent with the open-field activity, which indicates that there is no overall change in locomotor behavior (Engel and Allan 1999).
Figure 4.
5-HT3-OE mice are less anxious in the elevated plus-maze. Data are mean ± SEM. Black bars, 5-HT3-OE; gray bars, WT. *: A significant difference at P < 0.02 by unpaired t-test. (A) Number of entries into the open arms expressed as a percentage of total arm entries. (B) Time in sec spent in the open arms of the maze. (C) Time (sec) spent in the closed arms of the maze. (D) Time (sec) spent in the center of the maze.
Pain Sensitivity Is Not Different Between WT and 5-HT3-OE Mice
Table 1 shows the three behavioral responses assessed for increasing shock intensities for both 5-HT3-OE and WT mice. A three-way ANOVA indicated there was no significant difference in reactivity to footshock due to genotype. However, a gender difference was noted, F(1,60) = 17.5, P < 0.001, as was a significant effect of the behavior being assessed, F(2,60) = 17.2, P < 0.001. Females from both genotypes were more sensitive to the footshock (reached behavioral thresholds at lower mA). Adult female mice used in these experiments weigh less than males of both genotypes, as reflected by a significant main effect of gender, F(1,20) = 53.921, P < 0.001 in a two-way ANOVA of genotype × gender. Weight differences correlate with the difference noted in pain sensitivity and may be the reason for the gender difference in response to footshock. However, as there is no effect of genotype as a whole in sensitivity to footshock, the difference due to gender is not likely to influence the fear conditioning results. Additionally, the intensity of footshock used in fear conditioning is well above the highest intensity shock tested here and is sufficient to cause all animals trained to jump and vocalize.
Table 1.
Pain Thresholds Are Not Different Between Genotypes
| Movement | Vocalization | Jump | |
|---|---|---|---|
| 5-HT3-OE (N = 12) | 0.09 ± 0.004 | 0.12 ± 0.01 | 0.14 ± 0.01 |
| WT (N = 12) | 0.09 ± 0.004 | 0.11 ± 0.01 | 0.14 ± 0.01 |
Numbers are in mAmps. Data are mean ± SEM.
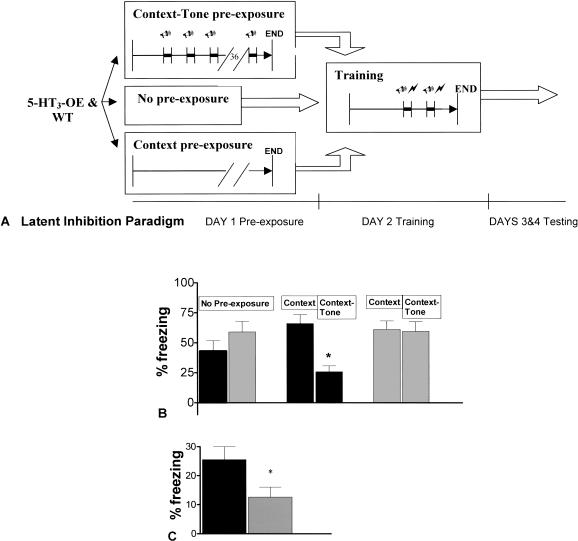
5-HT3-OE Mice Have Enhanced Latent Inhibition (LI)
Figure 5A illustrates the latent inhibition paradigm. A reduction in freezing of the context-tone pre-exposed group relative to the context pre-exposed group is indicative of LI. 5-HT3-OE mice have enhanced LI to the tone compared to WT mice, as evidenced by the results from the tone test shown in Figure 5B. A significant main effect of genotype was found using a two-way ANOVA for freezing to the tone, F(1,42) = 5.94, P = 0.02. A significant main effect of pre-exposure, F(2,42) = 3.88, P = 0.03 was also found. An interaction between genotype and pre-exposure, F(2,42) = 3.3, P = 0.05 was found, reflecting the fact that context-tone pre-exposed 5-HT3-OE mice have reduced freezing to the tone relative to all other groups. A main effect of genotype for freezing to the context, F(1,42) = 5.7, P = 0.02, suggests that 5-HT3-OE mice have enhanced contextual conditioning in this LI paradigm (Fig. 5C), similar to the enhancement seen in fear conditioning.
Figure 5.
Latent inhibition to the tone is enhanced in 5-HT3-OE mice, whereas contextual conditioning remains elevated. (A) Day 1 pre-exposure: Mice in the context-tone pre-exposure group received 40 presentations of a 5-sec tone (80-db, 6-Hz clicker, intertrial interval 30 sec). Mice in the no pre-exposure group remained in their home cages. Mice in the context pre-exposure group were placed in the training chamber for the same amount of time (23 min, 50 sec) as the context-tone group but received no exposure to the tone. Day 2 training: After 90 sec of habituation, the tone was presented for 5 sec and a footshock (0.6 mA) was delivered through the stainless steel grid floor for the last 2 sec of the tone. Thirty sec after the first footshock, the tone and footshock sequence was repeated and mice were removed from the context 30 sec after co-termination of the second tone/shock pairing. Day 3 testing: Mice were tested for freezing to the tone. Day 4 testing: Mice were tested for freezing to the context. (B) Conditioned responding to the tone. Data are mean ± SEM. Black bars, 5-HT3-OE; gray bars, WT. *: A significant difference at P = 0.05 for a genotype × pre-exposure interaction. (C) Conditioned responding to the context. Data are mean ± SEM. Black bar, 5-HT3-OE; gray bar, WT. *: P = 0.02.
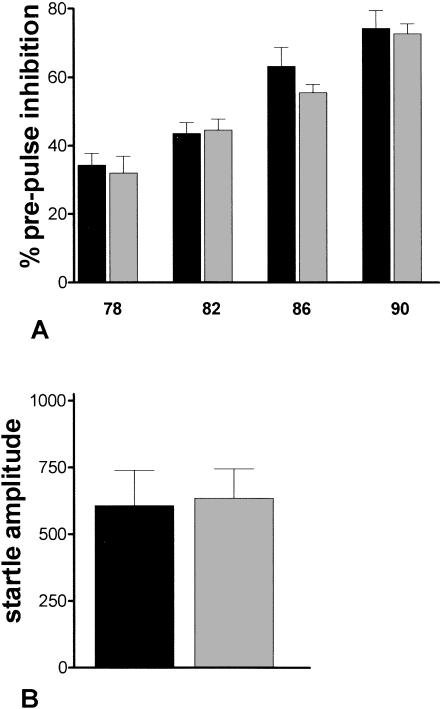
Pre-Pulse Inhibition (PPI) Is Not Different Between WT and 5-HT3-OE Mice
5-HT3-OE mice are not different from WT mice in either PPI or maximum startle amplitude (Fig. 6A,B). Additionally, as the number of females and males is balanced in this experiment and there is no significant effect of gender, it would appear that differences in weight did not effect the startle response overall. No significant effect of genotype on PPI was found using ANOVA. A significant main effect of prepulse intensity was detected, F(3,72) = 38.5, P < 0.001, signifying that increasing the prepulse intensity led to increased inhibition of the startle response for both transgenic and WT mice. Transgene presence had no significant effect on the maximum startle amplitude. Results suggest that overexpression of the 5-HT3 receptor has not altered sensorimotor gating or the startle response.
Figure 6.
5-HT3-OE mice do not have altered pre-pulse inhibition or startle. Data are mean ± SEM. Black bars, 5-HT3-OE; gray bars, WT. (A) PPI. x-axis labels indicate the intensity of the prepulse in decibels. (B) Max startle amplitudes.
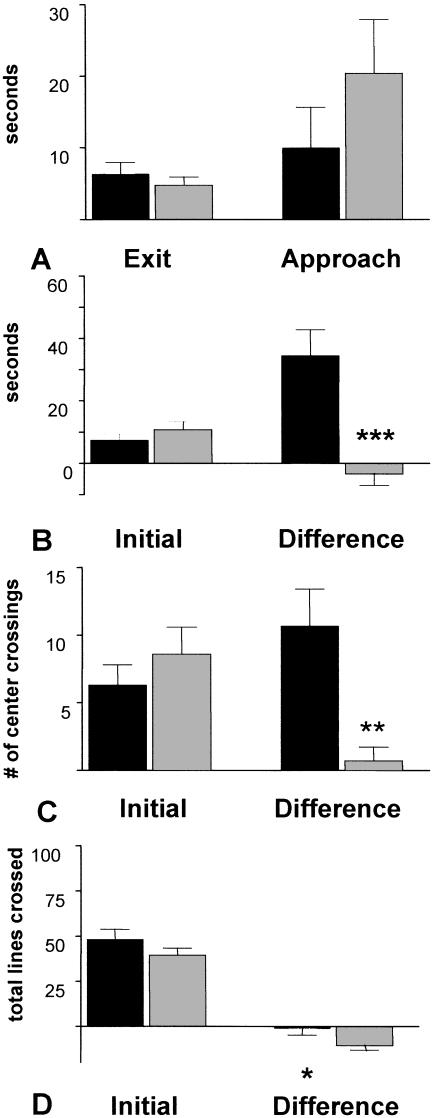
5-HT3-OE Mice Have Enhanced Inspective Behavior in Response to Novelty
Latency to leave the center of the maze during the initial 5-min segment and to approach a novel object in the second segment is illustrated in Figure 7A. Behavior within the testing environment (initial 5-min segment) and behavior in response to the novel object (second 5-min segment with novel object—initial segment without novel object) are quantified in Fig. 7B–D. Genotype did not significantly affect the latency to leave the center after placement in the open field, and latency to approach the novel object in the second segment of the test did not differ significantly, indicating that locomotor activity was similar in terms of latency for both genotypes in both segments (Fig. 7A). Time spent in the center during the initial 5-min segment did not differ significantly between WT and 5-HT3-OE mice; however, a significant effect of genotype was found for the difference score of time spent in the center during the second segment minus the time spent in the center during the initial segment, F(1,28) = 22.0 P < 0.001, indicating that 5-HT3-OE mice spend more time in the center area than WT mice when the novel object is present relative to behavior in the absence of the novel object (Fig. 7B). Although genotype did not significantly affect the number of center line crossings during the initial segment, an effect of genotype was significant for the measure of the difference in center line crossings, reflecting the observation that 5-HT3-OE mice displayed increased line crossings in the center during the second test segment compared to WT mice, F(1,29) = 15.0, P = 0.001 (Fig. 7C). Total transitions during the initial test segment did not differ significantly between WT and 5-HT3-OE mice, indicating that locomotor activity was similar between the two groups. However, a significant effect of genotype was found for the difference score between the first and second segments, F(1,29) = 5.0 P = 0.033 (Fig. 7D). Taken together, these results indicate that 5-HT3-OE mice demonstrate enhanced object-directed inspective activity in response to insertion of the novel object.
Figure 7.
5-HT3-OE mice have increased exploratory behavior in response to a novel object. Data are mean ± SEM. Black bars, 5-HT3-OE; gray bars, WT. “Exit”: reflects the latency to leave the center after placement in the open field. “Approach”: Latency to approach the novel object. “Initial”: Behavior in the first five min. “Difference”: The difference score between the first 5-min and second 5-min segments (segment 2 – segment 1). All * apply to difference groups. (A) Latency. (B) Time in center. *** P < 0.001. (C) Number of times center lines were crossed. ** P = 0.001. (D) Total lines crossed. * P = 0.033.
DISCUSSION
Behavioral responses of 5-HT3-OE mice were markedly different from WT mice in both contextual fear conditioning and latent inhibition tasks assessing learning and attention. PTS-trained 5-HT3-OE mice displayed enhanced conditioning to contextual cues compared to PTS-trained WT mice, whereas auditory conditioning remained at similar levels in conditioned-fear PTS groups. No differences between genotypes were noted for IS groups in either the context or tone tests, suggesting that overexpression of the 5-HT3 receptor did not affect freezing behavior in nonassociative paradigms. Furthermore, the increase in contextual conditioning evident in PTS-trained 5-HT3-OE mice relative to PTS-trained WT mice was also detected in the context test portion of the LI paradigm, revealing that contextual conditioning was enhanced in two learning paradigms with differing parameters. The difference in freezing levels between the LI context tests and the fear conditioning context test may be due to differences in the duration of the training protocols. Based on results from the PTS tone test in the fear conditioning paradigm, it is unlikely that 5-HT3-OE mice are simply better at freezing or are more sensitive to the footshock used for training than WT mice. Indeed, 5-HT3-OE mice were not different from WT in three measures of sensitivity to the footshock. Thus, it is unlikely that differences in pain sensitivity can account for the improvements noted in contextual conditioning.
Based on these observations, we conclude that hippocampal-dependent learning processes involved in fear conditioning are affected by 5-HT3 receptor transgene expression in the forebrain. It would be interesting to determine whether other hippocampal-dependent tasks such as the Morris water maze are affected similarly by receptor overexpression. Indeed, further experiments would be necessary to evaluate which hippocampal functions, specifically, are affected by transgene expression. Because serotonin is released preferentially in the hippocampus in response to stressful or fearful situations (Wilkinson et al. 1996), 5-HT3 receptor activation has the potential to connect emotionally salient events to hippocampal memory systems important for fear conditioning. The hippocampus possesses a large number of 5-HT3 receptor-expressing GABAergic interneurons (Morales and Bloom 1997). Interestingly, 5-HT3-OE mice have increased receptor expression in hippocampal GABAergic interneurons as well as principal excitatory neurons (M. Morales, pers. comm.). Although the noted improvement in fear conditioning for 5-HT3-OE mice may be due to an endogenous-like localization of the overexpressed 5-HT3 receptors to GABAergic interneurons of the hippocampus, it remains possible that ectopic expression on pyramidal neurons may have led to this augmentation. N-methyl-D-aspartate (NMDA) receptors within the hippocampus appear to be essential for contextual fear conditioning (Zhang et al. 2001). Overexpressed 5-HT3 receptors may be producing effects on learning and memory by causing the depolarization of pyramidal cells in which they are ectopically expressed, thus relieving the magnesium block of the NMDA channel. This scenario would require the release of serotonin onto pyramidal cells in order to activate the overexpressed 5-HT3 receptors. However, serotonergic projections from the median raphe predominantly innervate a subset of GABAergic interneurons within the hippocampus (Hornung and Celio 1992). Thus, it remains possible that overexpressed receptors on interneurons, which mimic endogenous receptor localization, contribute to the learning enhancement observed for 5-HT3-OE mice. This possibility, while arguing against some of the literature which indicates that antagonists of endogenous 5-HT3 receptors enhance learning (Costall and Naylor 1992), might explain why complex hippocampal-dependent learning and memory tasks are occasionally worsened by antagonism of this receptor (Bratt et al. 1994). The present results would support the argument that endogenous 5-HT3 receptors may in fact contribute to the acquisition of hippocampal-dependent fear conditioning. Still, it is entirely possible, and perhaps likely, that the effects on learning due to receptor overexpression are a result of ectopic expression.
Behavioral changes might also result from a compensatory alteration in neuronal physiology due to increased 5-HT3 receptor expression, either post- or prenatally. It has not been demonstrated that expression of the 5-HT3 transgene is limited to postnatal development. 5-HT3 receptor mRNA can be detected at embryonic day 10 in the rat and may play a role in secondary induction in fetal development (Johnson and Heinemann 1995). However, CaMKIIα protein is not highly expressed until after birth (Burgin et al. 1991), and fyn-transgene expression driven by the same CaMKIIα promoter used here is expressed strongly only postnatally (Kojima et al. 1997), so we speculate that the expression of the 5-HT3 receptor transgene is also limited to after birth (Engel et al. 1998). Indeed, transgene expression is limited spatially to the forebrain, as dictated by the expression profile of the CaMKIIα promoter. We therefore suggest that the interference of receptor overexpression in prenatal development is minimal or absent.
Although compensatory changes in the same or other neurotransmitter systems may have occurred in response to receptor overexpression which occurs postnatally, the ability of ondansetron to reduced freezing levels to that of WT in the context test argues that an acute effect of the receptor is a more likely reason for the learning improvement, and not compensatory changes. In previous experiments it was shown that 5-HT3 receptor overexpression did not produce compensatory changes in monoamine levels, dopamine receptors, or transporters (Allan et al. 2001), and so it remains possible that compensatory changes may not be the sole basis for the behavioral phenotypes we have observed in the transgenic mice.
The hippocampus influences attention and is necessary not simply for learning, but for discriminating; that is, for determining when it is appropriate to learn one thing in relation to another. Thus, an important role of the hippocampus is to inhibit unnecessary associations and allow for the formation of meaningful or predictive associations (Corcoran and Maren 2001). LI paradigms reflect decremental attention, or the ability to ignore previously irrelevant stimuli and thus inhibit unnecessary associations (Gould and Wehner 1999). 5-HT3-OE mice pre-exposed to the context and tone learned to ignore the irrelevant tone (of low predictive value) during pre-exposure and thus were less able, compared to WT mice exposed to the same stimuli, to use the stimulus as an associative cue the next day. Previous studies indicate that antagonism of the 5-HT3 receptor facilitates LI (Moran and Moser 1992). Here we report the enhancement of LI in a fear conditioning task as an effect of chronic 5-HT3 receptor transgene overexpression in the forebrain. Improved attention may contribute to the learning enhancement evident for 5-HT3-OE mice.
PPI and LI are disrupted in individuals with schizophrenia, so in light of LI results it was important to assess PPI. PPI is a measure of the ability to shut out stimuli that quickly follow a previous stimulus, and allows for mental integration (Dulawa and Geyer 2000). This mechanism is thought to be important in order to prevent the interruption of ongoing information processing routines by ensuing stimuli. Serotonin acts as a modulatory neurotransmitter in PPI paradigms (Paylor and Crawley 1997; Caldarone et al. 2000; Dulawa and Geyer 2000). Sensorimotor gating, as reflected by PPI to a tactile startle stimulus, appears to be normal in 5-HT3-OE mice and shows the characteristic dependence on prepulse intensity, as does WT PPI. Startle amplitude is also unaffected by transgene expression. Thus, overexpression of the 5-HT3 receptor did not appear to influence PPI. This might indicate that the enhancement in attention for 5-HT3-OE mice, revealed using LI, is independent of an overall antipsychotic effect of overexpression.
Extinction to the auditory cue and to the context was unaffected by overexpression of the 5-HT3 receptor. WT and 5-HT3-OE mice were able to learn that the tone and the context were no longer predictive of the aversive footshock at similar rates. As extinction in the context is context-specific and thus depends on the hippocampus (Corcoran and Maren 2001), 5-HT3-OE mice do not appear to display hippocampal-dependent learning differences in extinction paradigms. Results from this protocol suggest that enhanced learning and memory in 5-HT3-OE mice may depend on the novelty of the learning situation. Extinction is an active learning process that does not erase, but rather suppresses, the original learning. Similar to fear conditioning, extinction requires consolidation and retrieval of new memories (Berman and Dudai 2001). The main difference between fear conditioning and extinction experiments is that the situation is completely new, and therefore novel, in the original conditioned fear experiments. The absence of novelty in the extinction protocol and the lack of extinction enhancement for 5-HT3-OE mice might suggest that the enhancement of the context-footshock association depends on the novelty of the learning paradigm. The finding that 5-HT3-OE mice display heightened inspective and inquisitive behavior when exposed to a novel object in an open field further indicates that heightened attention to novelty may contribute to a specific enhancement in the attention to or acquisition of hippocampal-dependent cues. The 5-HT3 receptor may be important in the distinction between learning something new about a previous association and learning something completely new and therefore novel.
5-HT3-OE mice are less anxious in the elevated plus-maze. Using an ethological version of the elevated plus-maze, Rodgers et al. (1995) determined that anxiogenesis was indicated for a low concentration of the 5-HT3 receptor antagonist ondansetron, supporting an anxiolytic role of the 5-HT3 receptor. Other studies have reported conflicting results. It appears that differences in paradigms (Gonzalez et al. 1998) and even in parameters within a given paradigm (Nevins and Anthony 1994) are differentially sensitive to the anxiety modifying-effects at this receptor. Here we report that overexpression of the 5-HT3 receptor is anxiolytic in both the elevated plus-maze and the second segment of the novelty exploration paradigm. As behavior within the initial 5-min segment of the novelty exploration task can be considered a classic open field test, and because there was no difference in behavior within the center area due to genotype, it appears that overexpression of the 5-HT3 receptor is not inherently anxiolytic in all behavioral tests of anxiety. Although fearful reactions are generally considered to be controlled by the amygdala (Rogan and LeDoux 1996; Davis and Whalen 2001), the hippocampal formation also is important in anxious behaviors (Gonzalez et al. 1998; File et al. 2000). In fact, the hippocampal formation is thought to be capable of coding critical aspects of anxiety, allowing for the integration of anxiety states and learning cues. The present results suggest that enhanced hippocampal-dependent learning observed for 5-HT3-OE mice is not confounded by a concomitant increase in anxiety. Whereas anti-anxiety drugs that enhance the effects of GABA tend to reduce learning, antianxiety agents which act via the serotonin system do not suppress learning in humans (Korneyev 1997) or long-term potentiation (LTP) in rodent (Mori et al. 2001). Therefore, a learning enhancement in 5-HT3-OE mice, in the face of an anxiolytic affect, fits with the idea that anxiolytics that affect the serotonin system do not impair learning and memory.
It is becoming increasingly obvious that the 5-HT3 receptor is a valuable receptor to target for drug development (Reeta et al. 1999; Johnson et al. 2000; Ye et al. 2001), so it is necessary to understand the role of this receptor in behavior. Our present results suggest that overexpression of the 5-HT3 receptor results in decreased anxiety and subsequent, or independently enhanced, inquisitive behavior in response to novel stimuli, which may influence hippocampal-dependent learning and memory as well as attention. The overexpressed 5-HT3 receptor may contribute to hippocampal functioning and learning and memory by enhancing the contrast between predictive and irrelevant stimuli in learning paradigms.
MATERIALS AND METHODS
Animals
All of the procedures employed in the current studies were approved by the University of New Mexico Laboratory Animal Care and Use Committee. 5-HT3-OE mice were developed by our laboratory and are described elsewhere (Engel et al. 1998). WT mice are transgene-negative littermates. The genetic background of WT and 5-HT3-OE mice is B6SJL/F2 (B6xSJL/F1 × B6xSJL/F1). Mice were housed 2–4 per cage in a room with a 12-h light/dark cycle (lights on at 0800 h). Standard chow and water were available ad libitum. Male and female mice, 60–120-d-old, were used in the present experiments. Behavioral testing was conducted between 0800 and 1500 h.
Fear Conditioning
Fear conditioning took place in a Coulbourn Habitest Modular Test System with a stainless steel grid floor for administration of the footshock. The apparatus was located within a sound-attenuated chamber. After the removal of each mouse from the training context, 70% isopropanol was used to clean the walls and floor of the module. Conditioning protocols were adapted from those described by Paylor et al. (1994). Mice were assigned to two groups, paired tone shock (PTS) or immediate shock (IS). For PTS training, groups were composed of 5 naïve animals × 2 genders × 2 genotypes. For IS training, groups consisted of 4 naïve animals × 2 genders × 2 genotypes. Figure 1A,B depicts the training paradigms. The tone conditioned stimulus was an 80-db, 6-Hz clicker. An unconditioned stimulus of an electric footshock (0.6 mA) was used. The IS and PTS training took 4.5 min each. Twenty-four h after training, mice were reintroduced to the conditioning context for the context retention test and scored by an observer blind to genotype. The conditioning context was again cleaned with 70% isopropanol between animals. The tone and the footshock were not delivered during this test session. The animal's behavior was observed and contextual conditioning was assessed using a time-sampling procedure. Every 10 sec, the mouse was scored as either moving or freezing. Freezing is the conditioned response measured to reflect the amount of learning and is defined by the absence of movement other than that required for respiration (Paylor et al. 1994). Approximately 2 h later, freezing to an altered context (in a clear plastic container with orange-scented bedding) and to the tone in this altered context was assessed with the time-sampling procedure by an observer blind to genotype. The altered context was cleaned with 70% ethanol after each mouse instead of isopropanol to ensure that all olfactory cues were different in the altered context from the conditioning context. Freezing was scored for 3 min without presentation of the tone to determine levels of freezing in response to the altered context itself. The tone then came on and remained on while freezing was scored for an additional 3 min. The amount of freezing is represented as the % freezing (# of freezing intervals ÷ total intervals). Four mice were removed from the PTS contextual test data set. One OE male was removed as a jumping response predominated on the testing day, one OE female was removed due to a lack of movement in the corner of the context resulting in a 100% freezing response, and two WT female mice also exhibited jumping behavior on the testing day. A three-way (training × gender × genotype) analysis of variance (ANOVA) was used to analyze the context and tone test data.
Ondansetron, 0.5 and 1.0 mg/kg (crushed tablets of Zofran [ondansetron hydrochloride] Cerenex Pharmaceuticals in saline) and saline were administered intraperitoneally (i.p.) 45 min prior to training in a separate experiment using 4 animals × 2 genders × 2 genotypes × 3 treatments. Testing was carried out as described above. A three-way (gender × genotype × injection) ANOVA was used to analyze the data.
Extinction
5-HT3-OE and WT mice were trained as described for PTS fear conditioning. Two groups were assigned for extinction, one for extinction to the context and one for extinction to the tone. Groups were composed of 3 naïve animals × 2 genders × 2 genotypes × 2 extinction conditions. Freezing to the context was assessed as described for fear conditioning once every 24 h for the context extinction group. Freezing to the tone was assessed, again as previously described, once every 24 h for the tone extinction group. When mice displayed basal levels of freezing, the testing was halted. Data for each mouse were normalized to the level of freezing during the first test and expressed as a percentage of the first test. A three-way ANOVA (day × gender × genotype) was used to assess extinction to the context and to the tone.
Elevated Plus-Maze
Mice were placed in the center square (6 × 6 cm) of a Plexiglas maze shaped in a cross elevated 2 ft above the ground. The supports that elevate the maze were made of clear Plexiglas and were positioned in the middle of the arms so that the mouse was unable to detect them. The maze had two open arms (30 × 6 cm each) and was located in a moderately lit, sound attenuated room. Open arms consisted of the clear Plexiglas floor and no walls. The closed arms were covered with black contact paper and had walls 6-cm high, also covered with black contact paper. Groups were composed of 3 naïve animals × 2 genders × 2 genotypes. An observer blind to genotype monitored behavior for 5 min with the aid of a video camera. The percentage of time spent in the open arms, closed arms, and center area as well as the number of entries (two front paws) into the open and closed arms was recorded. Unpaired t-tests were used to assess the effect of genotype on anxious behaviors.
Pain Sensitivity
Procedures were previously described by Crawley (1999). Responses to the footshock were assessed after mice had been extinguished in the context. Mice had been given 2 wks of rest after the 24-h extinction paradigm reported here, as well as a 2-h extinction paradigm being explored concurrently and not reported here, and were used to assess sensitivity to footshock. Groups consisted of 6 animals × 2 genders × 2 genotypes. The intensity of shock required to elicit running, vocalization, and a jump was determined by an observer blind to genotype for each of the 24 animals by delivering a 1-sec shock every 30 sec starting at 0.08 mA and increasing the shock by 0.02 mA each time. The maximum intensity tested was 0.20 mA. A three-way ANOVA (response × gender × genotype) was used to analyze shock reactivity.
Latent Inhibition
Procedures were adapted from those described by Caldarone et al. (2000). Figure 4A illustrates the paradigm used. WT and 5-HT3-OE mice were separated into three groups and pre-exposed to the training context alone (context group), or to the context with presentation of the tone (context-tone group), or received no pre-exposure of context or tone. Groups consisted of 4 naïve animals × 2 genders × 2 genotypes × 3 pre-exposure conditions. The conditioning chamber was cleaned with 70% isopropanol after the removal of each mouse during pre-exposure. Twenty-four h after pre-exposure, mice from each pre-exposure condition and the no pre-exposure group were placed in the conditioning context for fear conditioning (2-min+40-sec training session) as described in Figure 4A. The apparatus was again cleaned with 70% isopropanol between animals. The day after training, mice were observed for a freezing response to the tone as described for fear conditioning. On the following day, mice were scored for freezing to the original conditioning context (where both the pre-exposure and training had occurred), also as described for fear conditioning. A three-way ANOVA (pre-exposure × gender × genotype) was used to assess freezing to the tone and to the context after training in the LI paradigm.
Acoustic Prepulse Inhibition of Tactile Startle Response
Testing was conducted in the SR-Lab System (San Diego Instruments) startle apparatus as previously described by Paylor and Crawley (1997). The background noise level of the chamber was 70 dB. Mice previously used for 2-h extinction tests not reported here were used in all PPI tests. Mice had been allowed 2 wks between the last day of extinction testing and the PPI test. Groups were composed of 5 animals × 2 gender × 2 genotypes. Each test session began with a 5-min acclimation period, after which each subject was presented with 60 trials (10 trial types repeated six times pseudorandomly). Trial types were: one 40-msec, 12-psi tactile (air puff to the back of the animal) startling stimulus; four prepulse trials of 78, 82, 86, and 90 dB (20 msec) followed by the tactile stimulus (100 msec after onset of the prepulse); four trials of 78, 82, 86, or 90 dB (40 msec); and one trial with no stimuli. The average intertrial interval (ITI) was 15 sec and ranged from 10–20 sec. The startle response was recorded for 65 msec beginning at the stimulus onset. Maximum startle values for this time period were recorded and analyzed using a macro created in Excel. Percent prepulse inhibition of the startle response was calculated as 100 – ([prepulse trial/tactile startling stimulus] × 100). A high percent prepulse inhibition value reflects strong prepulse inhibition. A three-way ANOVA (prepulse intensity × gender × genotype) was used to assess inhibition of the startle response in the presence of a prepulse. A two-way ANOVA (genotype × gender) was used to assess startle in the absence of the prepulse.
Novel Object Exploration
The test was adapted from the protocol described by Grailhe et al. (1999). An open field apparatus was used measuring 17″ × 17″ with 8″-high Plexiglas side-walls. The floor of the apparatus was black and divided into five areas: four equal quadrants and one center area with a diameter of 14 cm. The experiments were carried out in a dimly lit room with the aid of a video camera to minimize any effects of stress/anxiety. An observer blind to genotype analyzed the tapes. The floor and walls of the open field were wiped with 70% isopropanol before each test session. For 5-HT3-OE mice, 13 naïve animals were used, 6 female and 7 male. For WT mice, 18 naïve animals were used, 9 female and 9 male. The test session consisted of two 5-min periods. For the first five min, mice were placed into the center area and latency to leave the center was recorded, the time spent in the center was assessed, and the total number of center line crosses and the number of total lines crossed were tallied. During the second 5 min, the same measures were taken after introduction of a novel object into the center. Latency to approach the object was recorded. The object was a pink and green striped gray cube (1 inch3) with an open side. The cube was placed in the center with the open side facing the mouse. Two WT male mice were taken out of the analysis of latency as the initial measurement was not caught on tape. One WT male mouse was also removed from the analysis of time in the center due to observer error with a timer. A two-way ANOVA (genotype × gender) was used to analyze each of the four measures of reactivity to the novel environment (initial segment score, first five min) and to analyze the same four measures of reactivity to the novel object using a difference score of the second 5-min segment minus the initial 5-min segment.
Acknowledgments
This work was supported by an Army Grant, DAMD 17-01-1-0680.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18USC section 1734 solely to indicate this fact.
Article and publication are at http://www.learnmem.org/cgi/doi/10.1101/lm.56103.
References
- Allan, A.M., Galindo, R., Chynoweth, J., Engel, S.R., and Savage, D.D. 2001. Conditioned place preference for cocaine is attenuated in mice overexpressing the 5-HT3 receptor. Psychopharmacology 158: 18–27. [DOI] [PubMed] [Google Scholar]
- Barnes, J.M., Barnes, N.M., Champaneria, S., Costall, B., and Naylor, R.J. 1990a. Characterisation and autoradiographic localisation of 5-HT3 receptor recognition sites identified with [3H]-(S)-Zacopride in the forebrain of the rat. Neuropharmacology 29: 1037–1045. [DOI] [PubMed] [Google Scholar]
- Barnes, J.M., Costall, B., Coughlan, J., Domeney, A.M., Gerrard, P.A., Kelly, M.E., Naylor, R.J., Onaivi, E.S., Tomkins, D.M., and Tyers, M.B. 1990b. The effects of ondansetron, a 5-HT3 receptor antagonist, on cognition in rodents and primates. Pharmacol. Biochem. Behav. 35: 955–962. [DOI] [PubMed] [Google Scholar]
- Berman, D.E. and Dudai, Y. 2001. Memory extinction, learning anew, and learning the new: Dissociations in the molecular machinery of learning in cortex. Science 291: 2417–2419. [DOI] [PubMed] [Google Scholar]
- Bratt, A.M., Kelly, M.E., Domeney A.M., Naylor, R.J., and Costall, B. 1994. Ondansetron fails to attenuate a scopolamine-induced deficit in a Stone maze task. Neuroreport 5: 1921–1924. [DOI] [PubMed] [Google Scholar]
- Burgin, K.E., Waxham, M.N., Rickling, S., Westgate, S.A., Mobley, W.C., and Kelly, P.T. 1991. In situ hybridization histochemistry of Ca2+/calmodulin-dependent protein kinase in developing rat brain. J. Neurosci. 10: 1788–1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldarone, B.J., Duman, C.H., and Picciotto, M.R. 2000. Fear conditioning and latent inhibition in mice lacking the high affinity subclass of nicotinic acetylcholine receptors in the brain. Neuropharmacology 39: 2779–2784. [DOI] [PubMed] [Google Scholar]
- Campbell, A.D., Kohl, R.R., and McBride, W.J. 1996. Serotonin-3 receptor and ethanol-stimulated somatodendritic dopamine release. Alcohol 13: 569–574. [DOI] [PubMed] [Google Scholar]
- Carli, M., Luschi, R., and Samanin, R. 1997. Dose-dependent impairment of spatial learning by intrahippocampal scopolamine: Antagonism by ondansetron, a 5-HT3 receptor antagonist. Behav. Brain Res. 82: 185–194. [DOI] [PubMed] [Google Scholar]
- Consolo, S., Bertorelli, R., Russi, G., Zambelli, M., and Ladinsky, H. 1994. Serotonergic facilitation of acetylcholine release in vivo from rat dorsal hippocampus via serotonin 5-HT3 receptors. J Neurochem. 62: 2254–2261. [DOI] [PubMed] [Google Scholar]
- Corcoran, K.A. and Maren, S. 2001. Hippocampal inactivation disrupts contextual retrieval of fear memory after extinction. J. Neurosci. 21: 1720–1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costall, B. and Naylor, R.J. 1992. The psychopharmacology of 5-HT3 receptors. Pharmacol. Toxicol. 71: 401–415. [DOI] [PubMed] [Google Scholar]
- Crawley, J.N. 1999. Behavioral phenotyping of transgenic and knockout mice: Experimental design and evaluation of general health, sensory functions, motor abilities, and specific behavioral tests. Brain Res. 835: 18–26. [DOI] [PubMed] [Google Scholar]
- Davies, P.A., Pistis, M., Hanna, M.C., Peters, J.A., Lambert, J.J., Hales, T.G., and Kirkness, E.F. 1999. The 5-HT3B subunit is a major determinant of serotonin-receptor function. Nature 397: 359–363. [DOI] [PubMed] [Google Scholar]
- Davis, M. and Whalen, P.J. 2001. The amygdala: Vigilance and emotion. Mol. Psychiatry 6: 13–34. [DOI] [PubMed] [Google Scholar]
- Derkach, V., Surprenant, A., and North, R. 1989. 5-HT3 receptors are membrane ion channels. Nature 339: 706–709. [DOI] [PubMed] [Google Scholar]
- Dubin, A.E., Huvar, R., D'Andrea, M.R., Pyati, J., Zhu, J.Y., Joy, K.C., Wilson, S.J., Galindo, J.E., Glass, C.A., Luo, L., et al. 1999. The pharmacological and functional characteristics of the serotonin 5-HT3A receptor are specifically modified by a 5-HT3B receptor subunit. J. Biol. Chem. 274: 30799–30810. [DOI] [PubMed] [Google Scholar]
- Dulawa, S.C. and Geyer, M.A. 2000. Effects of strain and serotonergic agents on prepulse inhibition and habituation in mice. Neuropharmacology 39: 2170–2179. [DOI] [PubMed] [Google Scholar]
- Engel, S.R. and Allan, A.M. 1999. 5-HT3 receptor overexpression enhances ethanol sensitivity in mice. Psychopharmacology 144: 411–415. [DOI] [PubMed] [Google Scholar]
- Engel, S.R., Lyons, C.R., and Allan, A.M. 1998. 5-HT3 receptor overexpression decreases ethanol self-administration in transgenic mice. Psychopharmacology 140: 243–248. [DOI] [PubMed] [Google Scholar]
- File, S.E., Kenny, P.J., and Cheeta, S. 2000. The role of the dorsal hippocampal serotonergic and cholinergic systems in the modulation of anxiety. Pharmacol. Biochem. Behav. 66: 65–72. [DOI] [PubMed] [Google Scholar]
- Giovannini, M.G., Ceccarelli, I., Molinari, B., Cecchi, M., Goldfarb, J., and Blandina, P. 1998. Serotonergic modulation of acetylcholine release from cortex of freely moving rats. J. Pharmacol. Exp. Ther. 285: 1219–1225. [PubMed] [Google Scholar]
- Gonzalez, L.E., Ouagazzal, A.-M., and File, S.E. 1998. Stimulation of benzodiazepine receptors in the dorsal hippocampus and median raphe reveals differential GABAergic control in two animal tests of anxiety. Eur. J. Neurosci. 10: 3673–3680. [DOI] [PubMed] [Google Scholar]
- Gould, T.J. and Wehner, J.M. 1999. Genetic influences on latent inhibition. Behav. Neurosci. 113: 1291–1296. [DOI] [PubMed] [Google Scholar]
- Grailhe, R., Waeber, C., Dulawa, S.C., Hornung, J.P., Zhuang, X., Brunner, D., Geyer, M.A., and Hen, R. 1999. Increased exploratory activity and altered response to LSD in mice lacking the 5-HT5A receptor. Neuron 22: 581–591. [DOI] [PubMed] [Google Scholar]
- Hong, E. and Meneses, A. 1996. Systemic injection of p-chloroamphetamine eliminates the effect of the 5-HT3 compounds on learning. Pharmacol. Biochem. Behav. 53: 765–769. [DOI] [PubMed] [Google Scholar]
- Hope, A.G., Downie, D.L., Sutherland, L., Lambert, J.J., Peters, J.A., and Burchell, B. 1993. Cloning and functional expression of an apparent splice variant of the murine 5-HT3 receptor A subunit. Eur. J. Pharmacol. 245: 187–192. [DOI] [PubMed] [Google Scholar]
- Hornung, J. and Celio, M. 1992. The selective innervation by serotonergic axons of calbindin-containing interneurons in the neocortex and hippocampus of the marmoset. J. Comp. Neurol. 320: 457–467. [DOI] [PubMed] [Google Scholar]
- Johnson, B.A., Roache, J.D., Javors, M.A., DiClemente, C.C., Cloninger, C.R., Prihoda, T.J., Bordnick, P.A., Ait-Daoud, N., and Hensler, J. 2000. Ondansetron for reduction of drinking among biologically predisposed alcoholic patients: A randomized controlled trial. JAMA 284: 963–971. [DOI] [PubMed] [Google Scholar]
- Johnson, D.S. and Heinemann, S.F. 1995. Embryonic expression of the 5-HT3 receptor subunit, 5-HT3R-A, in the rat: An in situ hybridization study. Mol. Cell. Neurosci. 6: 122–138. [DOI] [PubMed] [Google Scholar]
- Khakh, B.S. and Henderson, G. 2000. Modulation of fast synaptic transmission by presynaptic ligand-gated cation channels. J. Auton. Nerv. Syst. 81: 110–121. [DOI] [PubMed] [Google Scholar]
- Kilpatrick, G.J., Jones, B.J., and Tylers, M.B. 1987. Identification and distribution of 5-HT3 receptors in rat brain using radioligand binding. Nature 330: 746–748. [DOI] [PubMed] [Google Scholar]
- Kim, J.J. and Fanselow, M.S. 1992. Modality-specific retrograde-amnesia of fear. Science 256: 675–677. [DOI] [PubMed] [Google Scholar]
- Kojima, N., Wang, J., Mansuy, I.M., Grant, S.G., Mayford, M., and Kandel, E.R. 1997. Rescuing impairment of long-term potentiation in fyn-deficient mice by introducing Fyn transgene. Proc. Natl. Acad. Sci. 94: 4761–4765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korneyev, A.Y. 1997. The role of the hypothalamic-pituitary-adrenocortical axis in memory-related effects of anxiolytics. Neurobiol. Learn. Mem. 67: 1–13. [DOI] [PubMed] [Google Scholar]
- Lattal, K.M. and Abel, T. 2001. An immediate-shock freezing deficit with discrete cues: A possible role for unconditioned stimulus processing mechanisms. J. Exp. Psychol. Anim. Behav. Process. 27: 394–406. [PubMed] [Google Scholar]
- MacDermott, A.B., Role, L.W., and Siegelbaum, S.A. 1999. Presynaptic ionotropic receptors and the control of transmitter release. Annu. Rev. Neurosci. 22: 443–485. [DOI] [PubMed] [Google Scholar]
- Maren, S. 2001. Neurobiology of Pavlovian fear conditioning. Annu. Rev. Neurosci. 24: 897–931. [DOI] [PubMed] [Google Scholar]
- Maricq, A.V., Peterson, A.S., Brake, A.J., Myers, R.M., and Julius, D. 1991. Primary structure and functional expression of the 5-HT3 receptor, a serotonin-gated ion channel. Science 254: 432–437. [DOI] [PubMed] [Google Scholar]
- Matsumoto, M., Yoshioka, M., Togashi, H., Tochihara, M., Ikeda, T., and Saito, H. 1995. Modulation of norepinephrine release by serotonergic receptors in the rat hippocampus as measured by in vivo microdialysis. J. Pharmacol. Exp. Ther. 272: 1044–1051. [PubMed] [Google Scholar]
- McMahon, L.L. and Kauer, J.A. 1997. Hippocampal interneurons are excited via serotonin-gated ion channels. J. Neurophys. 78: 2493–2502. [DOI] [PubMed] [Google Scholar]
- Monk, S.A., Desai, K., Brady, C.A., Williams, J.M., Lin, L., Princivalle, A., Hope, A.G., and Barnes, N.M. 2001. Generation of selective 5-HT3B subunit recognizing polyclonal antibody; identification of immunoreactive cells in rat hippocampus. Neuropharmacology 41: 1013–1016. [DOI] [PubMed] [Google Scholar]
- Morales, M. and Bloom, F.E. 1997. The 5-HT3 receptor is present in different subpopulations of GABAergic neurons in the rat telencephalon. J. Neurosci. 17: 3157–3167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales, M. and Wang, S.-D. 2002. Differential composition of 5-hydroxytryptamine3 receptors synthesized in rat CNS and peripheral nervous system. J. Neurosci. 22: 6732–6741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran, P.M. and Moser, P.C. 1992. MDL 73,147EF, a 5-HT3 antagonist, facilitates latent inhibition in the rat. Pharmacol. Biochem. Behav. 42: 519–522. [DOI] [PubMed] [Google Scholar]
- Mori, K., Togashi, H., Kojima, T., Matsumoto, M., Ohashi, S., Ueno, K., and Yoshioka, M. 2001. Different effects of anxiolytic agents, diazepam and the 5-HT1A agonist tandospirone, on hippocampal long-term potentiation in vivo. Pharmacol. Biochem. Behav. 69: 367–372. [DOI] [PubMed] [Google Scholar]
- Nayak, S.V., Ronde, P., Spier, A.D., Lummis, S.C.R., and Nichols, R.A. 1999. Calcium changes induced by presynaptic 5-hydroxytryptamine-3 serotonin receptors on isolated terminals from various regions of the rat brain. Neurosci. 91: 107–117. [DOI] [PubMed] [Google Scholar]
- Nevins, M.E. and Anthony, E.W. 1994. Antagonists at the serotonin-3 receptor can reduce the fear-potentiated startle response in the rat: Evidence for different types of anxiolytic activity? J. Pharmacol. Exp. Ther. 268: 248–254. [PubMed] [Google Scholar]
- Ortells, M.O. and Lunt, G.G. 1995. Evolutionary history of the ligand-gated ion-channel superfamily of receptors. Trends Neurosci. 18: 121–127. [DOI] [PubMed] [Google Scholar]
- Paylor, R. and Crawley, J.N. 1997. Inbred strain differences in prepulse inhibition of the mouse startle response. Psychopharmacology 132: 169–180. [DOI] [PubMed] [Google Scholar]
- Paylor, R., Tracy, R., Wehner, J., and Rudy, J.W. 1994. DBA/2 and C57BL/6 mice differ in contextual fear but not auditory fear conditioning. Behav. Neurosci. 108: 810–817. [DOI] [PubMed] [Google Scholar]
- Phillips, R.G. and LeDoux, J.E. 1992. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav. Neurosci. 106: 274–285. [DOI] [PubMed] [Google Scholar]
- Reeta, K.H., Handu, S.S., Sharma, D., and Bhargava, V.K. 1999. Effects of 5-HT3 antagonist ondansetron on learning and memory in rats. Indian J. Pharmacol. 31: 285–289. [Google Scholar]
- Rodgers, R.J., Cole, J.C., and Tredwell, J.M. 1995. Profile of action of 5-HT3 receptor antagonists, ondansetron and WAY 100289, in the elevated plus-maze test of anxiety of mice. Psychopharmacology 117: 306–312. [DOI] [PubMed] [Google Scholar]
- Rogan, M.T. and LeDoux, J.E. 1996. Emotion: Systems, cells, synaptic plasticity. Cell 85: 469–475. [DOI] [PubMed] [Google Scholar]
- Roychoudhury, M. and Kulkarni, S.K. 1997. Effects of ondansetron on short-term memory retrieval in mice. Methods Find. Exp. Clin. Pharmacol. 19: 43–46. [PubMed] [Google Scholar]
- Sung, K.-W., Engel, S.R., Allan, A.M., and Lovinger, D.M. 2000. 5HT3 receptor function and potentiation by alcohols in frontal cortex neurons from transgenic mice overexpressing the receptor. Neuropharmacology 19: 2346–2351. [DOI] [PubMed] [Google Scholar]
- Tecott, L.H., Maricq, A.V., and Julius, D. 1993. Nervous-system distribution of the serotonin 5-HT3 receptor mRNA. Proc. Natl. Acad. Sci. 90: 1430–1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hooft, J.A. and Vijverberg, H.P.M. 2000. 5-HT3 receptors and neurotransmitter release in the CNS: A nerve ending story? Trends Neurosci. 23: 605–610. [DOI] [PubMed] [Google Scholar]
- Werner, P., Kawashima, E., Reid, J., Hussy, N., Lundsrom, K., Buell, G., Humbert, Y., and Jones, K.A. 1994. Organization of the mouse 5-HT3 receptor gene and functional expression of two splice variants. Mol. Brain Res. 26: 233–241. [DOI] [PubMed] [Google Scholar]
- Wilkinson, L.S., Humby, T., Killcross, S., Robbins, T.W., and Everitt, B.J. 1996. Dissociations in hippocampal 5-hydroxytryptamine release in the rat following Pavlovian aversive conditioning to discrete and contextual stimuli. Eur. J. Neurosci. 8: 1479–1487. [DOI] [PubMed] [Google Scholar]
- Ye, J.H., Ponnudurai, R., and Schaefer, R. 2001. Ondansetron: A selective 5-HT3 receptor antagonist and its applications in CNS-related disorders. CNS Drug Rev. 7: 199–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, W.N., Bast, T., and Feldon J. 2001. The ventral hippocampus and fear conditioning in rats: Different anterograde amnesias of fear after infusion of N-methyl-D-aspartate or its noncompetitive antagonist MK-801 into the ventral hippocampus. Behav. Brain Res. 126: 159–174. [DOI] [PubMed] [Google Scholar]