Abstract
Purpose
RTOG protocol 95-17 was a phase I/II trial to evaluate multi-catheter brachytherapy as the sole method of adjuvant breast radiotherapy for stage I/II breast carcinoma following breast conserving surgery. Low or high dose rate sources were allowed. Dose prescription and treatment evaluation were based on recommendations in ICRU Report 58, and included the parameters mean central dose (MCD), average peripheral dose, dose homogeneity index (DHI), and the dimensions of the low and high dose regions.
Methods and Materials
Three levels of quality assurance were implemented: (1) Credentialing of institutions was required prior to entering patients onto the study. (2) Rapid review of each treatment plan was conducted prior to treatment, and (3) Retrospective review was performed by the Radiological Physics Center in conjunction with the study chairman and RTOG dosimetry staff.
Results
Credentialing focused on the accuracy of dose calculation algorithm and compliance with protocol guidelines. Rapid review was designed to identify and correct deviations from the protocol prior to treatment. The retrospective review involved recalculation of dosimetry parameters and review of dose distributions to evaluate the treatment. Specifying both central and peripheral doses resulted in uniform dose distributions, with a mean dose homogeneity index of 0.83 ±0.06.
Conclusions
Vigorous quality assurance resulted in a high-quality study with few deviations; only 4 of 100 patients were judged as minor variations from protocol and no patient was judged a major deviation. This study should be considered a model for quality assurance of future trials.
Keywords: partial breast irradiation, quality assurance, clinical trials, brachytherapy
Introduction
The treatment of breast cancer has evolved considerably over the last several decades, and techniques for conserving the breast have become the predominant methods of treating Stage I and II carcinoma. Several prospective randomized trials have demonstrated that breast conserving surgery and radiation therapy conferred comparable rates of overall survival and distant disease-free survival when compared with conventional mastectomy.[1,2] Breast conserving therapy is appealing to patients for the superior cosmetic results and reduced physical and emotional trauma compared to a mastectomy. However, as administered in the trials referred to above, the treatment regimen consists of surgery followed by a course of whole-breast radiation therapy of at least five weeks duration, which imposes substantial inconvenience on the patient.
Several studies were conducted subsequently to evaluate the pattern of recurrence after surgery alone. These studies indicated that relapses were invariably in the immediate vicinity of the lumpectomy cavity.[3-5] Consequently, the requirement for whole-breast irradiation was questioned and it was postulated that the benefit obtained from adjuvant radiation therapy might be preserved if the treatment were limited to the tissue immediately surrounding the lumpectomy bed. With a smaller treatment volume, larger doses per fraction could be utilized, and treatment could be delivered in a short time frame immediately after lumpectomy. Interstitial brachytherapy was postulated as a suitable treatment modality because it could deliver a high dose to the volume at risk for recurrence while maintaining the dose to the remainder of the breast at a low level.
Further, brachytherapy delivered at a constant low dose rate over several days was believed to provide superior clinical results in comparison to conventional fractionated radiation. [6] High-dose rate brachytherapy was believed to yield similar results when delivered in a fractionation scheme that mimicked low-dose rate regimens. [7] Interstitial brachytherapy was shown to be associated with acceptable cosmetic outcome [8] and low complication rates [9].
RTOG 95-17
The conclusions reached above encouraged the RTOG to develop and conduct protocol 95-17, for which a toxicity analysis has recently been reported. [10] This was a phase I/II trial to evaluate the technical feasibility, reproducibility of treatment delivery, cosmetic results, complication rates and local control rate of brachytherapy used as the sole method of adjuvant radiotherapy for patients with Stage I and II breast carcinoma following breast conserving surgery. Patients first received either tylectomy or reexcision of their original tumors, during which 6 clips were to be placed in the tylectomy cavity to define the target volume. Catheters then were placed for brachytherapy, arranged in two parallel planes along the superior and inferior aspects of the lumpectomy bed. The patients received either low-dose rate (LDR) or high-dose rate (HDR) brachytherapy. LDR brachytherapy was given with 192Ir seeds and a dose of 45 Gy was delivered over an elapsed time ranging from 3.5 to 6 days. HDR brachytherapy was given in a similar geometry, with 34 Gy delivered in 10 fractions, two fractions per day, in 5 to 7 days. The two fractions were spaced 6 hours apart.
Through discussions among the study chair, the RTOG headquarters dosimetry group and the Radiological Physics Center (RPC), the trial was designed with a three-tier QA program to ensure high quality and consistency of treatment delivered. The RPC as well as the protocol chair and physicist were responsible for the QA procedures.
This paper reviews the QA program associated with this trial, and describes the effectiveness of credentialing physicians and institutions prior to their participation.
The Radiological Physics Center
The RPC was established in the late 1960s as a resource in radiation dosimetry and physics for cooperative clinical trial groups and the radiation therapy facilities that deliver radiation treatment to patients entered into cooperative group protocols. The primary responsibility of the RPC is to assure NCI and the cooperative groups that participating institutions have adequate quality assurance procedures and no major systematic dosimetry discrepancies, so that they can be expected to deliver radiation treatments that are clinically comparable to those delivered by other institutions in the cooperative groups. To accomplish this, the RPC monitors the basic machine output and brachytherapy source strengths, the dosimetry data utilized by the institutions, the calculation algorithms used for treatment planning, and the institutions' QA procedures. The methods of monitoring include on-site dosimetry reviews by an RPC physicist and a variety of remote auditing tools. The remote auditing tools include (a) mailed dosimeters (TLD) evaluated on a periodic basis to verify output calibration and simple questionnaires to document changes in personnel, equipment, and dosimetry practices; (b) comparison of dosimetry data with RPC “standard data” to verify the compatibility and acceptability of dosimetry data; (c) evaluation of reference and actual patient calculations to verify the validity of treatment planning algorithms, the consistency of their application, and compliance with protocols; (d) review of the institutions' written quality assurance procedures and records; and (e) mailable anthropomorphic phantoms to verify tumor dose delivery for special treatment techniques.
Chart reviews conducted by the RPC have included rapid review of patient treatment plans prior to treatment, timely review of charts during the conduct of a trial, and retrospective review of patients following their treatment. The results of the reviews are provided to the study chairs, the cooperative groups, and when appropriate and constructive, to the institution.
Patient audit techniques also are essential components of the RPC's credentialing programs. Several cooperative groups have determined that the technologies used in certain clinical trials are sufficiently advanced to warrant credentialing of institutions that wish to participate in these trials. The credentialing procedures range in complexity from a simple registration process, to the completion of knowledge assessment questionnaires, to the performance of treatment planning benchmarks, and in certain situations, to the planning and irradiation of anthropomorphic phantoms.
The RPC works with all of the NCI-sponsored cooperative groups, either directly or in collaboration with the Advanced Technology QA Consortium (ATC), a consortium of five QA offices funded by the NCI.
Methods and Materials
A three-tier QA program was developed and implemented for this protocol. The three tiers consisted of (1) accreditation of institutions prior to participation in the protocol, (2) rapid review of each implant prior to insertion of the sources, and (3) retrospective review of each treatment. In addition, rigorous and quantifiable dose specification and reporting procedures were developed that were based on those recommended in ICRU report 58.[11] The parameters defined in Table I (see also Figures 1 and 2) were to be reported for each case, and were independently re-calculated at the RPC.
Table I.
The parameters used for dose specification and reporting for RTOG 95-17. The parameters are based on those recommended by the ICRU.[11]
| Target Volume, (TV): The TV is a volume enclosed by a surface 2 cm outside the surgical cavity peripherally and 1 cm superficially and 1 cm deep, as defined by the clips. |
| Prescribed Dose: The dose intended by the prescribing physician, and documented in the patient's chart. The Prescribed Dose was to be 45 Gy for LDR implants, and 34 Gy for HDR implants. |
| Peripheral Dose (PD): The minimum target dose, defined by the ICRU as the minimum dose at the periphery of the TV. This would ideally be equal to the Prescribed Dose. |
| Central Plane: The plane through the geometric center of the implant, passing perpendicularly through the catheters. |
| Coronal Plane: The plane parallel to and midway between the two implant planes. |
| Sagittal Plane: The plane orthogonal to the Central and Coronal planes through the center of the implant. |
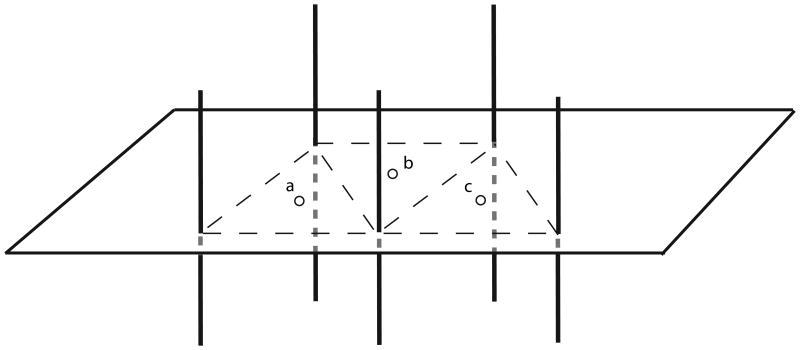
| Mean Central Dose (MCD): Average of all local dose minima, calculated by averaging the geometric center doses (GCD). The GCD is the dose at the center of the triangle formed by adjacent seed trains at their intersection with the central plane (see Figure 1). The MCD is the arithmetic mean of the GCDs. In this protocol, the GCD was modified for an obtuse triangle; the geometric center was determined at the boundary of the triangle rather than outside the triangle as required by the IRCU definition. |
| High Dose Region: The area encompassed by the isodose line corresponding to 150% of the MCD, as measured in the central plane. |
| Low Dose Region: The area within the target volume (TV) encompassed by an isodose line corresponding to 90% of the prescribed dose. |
| Dose Homogeneity Index (DHI): The ratio Prescribed Dose/MCD. |
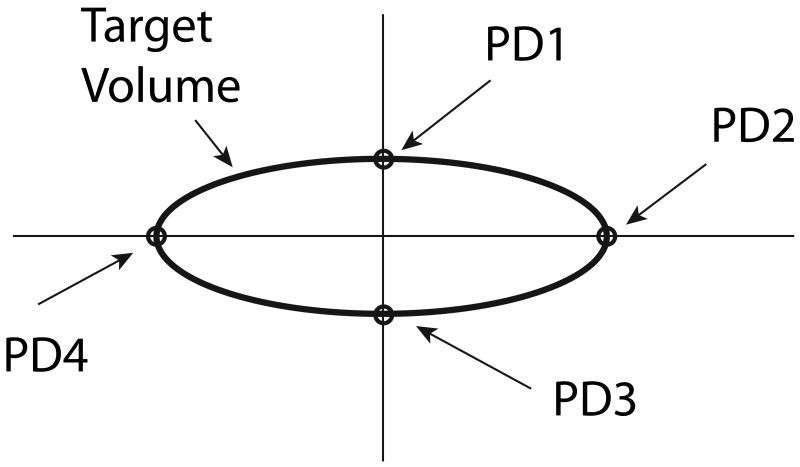
| Average Peripheral Dose (APD): The average of the four doses calculated at the intersections of the coordinate axes with the edges of the TV in a specific plane (see Figure 2). If the peripheral dose at any of the four points exceeded the prescribed dose, the prescribed dose was used in the sum. Therefore, only doses less than the prescribed dose impacted this parameter. |
| Percent Deviation (Dav): The difference between the APD and the Prescribed Dose, expressed as a percentage. D = 100(PD-APD)/PD. The Percent Deviation was calculated in the central plane (Dc) and sagittal plane (Ds) and the two were averaged to obtain Dav = (Dc + Ds)/2. |
1.
Sketch illustrating the determination of Mean Central Dose, MCD, as used in RTOG protocol 95-17. The centroids of the triangles formed by adjacent seed trains (catheters) correspond to the centers of the regions of low dose and low dose gradient between the seed trains, called Geometric Center Dose, GCD. The MCD was defined in the central plane of the implant and is the mean of the GCDs.
2.
Sketch illustrating the definition of Average Peripheral Dose (APD). The TV was depicted as an ellipse determined by the clips, and the axes were approximately centered on the TV. The four peripheral doses were averaged in both the central and sagittal planes.
Dose Specification
A comprehensive set of dose specifications and reporting criteria were established using the concept developed in ICRU report 58. [11] To support the specifications, explicit definitions were provided of the target volume (TV, on this trial the TV was the equivalent of the modern definition of the planning target volume - PTV), mean central dose (MCD), minimum target dose (called peripheral dose in the protocol), dose homogeneity index (DHI), average peripheral dose (APD), and percent deviation (Dav). The definitions are given in Table 1. These also provided quantifiable parameters to assess the treatment.
It should be noted that at the time of this trial, treatment planning for brachytherapy procedures was reported in two dimensions; most institutions at the time were not able to perform 3-D analyses such as dose-volume histograms. Consequently, the parameters identified in Table 1 that institutions were instructed to submit were needed to fully characterize the dose distributions.
Credentialing (Pre-Approval)
The RPC conducted an evaluation of each institution that applied to participate in the protocol. The evaluation consisted of a questionnaire and a benchmark case. The questionnaire requested the following information from each institution:
Institution name, radiation oncologist, medical physicist.
Treatment technique to be used (HDR or LDR, or both), source supplier, and equipment used for brachytherapy.
Dose calculation procedures.
QA procedures used to verify source strength and source position, and confirm dose calculations.
The institutions were also required to provide documentation indicating that they had a source strength verification system that was directly traceable to the National Institute of Standards and Technology (NIST), through an AAPM-Accredited Dosimetry Calibration Laboratory (ADCL). In the case of HDR 192Ir brachytherapy, for which no NIST standard exists, calibration systems were expected to be traceable to the de-facto national standard developed by the University of Wisconsin ADCL.[12]
The demographic items provided were recorded for comparison with patient cases submitted subsequently by each institution. The procedures for calculation and QA were compared by the RPC with published and recommended procedures [13,14,15] and deficiencies were discussed with the study chair and the institution. Institutions were required to provide evidence that deficiencies had been corrected before they were permitted to participate in the protocol.
The benchmark case consisted of an idealized two-plane implant (see figure 3). Each institution was instructed to submit their calculation of this geometric test case. The submitted calculations were compared by the RPC with standard calculations based on published and recommended data for the sources used.[14] These standard calculations were performed with software developed by the RPC, following the formalism expressed by the AAPM's Task Group 43. [14] The institution's calculations of peripheral dose (PD) and mean central dose (MCD) were expected to agree to within 15% with the RPC's calculations. This level of agreement was selected based on the RPC's experience with brachytherapy trials in which steep dose gradients mean more stringent criteria might be unrealistic. Discrepancies between the institution's calculations and the RPC review were resolved before the institution was permitted to participate in the protocol.
3.
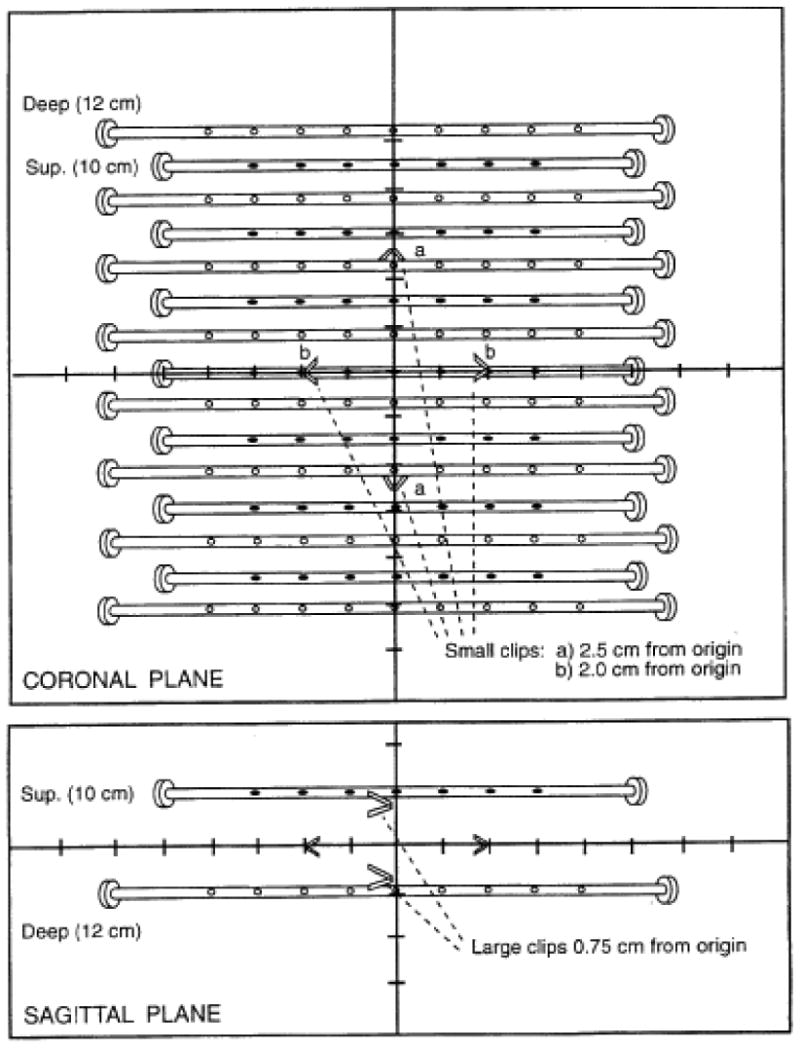
An idealized two-plane implant used as the benchmark case for RTOG protocol 95-17. The tylectomy cavity was marked by clips and provisions for both HDR and LDR were included.
Rapid Review
Each institution was required to submit, at least 24 hours before implanting the sources, the brachytherapy plans prepared for patients treated on this protocol for rapid review by the study chair and a medical physicist. The submitted plans were required to indicate the lumpectomy cavity, the locations of surgical clips, the TV, and the central, sagittal and coronal planes through the midpoint of the TV. The dose distributions were to indicate the prescribed dose, the isodose line corresponding to 90% of the PD, the MCD, and the isodose line corresponding to 150% of the MCD. The dose distributions were evaluated to assure that the reported PD, Dav, and DHI were within the constraints of the protocol as outlined in Table 2. These criteria were scored as either meeting protocol constraints, minor variations, or major deviations. When deficiencies were found, the institutions were contacted and improvements were required prior to initiating treatment.
Table 2.
Criteria for compliance with the protocol.
| Prescribed dose | DHI | Dav | |
|---|---|---|---|
| Per protocol | ± 5 % | ≥ 0.75 | ≤ 10 % |
| Minor protocol variation | 5 % < Rx D ≤ 10 % | 0.65 ≤ DHI < 0.75 | 10 % < Dav ≤ 20 % |
| Major protocol deviation | Rx D > 10 % | < 0.65 | > 20 % |
Retrospective Review
Following completion of treatment of each patient, institutions were required to submit the treatment plan actually used for therapy. This treatment plan was reviewed by the PI, the RPC, and by a dosimetrist at the RTOG. The three reviews were different, and were all directed toward an overall goal of assessing compliance with the protocol.
RPC review
The RPC reviewed the dosimetry submitted by the institution, but did not recalculate the dose distribution; the institution's dose calculations were accepted as the procedures had been reviewed at the time of credentialing. However, the RPC examined the images provided to verify that the margins drawn around the tylectomy cavity complied with the protocol. This review also assured that the sources were placed a minimum of 1 cm below the skin surface. The RPC recalculated the MCD, APD, Dc, Ds, Dav, and DHI using the institution's isodose distributions, and according to the descriptions of these parameters given in Table 1. The RPC then compared these values with the institution's calculations as well as with the requirements of the protocol. This RPC review also identified hot and cold spots.
Joint review
Together, the study chair, the RPC and the RTOG headquarters dosimetry group reviewed the source placement films submitted by each institution to ascertain the locations of the clips marking the tylectomy cavity. The distance between the implanted sources and the skin was again confirmed, and the coverage of the target volume by the implanted sources was verified. The joint review group also examined the hot and cold spots in the dose distribution, and reviewed the dose parameters listed in Table 1 for compliance with the protocol.
Results
Credentialing
The benchmark case was completed by 100% of participating institutions and all cases were evaluated by the RPC to verify that institutions were using adequate dose calculation algorithms and that they were able to correctly determine the MCD and other dosimetry parameters required for this study. In several cases, institutions failed to supply the required information. One institution determined HDR dwell times incorrectly, leading to an unacceptable distribution. One incorrectly accounted for the magnification of the benchmark, while another institution's digitizer was revealed to be incorrectly calibrated. An institution's well ionization chamber was discovered not to have an ADCL calibration. In several other cases, errors were made in calculating the required dosimetry parameters. The institutions were required to demonstrate that they had corrected the discrepancies before they could be credentialed. This exercise allowed the RPC to accept the dose distributions calculated by the participating institutions for each patient registered on the trial.
Twenty-seven institutions requested credentialing for one or both brachytherapy modalities. Of these, 18 institutions were ultimately credentialed to use HDR brachytherapy for the trial, although only 7 institutions actually entered patients on the study. 17 institutions were ultimately approved to use LDR brachytherapy, and 6 actually entered patients using this modality. Some results of the credentialing process are presented in Table 3.
Table 3.
Results of dose comparisons for the benchmark case, obtained during the credentialing process. The RPC calculated doses that were 2 % to 3 % higher on average, with standard deviations of 4 % to 6 %.
| MCD | APDc | APDs | ||
|---|---|---|---|---|
| HDR | Average (RPC/ Inst) | 1.029 | 1.032 | 1.022 |
| Std. Dev. (%) | 4.0 % | 4.2 % | 3.5% | |
| LDR | Average (RPC/ Inst) | 1.020 | 1.022 | 1.011 |
| Std. Dev. (%) | 6.0% | 6.0% | 5.8% | |
Patient Demographics
Implant geometry data for the 100 implants performed on the trial are shown in Table 4. Two-thirds of the patients were treated with HDR and one-third with LDR. On average, about 17 catheters were used regardless of modality. Most implants used two planes, and most HDR implants were optimized to produce ideal dose distributions.
Table 4.
Selected implant data.
| HDR | LDR | ||
|---|---|---|---|
| Patients | 68 | 32 | |
| # of catheters | 16.6 ± 3.3 | 17.0 ± 3.9 | |
| Optimized? | Yes | 40 (59%) | - |
| No | 17 (25%) | - | |
| Unclear | 11 (16%) | - | |
| # of planes | 1 | 0 | 1 (3%) |
| 2 | 48 (71%) | 26 (81%) | |
| 3 | 17 (25%) | 5 (16%) | |
| More | 1 (1.5%) | 0 | |
Rapid Review
Of all rapid reviews, recommendations for changes were made for six of the 68 patients (9%) treated with HDR and for 2 of the 32 patients (6%) treated with LDR. In these cases, the recommendations addressed the dwell times or the placement of dwell positions or sources, to help the institution meet the dose homogeneity requirements. These changes were recommended to bring the treatment plans into compliance with the dosimetry requirements of the protocol. The participating institutions made all requested changes before the patients were treated.
Final Review
A summary of the results of the final review is presented in Table 5. The table indicates excellent agreement in the calculation of mean central dose (MCD); on average the RPC agreed to within 1% with the institutions. No difference was seen in this agreement between HDR and LDR calculations.
Table 5.
Agreement between the RPC and the participating institutions on selected dosimetry parameters.
| HDR | LDR | |
|---|---|---|
| Ratio of MCD:
RPC/Inst. (Average ± st. dev.) |
0.992 ± 4 % | 0.995 ± 3 % |
| DHI (PD/MCD):
RPC/Inst. (Average ± st. dev.) |
0.84 ± 0.06 | 0.81 ± 0.06 |
| Dav:
RPC/Inst. (Average ± st. dev.) |
4.6 % ± 3.9 % | 3.4 % ± 4.5 % |
| Minor variations:
(Individual discrepancies) |
Dav = 16.4 % | DHI = 0.66 |
| Dav = 15.3 % | Cold spot, dead space | |
The large dose gradients often seen in brachytherapy frustrate the selection of a single objective parameter to describe dose. However, the close agreement seen between the RPC and the participating institutions in the calculation of MCD suggests that this parameter might serve as a useful comparison tool.
The RPC calculated the dose homogeneity index (DHI) for each patient. This parameter is an indication of the extent to which the minimum peripheral dose (PD) fell below the mean central dose. Only one patient submitted to the trial failed to meet the “per protocol” threshold of DHI ≥ 0.75 (see Table 2). In this case, the lumpectomy cavity collapsed, bringing two implant planes close together.
The table also indicates the Percent Deviation (Dav) of the peripheral doses from the prescribed dose. This is an indication of the discrepancy between the delivered dose at the periphery of the target, calculated on the major axes of both the coronal and sagittal planes, and the prescribed dose. Dav only reflects peripheral doses that fall below the prescribed dose, and therefore is an indication of a failure to deliver at least the prescribed dose at one or more of the peripheral calculation points. Two patients failed to meet the “per protocol” threshold of Dav ≤ 10 %.
Table 5 further indicates that of the 100 patients enrolled in the protocol, only 4 fell outside the ranges defined in Table 2 as “per protocol”. All qualified as minor variations, and none exceeded the threshold for major deviations. Three of the four were described in the paragraphs above; the fourth treatment plan demonstrated a cold spot exceeding 10 % below the prescribed dose, although the Dav met the protocol specifications. The cold spot resulted from an inadequate and non-uniform distribution of sources, and this poor source arrangement led to the classification of the case as a minor variation.
Discussion
The validity of a cancer clinical trial is dependent in large part on the quality of the treatments delivered by participating institutions. When a large number of submissions fail to meet the requirements of the protocol, the effectiveness of the trial to successfully evaluate its hypothesis is compromised. A large number of major protocol deviations results in an inefficient study and a risk of failure to meet accrual goals. A large number of minor protocol deviations can reduce the accuracy of the measured data, in that the deviations may reflect patients that were not treated in accordance with the protocol in ways that might affect their outcome. These minor deviations clearly can dilute the ability of the trial to prove or disprove the hypothesis with sufficient statistical confidence.
One way of improving the quality of data submitted is through credentialing of institutions in advance of their participation in a clinical trial. Credentialing can consist of very simple procedures, such as the completion of a questionnaire, or it can require more demanding activities such as submission of benchmark treatment plans, or irradiation of anthropomorphic phantoms containing dosimeters that allow comparison between the delivered dose distribution and the institution's own treatment plan.
RTOG 95-17 represents the first cooperative group breast brachytherapy trial in North America, and hence marks a unique milestone in clinical trial implementation and the application of QA procedures. In addition to the stated goals of the trial, RTOG protocol 95-17 evaluated the effectiveness of credentialing on the quality of patient treatments and compliance with the protocol. All participating institutions were required to complete several credentialing procedures to demonstrate their familiarity with the protocol, their ability to meet certain basic QA requirements, and their treatment planning capability. These credentialing procedures were not onerous, and were completed by a total of 27 institutions.
An important component of the QA of this trial, the rapid review process, identified suboptimal plans in only 8% of patients. In each case, the deficiencies were corrected prior to treatment delivery. This documents both the excellent treatment planning of participating institutions on this trial, and the benefit reaped by the patients and the trial itself through implementation of a rapid review QA process.
The implementation of the quality assurance procedures described above clearly enhanced the overall quality of this clinical trial. Overall statistics are not available describing institutional compliance with clinical trials operated by the RTOG and other study groups. However, a review of a number of reports of clinical trials in which figures indicating deviations were reported indicates that most trials experience considerably more than the approximately 4 % minor variations reported here, and many reported a significant rate of major deviations, which were not seen with this protocol [16].
RTOG 9517 used brachytherapy calculation and reporting procedures that, by today's standards, could be considered outdated. Modern treatment planning computer systems allow the automated optimization of brachytherapy plans, the registration of two or more sets of image data, and the calculation and reporting of dose distributions in 3D. The QA procedures described here have been incorporated into more recent trials, including the joint NSABP B-39/ RTOG 0413 partial breast irradiation trial.
The RPC's procedures have been augmented with evaluation of dose-volume parameters now that such capabilities are available at the RPC and at most study group member facilities. Such QA procedures should be considered the minimum necessary to support an advanced-technology clinical trial.
Acknowledgments
This work was supported by several Public Health Service Grants: RPC - U10 CA 10953; RTOG - U10 CA21661; and CCOP - U10 CA35272; awarded by the National Cancer Institute, Department of Health and Human Services.
Footnotes
Conflicts of Interest Notification No potential conflicts of interest exist.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fisher B, Redmond C, Poisson R, et al. NSABP Eight-year results of a randomized clinical trial comparing total mastectomy and lumpectomy with or without irradiation in the treatment of breast cancer. N Engl J Med. 1989;320:822–828. doi: 10.1056/NEJM198903303201302. [DOI] [PubMed] [Google Scholar]
- 2.Veronesi U, Saccozzi R, Del Vechio M, et al. Comparing radical mastectomy with quadrantectomy, axillary dissection, and radiotherapy in patients with small cancers of the breast. N Engl J Med. 1981;305:6–11. doi: 10.1056/NEJM198107023050102. [DOI] [PubMed] [Google Scholar]
- 3.Fisher ER, Sass R, Fisher B, et al. Pathologic findings from the National Surgical Adjuvant Breast Project (Protocol 6): II Relation of local breast recurrence to multicentricity. Cancer. 1986;57:1717–24. doi: 10.1002/1097-0142(19860501)57:9<1717::aid-cncr2820570902>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 4.Crile G, Jr, Esselstyn CB., Jr Factors influencing local recurrence of cancer after partial mastectomy. Cleveland Clin J Med. 1990;57:143–46. doi: 10.3949/ccjm.57.2.143. [DOI] [PubMed] [Google Scholar]
- 5.Liljegren G, Holmberg L, Adami H, et al. Sector resection with or without postoperative radiotherapy for Stage I breast cancer: five-year results of a randomized trial. J Natl CA Inst. 1994;86:717–22. doi: 10.1093/jnci/86.9.717. [DOI] [PubMed] [Google Scholar]
- 6.Hellman S, Harris JR, Levene MB. Radiation therapy of early carcinoma of the breast without mastectomy. Cancer. 1980;46:988. doi: 10.1002/1097-0142(19800815)46:4+<988::aid-cncr2820461323>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 7.Orton CC, Seyedsard M, Somnay A. A Comparison of high and low dose rate remote afterloading for cervix cancer and the importance of fractionation. Int J Radiat Oncol Biol Phys. 1991;21:1425–34. doi: 10.1016/0360-3016(91)90316-v. [DOI] [PubMed] [Google Scholar]
- 8.Vicini F, White J, Gustafson G, et al. The use of iodine-125 seeds as a substitute for iridium-192 seeds in temporary interstitial breast implants. Int J Rad Oncol Biol Phys. 1993;27:561–66. doi: 10.1016/0360-3016(93)90380-e. [DOI] [PubMed] [Google Scholar]
- 9.Kuske RR, Bolton JS, Wilenzick RM, et al. Brachytherapy As The Sole Method of Breast Irradiation in TIS, T1, T2, N0-1 Breast Cancer. Int J Radiat Oncol Biol Phys. 1994;30(S1):245. [Google Scholar]
- 10.Kuske RR, Winter K, Arthur DW, et al. Phase II trial of brachytherapy alone after lumpectomy for select breast cancer: Toxicity analysis of RTOG 95-17, 2006. Int J Radiat Oncol Biol Phys. 2006;65:45–51. doi: 10.1016/j.ijrobp.2005.11.027. [DOI] [PubMed] [Google Scholar]
- 11.Chassagne D, Dutreix A, Ash D, et al. Dose and Volume Specification for Reporting Interstitial Therapy. ICRU Report #58. 1997 [Google Scholar]
- 12.Goetsch SJ, Attix FH, Pearson DW, et al. Calibration of 192Ir high-dose-rate afterloading systems. Med Phys. 1991;18:462. doi: 10.1118/1.596649. [DOI] [PubMed] [Google Scholar]
- 13.Kutcher GJ, Coia L, Gillin M, et al. Comprehensive QA for radiation oncology: Report of the AAPM Radiation Therapy Committee Task Group 40. Med Phys. 1994;21:581–618. doi: 10.1118/1.597316. [DOI] [PubMed] [Google Scholar]
- 14.Nath R, Anderson LL, Luxton G, et al. Dosimetry of interstitial brachytherapy sources: Recommendations of the AAPM Radiation Therapy Committee Task Group No 43. Med Phys. 1995;22:209–234. doi: 10.1118/1.597458. [DOI] [PubMed] [Google Scholar]
- 15.Fraass B, Doppke K, Hunt M, et al. American Association of Physicists in Medicine Radiation Therapy Committee Task Group 53: Quality assurance for clinical radiotherapy treatment planning. Med Phys. 1998;25:1773–1829. doi: 10.1118/1.598373. [DOI] [PubMed] [Google Scholar]
- 16.Lowenstein J, Roll J, Followill D, et al. The value of credentialing. Int J Radiat Oncol Biol Phys. 2006 abstract. [Google Scholar]