Abstract
Autism is a neurodevelopmental disorder involving dysmaturation of widely distributed brain systems. Accordingly, behaviors that depend on distributed systems, such as higher level cognition and sensorimotor control, are compromised in the disorder. The current study investigated alterations in neural systems underlying sensorimotor disturbances in autism. An fMRI investigation was conducted using saccadic and pursuit eye movement paradigms with 13 high-functioning individuals with autism and 14 age- and IQ- matched typically developing individuals. Individuals with autism had reduced activation in cortical eye fields and cerebellar hemispheres during both eye movement tasks. When executing visually guided saccades, individuals with autism had greater activation bilaterally in a frontostriatal circuit including dorsolateral prefrontal cortex, caudate nucleus, medial thalamus, anterior and posterior cingulate cortex, and right dentate nucleus. The increased activation in prefrontal-striatal-thalamocortical circuitry during visually guided saccades indicates that systems typically dedicated to cognitive control may need to compensate for disturbances in lower-level sensorimotor systems. Reduced activation throughout visual sensorimotor systems may contribute to saccadic and pursuit disturbances that have been reported in autism. These findings document that neurodevelopmental disturbances in autism affect widely distributed brain systems beyond those mediating language and social cognition.
Keywords: Autism, Neuroimaging, Eye Movement, Attention, Frontostriatal Systems
1. Introduction
Autism is a neurodevelopmental disorder with multiple associated neurological impairments. MRI morphometry and postmortem studies have revealed altered gray matter volume and abnormal cell density and size in several cortical and subcortical regions (Bailey et al., 1998; Kemper and Bauman, 1998; Courchesne et al., 2001; Casanova et al., 2002; Sparks et al., 2002). Recent MRI studies of autism have documented abnormal white matter growth (Filipek et al., 1992; Herbert et al., 2004; Hendry et al., 2006), which might disrupt organization of long fiber tracts that are essential for integrating activity across brain regions for supporting adaptive behavior. These findings suggest that autism may affect the organization of both local neural circuity and the functional organization of distributed brain systems.
Widely distributed dysmaturation in complex functional brain systems is a pattern that could explain the diverse clinical manifestations, and their variability, in autism. This would include disturbances in sensorimotor as well as higher cognitive functions and social behaviors, because all are dependent upon effective functional organization across widely-distributed brain regions for their integrity. Because neural systems mediating sensorimotor behaviors, such as postural control and eye movements, are well understood, and the input and output to these systems are amenable to precise control and measurement, sensorimotor assessments are well suited to the task of delineating neurophysiological deficits in complex brain systems in autism.
Impairments in postural control have been documented using quantitative laboratory methodology (Molloy et al., 2003; Minshew et al., 2004). Eye movement abnormalities have been also reported in individuals with autism. The relevant brain circuitry supporting eye movements includes cortical eye fields and cerebellum that translate sensory information to motor commands, dorsolateral prefrontal cortex and anterior cingulate cortex that provide top-down higher-cognitive control of attention and eye movements, and striatum and brainstem which initiate eye movements. Individuals with autism have robust deficits in the voluntary or endogenous control of saccades. This has been observed as a difficulty inhibiting saccades to targets when instructed to do so, and a reduced accuracy of saccades made to remembered locations (Minshew et al., 1999; Goldberg et al., 2002). An fMRI study from our group demonstrated that the abnormality in memory-guided saccades was related to reduced recruitment of prefrontal cortex during task performance (Luna et al., 2002). Less robust deficits in visually guided, reflexive saccadic eye movements also have been reported. While the peak velocity and latency of visually guided saccades appear to be unimpaired, a mild dymetria (overshooting and undershooting) of saccades has been observed (Takarae et al., 2004b). These findings indicate that brainstem circuitry mediating reflexive saccadic eye movements is relatively intact in this population, but that cerebellar functions may be compromised. Smooth pursuit eye movement deficits have been also documented in laboratory studies of individuals with autism (Takarae et al., 2004a). The neurological basis of disturbances in visually guided pursuit and saccadic eye movements remains to be established, particularly with regard to whether they are caused by regional or systems-level dysfunction, and whether there are any compensatory or fundamental alterations in brain circuitry supporting these behaviors in individuals with autism.
The current study examined brain activation during execution of visually guided saccades and smooth pursuit tracking in high functioning individuals with autism and age- and IQ- matched typically developing individuals. The aim was to define the neural basis of abnormalities in the sensorimotor control of eye movements that were observed in previous laboratory studies.
2. Methods
2.1. Participants
Participants included groups of 17 individuals with autism and 19 typically developing individuals that were matched on age and Full-Scale IQ. Four subjects with autism and 5 typically developing subjects were excluded because of excessive head movement during imaging studies. Mean ages of the remaining participants were 24.5±7.7 years old (range: 17-44) for the autism group and 26.6±7.8 years (range: 18-40) for the typically developing control group, t(25)=0.68, n.s. All participants were given the Wechsler Adult Intelligence Scale-III to access general intellectual functioning. The mean Full-Scale IQ score was 105.9±12.3 (range: 87-129) for the autism group, and 110.3±13.7 (range: 90-138) for the control group, t(25)=0.87, n.s. The Verbal and Performance IQ scores were 107.5±11.5 and 103.1±12.5 in the autism group and 108.5±12.0 and 110.9±14.4 in the control group.
The diagnosis of autism was established according to DSM-IV criteria using two structured research diagnostic instruments, the Autism Diagnostic Interview-Revised (Lord et al., 1994) and the Autism Diagnostic Observation Schedule-General (Lord et al., 2000). Diagnosis was confirmed independently by expert clinical opinion (Nancy J. Minshew or Diane L. Williams of the Pittsburgh CPEA Subject Core). Individuals with autism were excluded if they had an associated disorder known to cause autistic features such as fragile X syndrome or tuberous sclerosis. None of the participants with autism had comorbid ADHD.
Potential control participants were screened with a questionnaire, which they or their parents completed, to rule out a personal history of psychiatric or neurological disorder, family history of autism, and first-degree relatives with any neuropsychiatric disorder considered to have a genetic component. This information was confirmed by telephone review of the completed questionnaire and personal interview. Screening tests were used to rule out learning disabilities as evidenced by significant disparities in Verbal and Performance IQ scores or academic achievement scores significantly below IQ expectations.
No participants were taking medications known to affect cognitive or oculomotor abilities at the time of testing, including methylphenidate, amphetamines and anti-epileptic medications, and none had a history of head injury, birth injury or seizure disorder. Informed consent was obtained from all participants, with children and adolescents providing informed assent along with the consent of their parent or guardian. Far visual acuity of all participants was normal or corrected to at least 20/40. The study was approved by the Institutional Review Boards of the University of Pittsburgh and the University of Illinois at Chicago.
2.2. Tasks
2.2.1. Visually Guided Saccade Task
A white circle subtending 1.0° of visual angle was presented against a homogeneous dark gray background and displaced every 750 ms in 4° steps along the horizontal plane (0°, ±4°, ±°8 positions). The direction of target movement (right or left) was randomly assigned and thus unpredictable except at the ±8° locations after which the target always moved back toward the center of the screen. This saccade condition alternated with a central fixation condition in 30 s blocks for a total paradigm duration of 8.5 minutes. Stimuli were projected onto a rear projection screen that participants viewed from an angled mirror fixed to the head coil.
2.2.2. Smooth Pursuit Task
The target (white circle with a diameter of 1°) moved at an average speed of 10°/s along the horizontal meridian. Target velocity varied in a sinusoidal fashion moving between ± 10°. This pursuit condition also alternated with a central fixation condition in 30 s blocks for 8.5 minutes.
2.3. Procedures
Before fMRI studies, all participants performed pursuit and saccade tasks in the laboratory setting to evaluate and verify their ability to perform oculomotor tasks (Takarae et al., 2004a; Takarae et al., 2004b) (Table 1). Eye movements were not monitored during scans as in several other clinical fMRI studies (Keedy et al., 2006) because of the lack of a technical capacity to obtain high resolution data in scanners, especially to accurately quantify pursuit eye movements, when these studies were initiated. However, all participants had performed the tasks consistently in a cooperative manner in the laboratory and were extensively trained with the tasks prior to the scans. Participants were also retrained in the tasks immediately before beginning the imaging studies, at which time task comprehension and cooperation were reconfirmed. Participants spent approximately 20 minutes in a mock scanner to gain familiarity with the noise and confinement of an MRI scanner before beginning imaging studies.
Table 1.
Eye movement measures during visually guided saccade and visual pursuit tasks obtained in laboratory studies prior to brain imaging investigations. Means and standard deviations are listed. No group differences are statistically significant.
| Control | Autism | |
|---|---|---|
| Visually Guided Saccades with 10 degree targets | ||
|
| ||
| Gain | 0.96 (0.06) | 0.91 (0.08) |
| Latency (ms) | 211 (31) | 216 (30) |
|
| ||
| Visual Pursuit | ||
|
| ||
| Gain at 8 degrees/s | 0.90 (0.05) | 0.86 (0.09) |
| Gain at 16 degrees/s | 0.89 (0.06) | 0.84 (0.10) |
2.4. MRI Parameters
fMRI studies were performed on a 1.5 Tesla Signa whole body MR scanner (General Electric Medical Systems, Milwaukee, WI) with echo-planar imaging (EPI) capability (Advanced NMR Systems, Inc., Wilmington, MA) at the University of Pittsburgh. Gradient-echo echo-planar imaging, sensitive to blood oxygen level dependent (BOLD) effects (Kwong et al., 1992), was performed using a commercial head RF coil. Acquisition parameters were: TE = 50 ms; TR = 3 s; flip angle = 90°; single shot; full k-space; 128 × 64 acquisition matrix with a field of view (FOV) = 40 × 20 cm2, generating an in-plane resolution of 3.125 mm2. Fourteen oblique 5mm slices with 1mm gap, parallel to the AC-PC line, were acquired to cover the whole brain with the exception of the most posterior dorsal/posterior parietal lobe and the base of the frontal lobe and cerebellum. For registration of the functional data, T1 weighted images were acquired of the whole brain with 3D gradient echo imaging with TR=25 ms, TE=5ms, 30° flip angle, 256 × 256 × 192 acquisition matrix, FOV = 24 × 18 cm2, 1.5 mm thick axial slices with no gap.
2.5. Image Analysis
FIASCO software (Functional Imaging Analysis Software - Computational Olio) (Eddy et al., 1996) was used to correct for signal drift and head movement. Correction for head motion was performed in three dimensions using a two level optimization algorithm to estimate rotation and translation values. For each subject, only volumes with displacement of less than 1.5 mm from the median head position over the time series were included in statistical analyses. The numbers of images that met this criterion were similar across groups for both tasks, p's > 0.3.
The T1 structural images were rotated so that the AC-PC plane in the structural images was parallel to the AC-PC plane in functional images. Structural images were then co-registered with maps of brain activation obtained from each individual subject. Both image sets were then transformed into Talairach coordinate space (Talairach and Tournoux, 1988) for group comparison using Analysis of Functional NeuroImages software (Cox, 1996). A modest Gaussian filter with sigma of 0.5mm (1.2 mm FWHM) was then applied to individual functional maps. The time series data were shifted by 6 s to compensate for delay in the BOLD response before statistical analysis for activation effects. Voxels were selected for statistical analyses if they were in the brain of at least 75% of cases in both groups. Maps of within-group activation for each task were created using Fisher's method (Lazar et al., 2002) for visual inspection in order to help interpret significant group differences.
In order to quantify between-group differences in brain activation, F-statistic maps were created in the following way. Voxel-wise chi-square values from the within-group activation maps were divided by corresponding degrees of freedom appropriate for each group. The ratio of chi-squares (divided by degrees of freedom) from each within-group map were used to compute F values in order to identify significant between group differences in task related activation. For instance, to identify brain areas that were more active in the autism group than in the control group during the VGS task, the voxelwise chi-square to degrees of freedom ratio for the autism group from the VGS task was divided by the chi-square to degrees of freedom ratio for the control group from the same task. The resulting F maps were resampled to 2 mm × 2 mm 2 mm space before statistical thresholds were applied.
A contiguity threshold for the whole brain (Forman et al., 1995) was used (minimum of 67 contiguous voxels, each with a group difference effect significant at the p<.005 level). This procedure maintained an experiment-wise Type 1 error rate of p < .025. Since F tests are 1 tailed tests, this threshold was applied separately to determine whether activation was greater or less in the autism group than the control group.
3. Results
3.1. Visually Guided Saccades
Both groups had robust activation in frontal and supplementary eye fields, posterior parietal cortex, visual cortex, and cerebellum during the visually guided saccade task. However, individuals with autism had significantly less activation bilaterally in frontal and supplementary eye fields, posterior parietal cortex and in the cerebellar hemispheres compared to typically developing individuals (Figures 1, 3 and 4).
Figure 1.
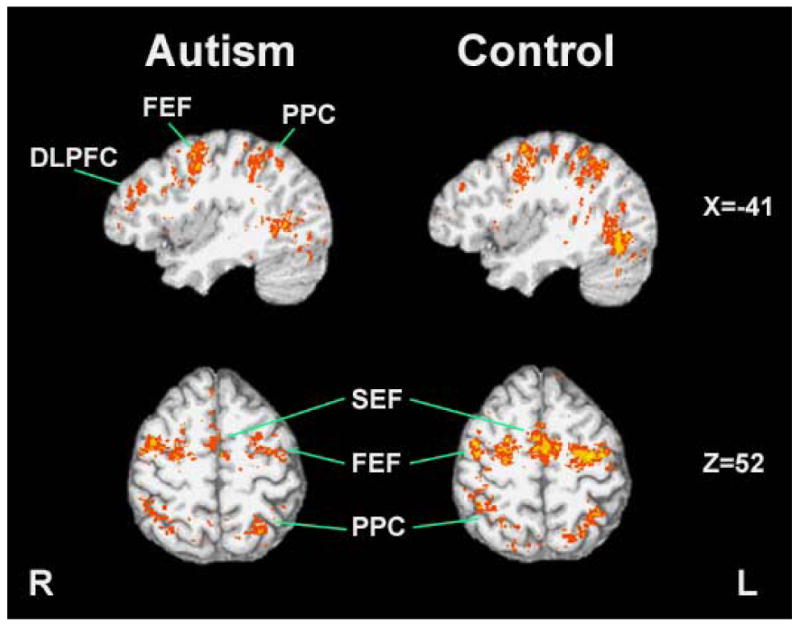
Group level activation during the visually guided saccade task. The autism group showed more pronounced activation in DLPFC during this task while the control group had higher activation in cortical eye fields. (FEF: Frontal Eye Field; DLPFC: Dorsolateral Prefrontal Cortex; PPC: Posterior Parietal Cortex; SEF: Supplementary Eye Field)
Figure 3.
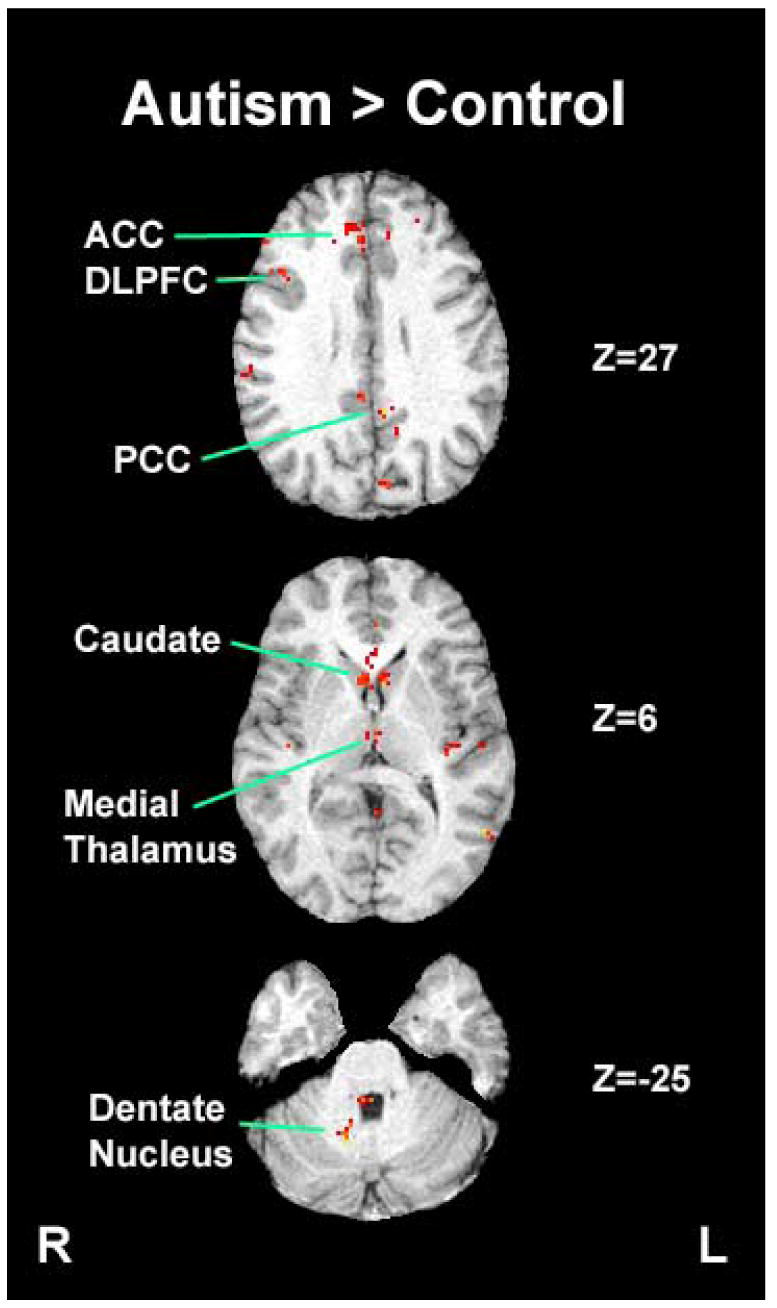
Brain regions that showed statistically higher task-related activation during the visually guided saccade task in the autism group compared to the control group (ACC: Anterior Cingulate Cortex; PCC: Posterior Cingulate Cortex; DLPFC: Dorsolateral Prefrontal Cortex)
Figure 4.
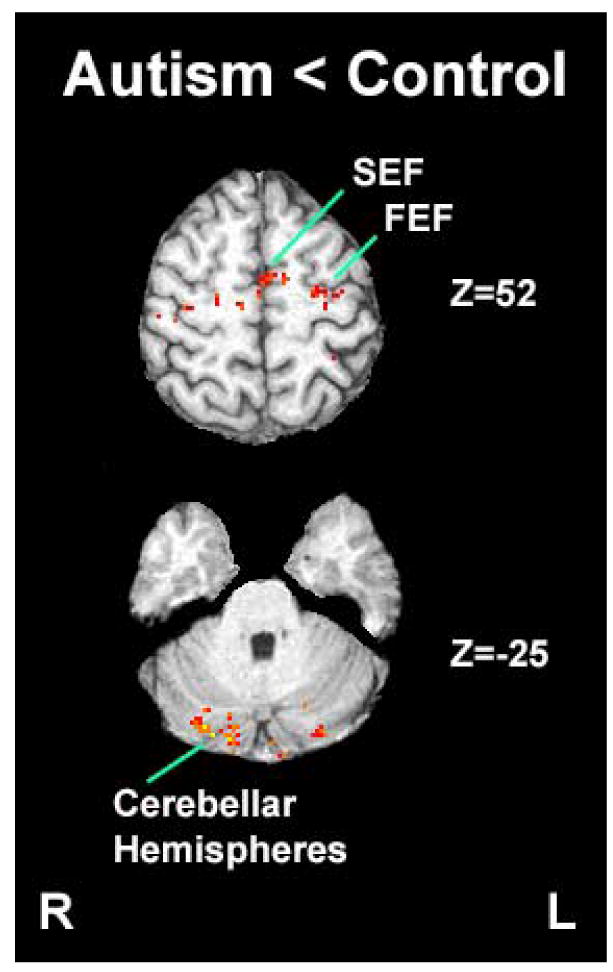
Brain regions that showed statistically higher task-related activation during the visually guided saccade task in the control group compared to the autism group (FEF: Frontal Eye Field; SEF: Supplementary Eye Field)
While individuals with autism had less activation in these sensorimotor areas, they had greater activation bilaterally in dorsolateral prefrontal cortex, anterior and posterior cingulate cortex, medial thalamus, caudate nucleus, and right dentate nucleus. These differences all stemmed from greater task-related activation in the autism group, except for posterior cingulate cortex, where typically developing individuals showed lower activation during the saccade than fixation condition while individuals with autism did not. Location of peak activation and volumes of significantly activated tissue in brain areas of interest are presented in Table 2.
Table 2.
Brain regions showing statistically greater task-related activation in individuals with autism or matched typically developing control participants. This table shows the z value for the peak activation in a priori regions of interest, its corresponding coordinates in Talairach stereotaxic space, and the volume of tissue in regions of interest in which there was statistically greater activation in one group relative to the other. Since clusters of activation identified by the contiguity threshold sometimes extended beyond predetermined regions of interest, reported volumes of activation in regions of interest are in some cases less than the cluster volume required to identify significant effects. F values computed during the analyses were converted to equivalent z values to allow direct comparisons with other studies.
| Left hemisphere | Right hemisphere | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Visually Guided Saccades | Volume (mm3) | Peak z | X | Y | Z | Volume (mm3) | Peak z | X | Y | Z |
| Control > Autism | ||||||||||
| Frontal Eye Field / Superior Precentral Sulcus | 704 | 4.03 | 23 | -15 | 55 | 296 | 4.46 | -37 | -17 | 48 |
| Supplementary Eye Field/ Superior Frontal Gyrus | 296 | 4.12 | 5 | -3 | 52 | 80 | 4.75 | -1 | -11 | 48 |
| Dorsolateral Prefrontal Cortex/ Middle Frontal | 176 | 4.59 | 41 | 26 | 28 | - | - | - | - | - |
| Gyrus | ||||||||||
| Posterior Parietal Cortex/ Intraparietal Sulcus | 856 | 4.37 | 25 | -75 | 36 | 352 | 5.20 | -21 | -51 | 44 |
| Precuneous | 320 | 4.34 | 3 | -57 | 32 | 240 | 3.96 | -10 | -67 | 42 |
| MT/V5/ Inferior Temporal Gyrus | 112 | 3.89 | 55 | -63 | -8 | 552 | 5.44 | -46 | -67 | -6 |
| Cerebellar Hemispheres | 392 | 4.86 | 27 | -85 | -22 | 1040 | 4.94 | -15 | -83 | -28 |
|
| ||||||||||
| Autism > Control | ||||||||||
| Frontal Eye Field / Inferior Precentral Sulcus | 344 | 4.95 | 47 | 11 | 38 | 120 | 3.84 | -41 | 11 | 30 |
| Pre-Supplementary Motor Area/ Superior Frontal Gyrus | 160 | 3.85 | 3 | 11 | 64 | - | - | - | - | - |
| Dorsolateral Prefrontal Cortex/ Middle Frontal Gyrus | 448 | 4.71 | 33 | 57 | 20 | 568 | 4.66 | -21 | 67 | 18 |
| Anterior Cingulate Cortex/ Cingulate Sulcus | 120 | 4.03 | 11 | 39 | 32 | 472 | 4.65 | -2 | 29 | 34 |
| Posterior Cingulate Cortex/ Cingulate Sulcus | 256 | 5.88 | 7 | -47 | 26 | 280 | 4.26 | -1 | -27 | 26 |
| MT/V5/ Inferior Temporal Gyrus | 72 | 4.68 | 53 | -61 | 6 | - | - | - | - | - |
| Medial Thalamus | 136 | 4.26 | 1 | -17 | 4 | 96 | 3.40 | -1 | -13 | 4 |
| Caudate Nucleus | 120 | 4.68 | 4 | 11 | 6 | 88 | 4.27 | -5 | 11 | 6 |
| Dentate Nucleus | - | - | - | - | - | 128 | 4.74 | -13 | -60 | -25 |
|
| ||||||||||
| Left hemisphere | Right hemisphere | |||||||||
| Visual Pursuit | Volume (mm3) | Peak z | X | Y | Z | Volume (mm3) | Peak z | X | Y | Z |
|
| ||||||||||
| Control > Autism | ||||||||||
| Frontal Eye Field/ Superior Precentral Sulcus | 496 | 4.84 | 41 | -17 | 46 | 240 | 4.60 | -35 | -11 | 52 |
| Pre-Supplementary Motor Area/ Superior Frontal | 216 | 4.28 | 2 | 17 | 66 | 208 | 4.28 | -7 | 15 | 64 |
| Gyrus | ||||||||||
| Dorsolateral Prefrontal Cortex/ Middle Frontal | 248 | 3.30 | 35 | 22 | 44 | 160 | 4.73 | -35 | 33 | 44 |
| Gyrus | ||||||||||
| Posterior Parietal Cortex/ Intraparietal Sulcus | 1272 | 5.08 | 45 | -58 | 36 | 1120 | 4.06 | -44 | -54 | 48 |
| Precuneous | 568 | 4.58 | 1 | -69 | 32 | 728 | 4.77 | -1 | -73 | 42 |
| Anterior Cingulate Cortex/ Cingulate Sulcus | - | - | - | - | - | 64 | 3.31 | -1 | 43 | 16 |
| Cingulate Motor Area/ Cingulate Sulcus | 72 | 4.28 | 3 | 13 | 40 | 64 | 3.40 | -1 | 9 | 40 |
| Posterior Cingulate Cortex/ Cingulate Sulcus | - | - | - | - | - | 136 | 3.56 | -1 | -37 | 38 |
| MT/V5/ Inferior Temporal Gyrus | - | - | - | - | - | 128 | 3.49 | -51 | -67 | -12 |
| Cerebellar Hemispheres | 1376 | 4.69 | 19 | -73 | -22 | 2488 | 5.68 | -39 | -63 | -28 |
|
| ||||||||||
| Autism > Control | ||||||||||
| Posterior Parietal Cortex/ Intraparietal Sulcus | 272 | 4.07 | 27 | -55 | 42 | - | - | - | - | - |
| MT/V5/ Inferior Temporal Gyrus | 176 | 3.62 | 43 | -79 | 6 | 304 | 4.38 | -47 | -61 | -2 |
| Caudate Nucleus | - | - | - | - | - | 80 | 3.61 | -9 | 7 | 20 |
3.2. Smooth Pursuit
During the pursuit task, individuals with autism had less activation than typically developing individuals bilaterally in the frontal eye fields, posterior parietal cortex, posterior cingulate cortex, cingulate motor area, and lobules VI and VII of the cerebellar hemispheres (Figures 2 and 5). Individuals with autism also demonstrated less activation in dorsolateral prefrontal cortex, precuneus, and the pre-supplementary motor area. The right caudate nucleus was activated more during the pursuit task in individuals with autism than in typically developing individuals. Small areas in left posterior parietal cortex were also more activated in individuals with autism, but they were anterior and superior to areas showing greater activation in typically developing individuals.
Figure 2.
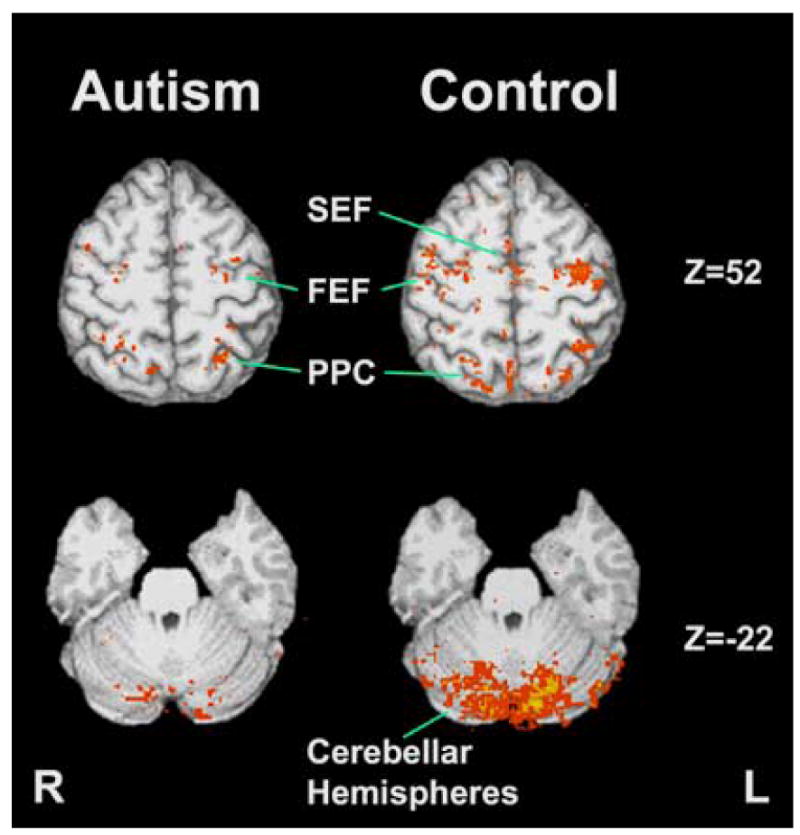
Group level activation during the smooth pursuit task. The control group had higher activation in cortical eye fields and cerebellar hemispheres during this task. (FEF: Frontal Eye Field; PPC: Posterior Parietal Cortex; SEF: Supplementary Eye Field)
Figure 5.
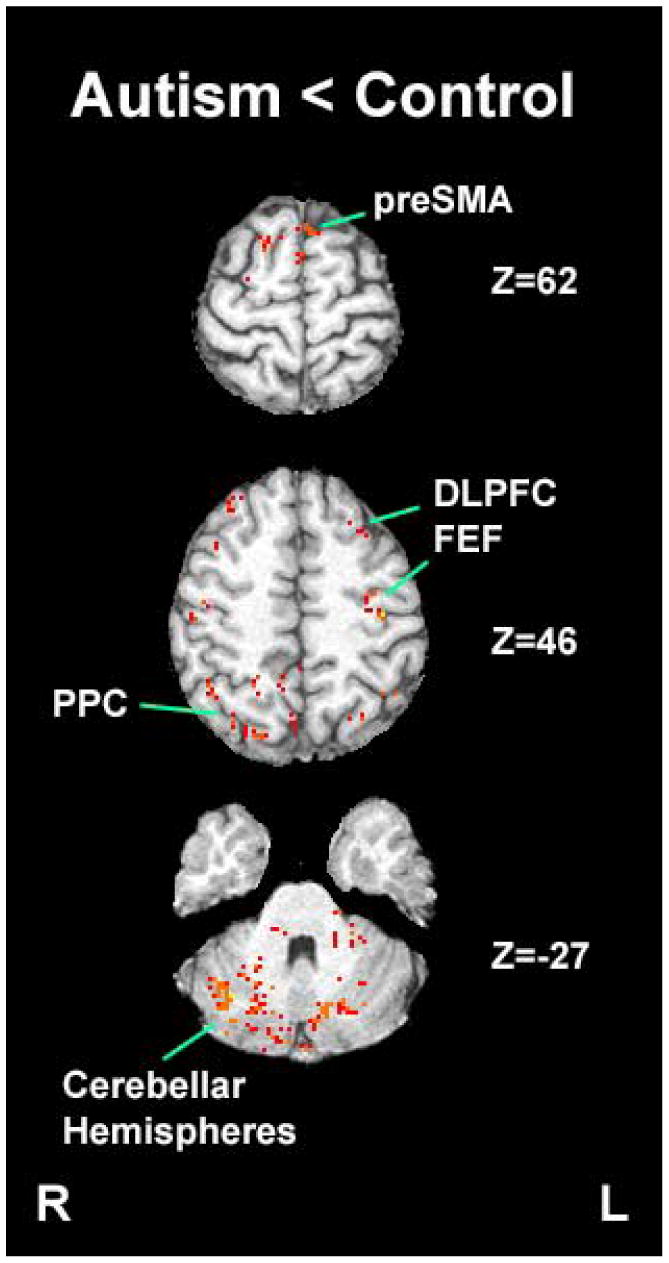
Brain regions that showed statistically higher task-related activation during the smooth pursuit task in the control group compared to the autism group (Pre-SMA: Pre-Supplementary Motor Area; FEF: Frontal Eye Field; DLPFC: Dorsolateral Prefrontal Cortex; PPC: Posterior Parietal Cortex).
4. Discussion
The current study provides new evidence about disturbances in widely-distributed neural system supporting sensorimotor processes in autism. During both saccade and pursuit eye movement tasks, individuals with autism showed reduced activation across several neocortical and subcortical brain areas that support sensorimotor functions. This indicates that a widely distributed dysfunction rather than localized pathology is the likely explanation for previously reported visuomotor disturbances in autism. Importantly, these observations suggest that neurophysiological disturbances in autism extend outside the neural systems related to the classic triad of diagnostic symptoms (impairments in social interactions and language, and stereotypical behaviors) and point to a pattern of dysmaturation that affects the organization of brain systems in a more generalized way.
During the visually guided saccade task, both typically developing control participants and those with autism showed significant activation in sensorimotor areas, including frontal and supplementary eye fields and cerebellar hemispheres as reported in typically developing subjects performing similar tasks in previous studies (Luna et al., 1998; Rosano et al., 2002; Nitschke et al., 2004). Activation in these areas was reduced in individuals with autism. Coordinates of the peak activation we observed in these regions of interest were similar to those in other published studies (Supplementary Table 1 online).
The most notable difference during this task, however, was the greater activation of rostral frontostriatal circuitry in individuals with autism, including bilateral dorsolateral prefrontal, anterior cingulate cortex, caudate nucleus, and medial thalamus. The right dentate nucleus was also more active, suggesting increased activity in cerebello-thalamic circuitry as well as frontostriatal systems. Regions in this frontostriatal and cerebello-thalamic circuitry are typically involved in the execution of intentional behaviors based on internalized representations and cognitive plans rather than automatic responses to external sensory stimuli (Sweeney et al., 1996; DeSouza et al., 2003; Nitschke et al., 2004).
One possible interpretation of the increased activation in frontostriatal circuitry during visually guided saccades is that individuals with autism may make saccades to unpredictable targets in a more intentional manner, rather than as an automatic reflexive response to target appearance as is typical in typically developing individuals. This could account for the greater dependence on the rostral frontostriatal pathways that are known to support saccades made on the basis of internally generated plans (Sweeney et al., 1996; DeSouza et al., 2003; Nitschke et al., 2004). However, when saccades are based on voluntary decisions, response latencies increase considerably relative to reflexive saccades, typically by more than 100 ms, in both typically developing individuals and individuals with autism (Munoz et al., 1998; Luna et al., 2002). Studies of visually guided saccades in individuals with autism do not indicate any such increase in response latency (Minshew et al., 1999; van der Geest et al., 2001; Takarae et al., 2004b), and saccade latencies obtained in the laboratory for participants in the present study were also in normal range (Table 1). Thus, findings with regard to saccade reaction times do not suggest that individuals with autism utilize a voluntary strategy to perform visually guided saccades. Rather, their reflexive visually guided saccades appear to be generated with greater reliance on brain systems that are typically specialized to support higher cognitive functions.
Increased activation in frontostriatal circuitry might occur to provide compensatory input to sensorimotor areas whose function appears to be compromised. Previous studies have shown that when a motor pathway is compromised by disease, an alternative circuitry that performs a related function can be recruited to compensate for dysfunction in the primary circuitry. This has been demonstrated in Parkinson's disease (Sabatini et al., 2000) and cerebellar degeneration (Wessel et al., 1995), where atypical or additional brain areas can be recruited to support performance of manual motor tasks. Similar findings have been reported in autism where non-motor areas can be recruited during manual motor tasks (Müller et al., 2001; Allen et al., 2004). Compensation could occur in the form of increased effort to maintain attention during the tasks. However, increased activation in the frontostriatal system was not observed during the pursuit task which has higher demands for sustained attention, suggesting that the finding does not represent general difficulty maintaining attention to visual information. In fact, the opposite pattern of reduced activity relative to typically developing subjects was seen in individuals with autism during the pursuit task.
We recently reported reduced prefrontal activation in autism using an oculomotor delayed response task in which saccades are made to remembered locations without sensory guidance (Luna et al., 2002). This task typically recruits robust dorsolateral prefrontal activation in typically developing individuals (Sweeney et al., 1996; Brown et al., 2004). Our findings in autism with the oculomotor delayed response task suggested an impairment in the ability of prefrontal cortex to support working memory systems. A more recent study by Haist and others (Haist et al., 2005) used a covert attention task in which eye movements need to be suppressed via endogenous control and reported that multiple areas in prefrontal cortex showed reduced activation in autism. This study provides additional evidence that when eye movements are under endogenous controls, prefrontal cortex was less active in individuals with autism. These findings, combined with observations from the present study, illustrate a pattern of prefrontal function in autism in which reduced task related activity is observed during tasks that require endogenous cognitive control, while greater activity is seen during sensorimotor tasks where exogenous sensory information elicits reflexive discrete shifts of attention and gaze with much lower cognitive load. This pattern of activation may be analogous to that seen in other domains in autism, where circuitry subserving basic functions can be enhanced relative to deficits in more complex abilities (Minshew et al., 1997; Belmonte and Yurgelun-Todd, 2003; Just et al., 2004).
If rostral frontostriatal systems are required to provide ongoing compensatory support for sensorimotor systems involving exogenous shifts of attention and gaze, as suggested by their enhanced function during our visually guided saccade task, this could potentially have an adverse neurodevelopmental impact on the functional specialization in prefrontal systems. The impact of such compensatory reorganization could interact with disorder-related neocortical abnormalities, such as intrinsic local circuit pathology or disturbances in long fiber tracts (Casanova et al., 2002; Herbert et al., 2004), to alter the course of maturation in prefrontal systems (Luna et al., 2007). However, it remains to be determined whether the increased activation we observed during the visually guided saccade task is compensatory in nature. Its selective presence during the saccade task is consistent with this possibility. However, while atypical recruitment of prefrontal circuitry could represent a functional compensation for disturbances in sensorimotor systems in other brain areas, it is also possible that they could be a direct result of neurodevelopmental perturbations in the intrinsic organization of prefrontal circuitry or even alterations in the rostral/caudal pattern of thalamocortical innervation.
The increased activation within prefrontal systems was not present during pursuit tracking. In this context, it is important to note that pursuit of predictable target motion, as examined in the present study, is fundamentally different from making visually guided saccades to unpredictable targets in its cognitive requirements as well as in motor output. Sustained visual tracking depends primarily upon the ability to modulate the pursuit response in relation to an internal representation of predicted target speed and trajectory. In contrast, the saccade task elicits discrete reflexive responses to unpredictable target displacements with less requirement for endogenous control. Thus, the reduced prefrontal activation during the pursuit task in individuals with autism might in part reflect a deficit in executing behavior based on internal representations as has been reported in neuropsychological studies of the disorder (Hill, 2004) and in our previous work with oculomotor tasks assessing spatial working memory (Luna et al., 2002).
Consistent with the idea that individuals with autism have difficulty executing motor responses requiring the establishment and use of internal representations, the autism group had less activation than the control group in areas involved in motor learning during the pursuit task. They had reduced activity in pre-supplementary motor area, cingulate motor area, and cerebellar hemispheres, all of which are known to support the acquisition of skilled motor responses (Picard and Strick, 2001; Pierrot-Deseilligny et al., 2002; Floyer-Lea and Matthews, 2004; Simo et al., 2005). Deficits in implicit sequence learning and initiating responses to predictable target sequences have been reported in autism (Mostofsky et al., 2000; Rinehart et al., 2001). Thus, dysfunction in the neural circuitry supporting motor learning may play a role in pursuit eye movement deficits in autism. In addition to the areas involved in the acquisition of skilled motor responses during pursuit, individuals with autism showed less activation in frontal and parietal eye fields and cerebellum that are central to the sensorimotor control of pursuit tracking (Keller and Heinen, 1991; Krauzlis, 2004). Dysfunction in sensorimotor abilities supported by these integrated brain regions was seen during both the saccade and pursuit tasks.
It is widely recognized that a complex pattern of brain dysmaturation occurs in autism. The current study documents reduced activation in sensorimotor areas during eye movement tasks, which indicate that neural system deficits in autism extend beyond brain areas mediating language and social cognition. While some models of autism have proposed hemisphere or lobe specific pathophysiology, the present study demonstrates that brain disturbances exist throughout multiple brain regions to include both neocortical and subcortical regions. Thus, our findings are not consistent with hemisphere or lobe specific pathology. In contrast to previous reports that rostral frontostriatal circuitry is less activated during tasks that rely on planning and behaviors based on internal representations (Luna et al., 2002), in the present study, rostoral frontostriatal circuitry showed an atypical increase in activity during simple sensorimotor tasks that elicit automatic reflexive motor responses. These findings are consistent with a model of autism which characterizes the disorder as having a pathophysiology involving a complex brain dysmaturation that affects the general architecture of widely distributed functional brain systems and their functional specialization. This type of dysmaturation would have greatest impact on complex behaviors supported by functional integration within widely distributed systems, providing an overarching model to explain how higher cognitive processes and basic sensorimotor control would be compromised in autism, and why more complex cognitive abilities are selectively affected in the disorder (Minshew et al., 1997).
Supplementary Material
Acknowledgments
This research was funded by a NICHD/NIDCD University of Pittsburgh-Carnegie Mellon University-University of Illinois at Chicago Collaborative Program of Excellence in Autism HD 35469, NS33355, MH01433, and by grants from the Edith L. Trees Charitable Trust and the National Alliance for Autism Research (now Autism Speaks).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allen G, Müller RA, Courchesne E. Cerebellar function in autism: functional magnetic resonance image activation during a simple motor task. Biological Psychiatry. 2004;56(4):269–278. doi: 10.1016/j.biopsych.2004.06.005. [DOI] [PubMed] [Google Scholar]
- Bailey A, Luthert P, Dean A, Montgomery MRM, Lantos S. A clinicopathological study of autism. Brain. 1998;121:889–905. doi: 10.1093/brain/121.5.889. [DOI] [PubMed] [Google Scholar]
- Belmonte MK, Yurgelun-Todd DA. Functional anatomy of impaired selective attention and compensatory processing in autism. Cognitive Brain Research. 2003;17(3):651–664. doi: 10.1016/s0926-6410(03)00189-7. [DOI] [PubMed] [Google Scholar]
- Brown MR, DeSouza JF, Goltz HC, Ford K, Menon RS, Goodale MA, Everling S. Comparison of memory- and visually guided saccades using event-related fMRI. Journal of Neurophysiology. 2004;91(2):873–889. doi: 10.1152/jn.00382.2003. [DOI] [PubMed] [Google Scholar]
- Casanova MF, Buxhoeveden DP, Switala AE, Roy E. Minicolumnar pathology in autism. Neurology. 2002;58(3):428–432. doi: 10.1212/wnl.58.3.428. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Karns CM, Davis HR, Ziccardi R, Carper RA, Tigue ZD, Chisum HJ, Moses P, Pierce K, Lord C, Lincoln AJ, Pizzo S, Schreibman L, Haas RH, Akshoomoff NA, Courchesne RY. Unusual brain growth patterns in early life in patients with autistic disorder: an MRI study. Neurology. 2001;57(2):245–254. doi: 10.1212/wnl.57.2.245. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- DeSouza JF, Menon RS, Everling S. Preparatory set associated with pro-saccades and anti-saccades in humans investigated with event-related FMRI. Journal of Neurophysiology. 2003;89(2):1016–1023. doi: 10.1152/jn.00562.2002. [DOI] [PubMed] [Google Scholar]
- Eddy WF, Fitzgerald M, Genovese CR, Mockus A, Noll DC. Functional image analysis software - computational olio. In: Prat A, editor. Proceedings in Computational Statistics. Heidelberg: Physica-Verlag; 1996. pp. 39–49. [Google Scholar]
- Filipek PA, Richelme C, Kennedy DN, Rademacher J, Pitcher DA, Zidel S, Caviness VS. Morphometric analysis of the brain in developmental language disorders and autism. Annals of Neurology. 1992;32:475. [Google Scholar]
- Floyer-Lea A, Matthews PM. Changing brain networks for visuomotor control with increased movement automaticity. Journal of Neurophysiology. 2004;92(4):2405–2412. doi: 10.1152/jn.01092.2003. [DOI] [PubMed] [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magnetic Resonance Medicine. 1995;33:636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- Goldberg MC, Lasker AG, Zee DS, Garth E, Tien A, Landa RJ. Deficits in the initiation of eye movements in the absence of a visual target in adolescents with high functioning autism. Neuropsychologia. 2002;40:2039–2049. doi: 10.1016/s0028-3932(02)00059-3. [DOI] [PubMed] [Google Scholar]
- Haist F, Adamo M, Westerfield M, Courchesne E, Townsend J. The functional neuroanatomy of spatial attention in autism spectrum disorder. Developmental Neuropsychology. 2005;27(3):425–458. doi: 10.1207/s15326942dn2703_7. [DOI] [PubMed] [Google Scholar]
- Hendry J, Devito T, Gelman N, Densmore M, Rajakumar N, Pavlosky W, Williamson PC, Thompson PM, Drost DJ, Nicolson R. White matter abnormalities in autism detected through transverse relaxation time imaging. Neuroimage. 2006;29(4):1049–1057. doi: 10.1016/j.neuroimage.2005.08.039. [DOI] [PubMed] [Google Scholar]
- Herbert MR, Ziegler DA, Makris N, Filipek PA, Kemper TL, Normandin JJ, Sanders HA, Kennedy DN, Caviness VS., Jr Localization of white matter volume increase in autism and developmental language disorder. Annals of Neurology. 2004;55(4):530–540. doi: 10.1002/ana.20032. [DOI] [PubMed] [Google Scholar]
- Hill EL. Executive dysfunction in autism. Trends in Cognitive Science. 2004;8(1):26–32. doi: 10.1016/j.tics.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Just MA, Cherkassky VL, Keller TA, Minshew NJ. Cortical activation and synchronization during sentence comprehension in high-functioning autism: evidence of underconnectivity. Brain. 2004;127:1811–1821. doi: 10.1093/brain/awh199. [DOI] [PubMed] [Google Scholar]
- Keedy SK, Ebens C, Keshavan MS, Sweeney JA. FMRI studies of eye movements in first-episode schizophrenia: smooth pursuit, visually guided saccades, and the oculomotor delayed response task. Psychiatry Research: Neuroimaging. 2006;146:199–211. doi: 10.1016/j.pscychresns.2006.01.003. [DOI] [PubMed] [Google Scholar]
- Keller EL, Heinen SJ. Generation of smooth-pursuit eye movements: neuronal mechanisms and pathways. Neuroscience Research. 1991;11:79–107. doi: 10.1016/0168-0102(91)90048-4. [DOI] [PubMed] [Google Scholar]
- Kemper TL, Bauman M. Neuropathology of infantile autism. Journal of Neuropathology and Experimental Neurology. 1998;57(7):645–652. doi: 10.1097/00005072-199807000-00001. [DOI] [PubMed] [Google Scholar]
- Krauzlis RJ. Recasting the smooth pursuit eye movement system. Journal of Neurophysiology. 2004;91(2):591–603. doi: 10.1152/jn.00801.2003. [DOI] [PubMed] [Google Scholar]
- Kwong KK, Belliveau JW, Chesler DA, Goldberg IE, Weisskoff RM, Poncelet BP, Kennedy DN, Hoppel BE, Cohen MS, Turner R, et al. Dynamic magnetic resonance imaging of human brain activity during primary sensory stimulation. Proceedings of the National Academy of Sciences of the United States of America. 1992;89(12):5675–5679. doi: 10.1073/pnas.89.12.5675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazar NA, Luna B, Sweeney JA, Eddy WF. Combining brains: a survey of methods for statistical pooling of information. Neuroimage. 2002;16(2):538–550. doi: 10.1006/nimg.2002.1107. [DOI] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH, Jr, Leventhal BL, DiLavore PC, Pickles A, Rutter M. The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. Journal of Autism and Developmental Disorders. 2000;30(3):205–223. [PubMed] [Google Scholar]
- Lord C, Rutter M, Le Couteur A. Autism diagnostic interview-revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism & Developmental Disorders. 1994;24(5):659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- Luna B, Doll SK, Hegedus SJ, Minshew NJ, Sweeney JA. Maturation of executive function in autism. Biological Psychiatry. 2007;61(4):474–481. doi: 10.1016/j.biopsych.2006.02.030. [DOI] [PubMed] [Google Scholar]
- Luna B, Minshew NJ, Garver K, Lazar NA, Thulborn KR, Eddy WF, Sweeney JA. Neocortical system abnormalities in autism: an fMRI study of spatial working memory. Neurology. 2002;59(6):834–840. doi: 10.1212/wnl.59.6.834. [DOI] [PubMed] [Google Scholar]
- Luna B, Thulborn KR, Strojwas MH, McCurtain BJ, Berman RA, Genovese CR, Sweeney JA. Dorsal cortical regions subserving visually-guided saccades in humans: an fMRI study. Cerebral Cortex. 1998;8:40–47. doi: 10.1093/cercor/8.1.40. [DOI] [PubMed] [Google Scholar]
- Minshew NJ, Goldstein G, Siegel DJ. Neuropsychologic functioning in autism: profile of a complex information processing disorder. Journal of the International Neuropsychological Society. 1997;3:303–316. [PubMed] [Google Scholar]
- Minshew NJ, Luna B, Sweeney JA. Oculomotor evidence for neocortical systems but not cerebellar dysfunction in autism. Neurology. 1999;52(5):917–922. doi: 10.1212/wnl.52.5.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minshew NJ, Sung K, Jones BL, Furman JM. Underdevelopment of the postural control system in autism. Neurology. 2004;63(11):2056–2061. doi: 10.1212/01.wnl.0000145771.98657.62. [DOI] [PubMed] [Google Scholar]
- Molloy CA, Dietrich KN, Bhattacharya A. Postural stability in children with autism spectrum disorder. Journal of Autism and Developmental Disorders. 2003;33(6):643–652. doi: 10.1023/b:jadd.0000006001.00667.4c. [DOI] [PubMed] [Google Scholar]
- Mostofsky SH, Goldberg MC, Landa RJ, Denckla MB. Evidence for a deficit in procedural learning in children and adolescents with autism: implications for cerebellar contribution. Journal of the International Neuropsychological Society. 2000;6(7):752–759. doi: 10.1017/s1355617700677020. [DOI] [PubMed] [Google Scholar]
- Müller RA, Pierce K, Ambrose JB, Allen G, Courchesne E. Atypical patterns of cerebral motor activation in autism: a functional magnetic resonance study. Biological Psychiatry. 2001;49(8):665–676. doi: 10.1016/s0006-3223(00)01004-0. [DOI] [PubMed] [Google Scholar]
- Munoz DP, Broughton J, Goldring J, Armstrong I. Age-related performance of human subjects on saccadic eye movement tasks. Experimental Brain Research. 1998;121(4):391–400. doi: 10.1007/s002210050473. [DOI] [PubMed] [Google Scholar]
- Nitschke MF, Binkofski F, Buccino G, Posse S, Erdmann C, Kompf D, Seitz RJ, Heide W. Activation of cerebellar hemispheres in spatial memorization of saccadic eye movements: an fMRI study. Human Brain Mapping. 2004;22(2):155–164. doi: 10.1002/hbm.20025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard N, Strick PL. Imaging the premotor areas. Current Opinion in Neurobiology. 2001;11(6):663–672. doi: 10.1016/s0959-4388(01)00266-5. [DOI] [PubMed] [Google Scholar]
- Pierrot-Deseilligny C, Ploner CJ, Muri RM, Gaymard B, Rivaud-Pechoux S. Effects of cortical lesions on saccadic eye movements in humans. Annals of New York Academy of Science. 2002;956:216–229. doi: 10.1111/j.1749-6632.2002.tb02821.x. [DOI] [PubMed] [Google Scholar]
- Rinehart NJ, Bradshaw JL, Brereton AV, Tonge BJ. Movement preparation in high-functioning autism and Asperger disorder: a serial choice reaction time task involving motor reprogramming. Journal of Autism and Developmental Disorders. 2001;31(1):79–88. doi: 10.1023/a:1005617831035. [DOI] [PubMed] [Google Scholar]
- Rosano C, Krisky CM, Welling JS, Eddy WF, Luna B, Thulborn KR, Sweeney JA. Pursuit and saccadic eye movement subregions in human frontal eye field: a high-resolution fMRI investigation. Cerebral Cortex. 2002;12(2):107–115. doi: 10.1093/cercor/12.2.107. [DOI] [PubMed] [Google Scholar]
- Sabatini U, Boulanouar K, Fabre N, Martin F, Carel C, Colonnese C, Bozzao L, Berry I, Montastruc JL, Chollet F, Rascol O. Cortical motor reorganization in akinetic patients with Parkinson's disease: a functional MRI study. Brain. 2000;123:394–403. doi: 10.1093/brain/123.2.394. [DOI] [PubMed] [Google Scholar]
- Simo LS, Krisky CM, Sweeney JA. Functional neuroanatomy of anticipatory behavior: dissociation between sensory-driven and memory-driven systems. Cerebral Cortex. 2005;15(2):1982–1991. doi: 10.1093/cercor/bhi073. [DOI] [PubMed] [Google Scholar]
- Sparks BF, Friedman SD, Shaw DW, Aylward EH, Echelard D, Artru AA, Maravilla KR, Giedd JN, Munson J, Dawson G, Dager SR. Brain structural abnormalities in young children with autism spectrum disorder. Neurology. 2002;59(2):184–192. doi: 10.1212/wnl.59.2.184. [DOI] [PubMed] [Google Scholar]
- Sweeney JA, Mintun MA, Kwee S, Wiseman MB, Brown DL, Rosenberg DR, Carl JR. Positron emission tomography study of voluntary saccadic eye movements and spatial working memory. Journal of Neurophysiology. 1996;75(1):454–468. doi: 10.1152/jn.1996.75.1.454. [DOI] [PubMed] [Google Scholar]
- Takarae Y, Minshew NJ, Luna B, Krisky CM, Sweeney JA. Pursuit eye movement deficits in autism. Brain. 2004a;127:2584–2594. doi: 10.1093/brain/awh307. [DOI] [PubMed] [Google Scholar]
- Takarae Y, Minshew NJ, Luna B, Sweeney JA. Oculomotor abnormalities parallel cerebellar histopathology in autism. Journal of Neurology, Neurosurgery and Psychiatry. 2004b;75(9):1359–1361. doi: 10.1136/jnnp.2003.022491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. New York: Thieme Medical Publishers; 1988. [Google Scholar]
- van der Geest JN, Kemner C, Camfferman G, Verbaten MN, van Engeland H. Eye movements, visual attention, and autism: a saccadic reaction time study using the gap and overlap paradigm. Biological Psychiatry. 2001;50(8):614–619. doi: 10.1016/s0006-3223(01)01070-8. [DOI] [PubMed] [Google Scholar]
- Wessel K, Zeffiro T, Lou JS, Toro C, Hallett M. Regional cerebral blood flow during a self-paced sequential finger opposition task in patients with cerebellar degeneration. Brain. 1995;118:379–393. doi: 10.1093/brain/118.2.379. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


