Abstract
Nonvisual arrestins are a family of multifunctional adaptor molecules that regulate the activities of diverse families of receptors including G protein–coupled receptors, frizzled, and transforming growth factor-β receptors. These activities indicate broad roles in both physiology and development for nonvisual arrestins. Drosophila melanogaster has a single nonvisual arrestin, kurtz, which is found at high levels within the adult olfactory receptor neurons (ORNs), suggesting a role for this gene in modulating olfactory sensitivity. Using heat-induced expression of a krz cDNA through development, we rescued krz1 lethality. The resulting adults lacked detectable levels of krz in the olfactory system. The rescued krz1 homozygotes have an incompletely penetrant antennal structural defect that was completely rescued by the neural expression of a krz cDNA. The krz1 loss-of-function adults without visible antennal defects displayed diminished behavioral responsiveness to both aversive and attractive odors and also demonstrated reduced olfactory receptor potentials. Both the behavioral and electrophysiological phenotypes were rescued by the targeted expression of the krz cDNA within postdevelopmental ORNs. Thus, krz is required within the nervous system for antennal development and is required later in the ORNs for the maintenance of olfactory sensitivity in Drosophila. The reduced receptor potentials in krz1 antenna indicate that nonvisual arrestins are required for the early odor-induced signaling events within the ORNs.
Keywords: adaptation, antenna, arrestin, Drosophila, electroantennogram, olfaction
Introduction
For many species, odors are a rich source of information on their immediate environment. Odors may lead an animal to food sources or mating partners, and they may allow the animal to recognize potential hazards such as toxins or predators. The amount of information conveyed by these odors is frequently dependent on the ability to detect gradients of dilute odors. Thus, effective odor detection for these animals requires a high sensitivity maintained across a wide range of odor concentrations.
In both vertebrates and invertebrates, odors are detected by a large family of G protein–coupled receptors (GPCRs; Buck and Axel, 1991; Clyne et al., 1999). Most GPCRs have an extremely high gain with enormous sensitivity (Menini et al., 1995; Lamb, 1996). A broad and dynamic range for signaling by GPCRs is typically sustained by the process of agonist-dependent desensitization. This process limits the magnitude of signaling through GPCRs, keeping weak stimuli from saturating the cellular responses. Agonist-dependent desensitization occurs through the successive activities of G protein–coupled receptor kinases (GRKs) and nonvisual arrestins (Ferguson et al., 1996; Krupnick and Benovic, 1998). The agonist-bound GPCR is phosphorylated by a GRK; the phosphorylated GPCR is then bound by the non-visual arrestin. The arrestin will sterically inhibit any further interactions with the heterotrimeric G protein, desensitizing the receptor. The nonvisual arrestins will then shuttle the activated GPCR to a coated pit, promoting the internalization of this receptor. The internalized receptor will then move from the clathrin-coated vesicle through the early endosome and frequently recycle back to the cell surface, returning the cell to its prior level of responsiveness (Pitcher et al., 1995; Krueger et al., 1997). Despite the tremendous progress in understanding the mechanism and regulation of GPCR agonist-dependent desensitization, much less is known of the physiological requirements for this process in living animals, especially in the context of odor perception. The genetic dissection of agonist-dependent desensitization within the olfactory system should elucidate the role of this process in safeguarding olfactory sensitivity.
In the Drosophila genome, there are 60 odorant receptor (OR) genes (Clyne et al., 1999; Gao and Chess, 1999; Vosshall et al., 1999). The mechanism of Drosophila OR transduction is presently unknown; however, vertebrate ORs are known to couple to heterotrimeric G proteins. The Drosophila ORs are inserted into the cilia membranes of the olfactory receptor neurons (ORNs). These cilia are located in sensilla that are distributed on the third antennal segment and the maxillary palps. The sensilla contain the cilia from one to four ORNs, each of which expresses one to two ORs (Shanbhag et al., 1999; Goldman et al., 2005). The ORNs project back to the antennal lobes where they synapse with antennal lobe projection neurons and local inhibitory neurons within the glomeruli (Stocker, 1994). Drosophila has a single nonvisual arrestin, kurtz (krz; Roman et al., 2000). Herein, we show that krz is expressed throughout the antenna, including within the ORNs. The absence of krz within the antenna of loss-of-function krz mutants leads to a marked decrease in olfactory sensitivity, measured both behaviorally and electrophysiologically. The targeted expression of krz within the ORNs, beginning shortly before eclosion, can rescue these defects.
Materials and methods
Fly stocks and genetics
All the stocks were raised at room temperature on standard cornmeal, sucrose, and yeast food. The flies used for the behavioral assays were raised at 25° C, 60% humidity, and with 12 h of light per day during the first 4 days and then followed by twice daily 37° C heat shocks in a cycling incubator for 10 days. Each heat shock lasted 1.5 h. After this period, the bottles were placed at room temperature, and the flies were allowed to eclose. The generated flies were harvested immediately after eclosion, their antennae checked for structural defects, and placed at 18° C for 4 days before the behavioral assay, electroantennogram (EAG) measurement, and immunohistochemistry. Flies with detectable structural defects were not used for behavior or electrophysiology. These same conditions were also used for all control genotypes. The krz1 allele, the b5.8T4 or b5.8T12 genomic krz trans-genes, and the UASkrzT12 cDNA transgene were originally described in Roman et al. 2000. The third multiple 3 (TM3) P{hs-hid}14, P{hspGal489-2-1}, and c155elavGal4 lines were obtained from Bloomington Stock Center. The P{Or83b-Gal4:vp16} lines were obtained from Dean Smith (University of Texas Southwestern Medical Center). The P{Or83b-Gal4:vp16} lines drive expression from upstream activation sequence (UAS) transgenes in approximately 70% ORNs (Kalidas and Smith, 2002). In order to generate adult flies that are deficient in krz expression, we used heat shock Gal4-induced krz activity to rescue the developmental lethality of the krz1 allele. The krz1 homozygotes (krz1: P{UASkrz}T12/+; krz1, P{hspGal4}/krz1) were generated from the cross of P{UASkrzT12}; krz1/TM3 hshid by krz1, P{hspGal489-2-1}/TM3 hshid. The c155-rescued homozygotes (c155; krz1: c155/+; P{UASkrz}T12/+; krz1/krz1) were generated from the cross of c155elavGal4; krz1/TM3 hshid by P{UASkrz}T12; krz1/TM3 hshid. The Or83b-Gal4-rescued homozygotes were generated from either Or83bGal4; krz1, P{hspGal489-2-1}/TM3 by P{UASkrzT12}; krz1/TM3 hshid (Or83b on the second chromosome: P{UASkrz}T12/+, Or83bGal4; krz1) or P{UASkrzT12}; Or83b, krz1/TM3 hshid by krz1, P{hspGal489-2-1}/TM3 hshid (Or83b on the third chromosome: P{UASkrz}T12/+; Or83bGal4; krz1).
Behavioral assay
The behavioral responses of the flies to different aversive and attractive odors were examined in an olfactory T-maze (Tully and Quinn, 1985). The testing was performed under dim red light at 24–25° C and 63–68% relative humidity. Odors were diluted in 10 ml of light mineral oil to the stated concentrations. In the T-maze apparatus, the odor was delivered into one arm after the airstreams were bubbled through the odorant diluted in mineral oil; fresh air was blown into the other arm after bubbling through light mineral oil. The air-flow in these experiments was maintained at 500 ml/min. Groups of ~30 flies were placed between arms and allowed 2 min to move to a preferred side. The response index was calculated as the number of flies in the odorant-containing arm minus that of flies in the air arm and then divided by the total number of flies found in both arms (Devaud et al., 2001). The flies trapped in the central compartment were counted but not included in the calculation. A minimum of 16 groups were assayed for each data point. The odorants 4-methylcyclohexanol(MCH),benzaldehyde(BEN),1-butanol, propionic acid (PA), isoamyl acetate (IA), and ethyl acetate (EA) were obtained from Sigma-Aldrich (St Louis, MO). Fluka brand 3-octanol was also obtained from Sigma-Aldrich. The maximum purity available was used.
Several changes were made for the test of behavioral responsiveness EA. The odorant was diluted in 40 ml of mineral oil instead of 10 ml. The odor was delivered into the T-maze after the airstream was wafted over the surface of the diluted odor at a rate of 200 ml/min. The flies were given 3 min to choose between arms in this experiment. Statistical analysis was carried out by using StatView v5.01 (SAS Institute Inc., Cary, NC). One-way analysis of variance (ANOVA) was used to analyze the mean avoidance responses of different genotypes in the T-maze behavioral assay. Significant genotype effects in these ANOVAs were followed by Bonferroni–Dunn post hoc analysis.
EAG measurements
The entrainment of flies and the method of recording were as described previously (Krishnan et al., 1999; Tanoue et al., 2004). Odor stimulation was achieved with diluted MCH (1:104) or octanol (OCT) (1:106). For measuring the kinetics of recovery, the response of each fly to a 1-s pulse of MCH or OCT was measured at the beginning of each experiment. The fly was then exposed to an adapting stimulus of a 30-s pulse of MCH or OCT. Recovery from adaptation was assessed at 5, 30, and 60 s and every 1 min thereafter, by measuring EAG response to a 1-s pulse of the odorant. The percentage of recovery with respect to the preadaptation stimulus was calculated for each time point. Statistical analysis for differences between time and genotype (two-way ANOVA) was performed using StatView software v5.01 (SAS Institute Inc.).
Immunohistochemistry
Two polyhistidine-tagged KRZ fusion proteins were made by cloning krz cDNA fragments into pRSETB (Invitrogen, Carlsbad, CA). One fusion protein (BEARR) was made by cloning the BamHI–EcoRI p478c fragment into the corresponding sites of pRSETB, and the second (SEARR) was made by cloning the SalI–EcoRI fragment into the XhoI/EcoRI sites of pRSETB. These fusion proteins were produced and purified as previously described (Roman et al., 1998). One New Zealand White female rabbit was injected for each fusion protein. Both fusion proteins were also coupled to Reacti-Gel 6X CDI-agarose according to the manufacturer’s recommendations (Pierce, Rockford, IL). These agarose-coupled fusion proteins were used to purify antibodies according to the methods of Smith and Fisher (1984).
The immunohistochemistry experiments were performed as previously described with some modifications (Roman et al., 1998). Fly heads were mounted in “Heisenberg collars,” frozen in optimal cutting media (Sakura Finetek, Torrence, CA), and cut into 12-μm frontal cryosections. The sections were placed onto Superfrost Plus slides (Fisher Scientific, Hampton, NH) and warmed for 5 min on a slide warmer. Sections were then fixed in 4% paraformaldehyde in phosphate buffered saline, high salt with Tween 20 (PBHT) for 20 min at room temperature. The fixed sections were rinsed with three consecutive 10-min incubations in PBHT. The sections were first blocked by 5% normal goat sera in PBHT for 3 h and then incubated with primary antibodies diluted in PBHT plus 5% normal goat sera overnight at room temperature. Unbound antibodies were washed off by three successive 10-min PBHT incubations. The secondary antibodies were incubated at room temperature for 3 h. The affinity-purified anti-KURTZ rabbit polyclonal antibody was used at a 1:200 dilution, and the monoclonal anti-elav (early embryonic abnormal vision; DSHB, University of Iowa) was used at a 1:25 dilution. The anti-rabbit–Alexa594 and anti-mouse–Alexa488 (Molecular Probes, Portland, OR) secondary antibodies were used at a dilution of 1:200. For immunohistochemistry on paraffin sections shown in Figure 3C,D, paraffin was removed from the sections with 2-min incubations in xylenes (Fisher Scientific). The sections were next hydrated by successive 5-min incubations in a series of decreasing ethanol concentrations (100%, 80%, 60%, 40%, 20%, 0% ethanol). It was also necessary to perform antigen retrieval in order to detect krz. To uncover the KRZ antigen, the hydrated slides were placed in 1 × phosphate-buffered saline (PBS) heated to 95° C for 5 min The slides were then removed and placed into 1 × PBS at room temperature for 10 min prior to immunoblocking. Nonspecific antibody binding was blocked with a 4-h incubation at room temperature in 5% normal goat sera (Sigma Chemicals, St Louis, MO) in 1 × PBS. For these experiments, affinity-purified antibody was used at a concentration of 1:100. Antibody was detected using the Vectastain ABC kit (Vector Laboratories, Burlingame, CA).
Figure 3.

KURTZ is found in axonal tracts and at low levels in sensilla. (A) A higher magnification of the w1118 antenna shown in Figure 2A. KRZ immunoreactivity is shown in green, and the nuclear elav protein is shown in magenta. The white arrowheads point to lightly stained sensilla. (B) A higher magnification of the c155; krz1 antenna shown in Figure 2C. α-KRZ labels all cells in wild-type third antennal segments but only a subset in the c155; krz1 antenna. (C) KRZ immunoreactivity is shown in brown in this paraffin section of a w1118 antenna. The staining is found throughout the third antennal segments, including the axonal tracts leaving the third segment, which are indicated by the black arrowheads. (D) α-KRZ antibody does not stain paraffin sections of krz1 (P{UASkrz}T12/+; krz1, P{hspGal4}/krz1) antenna.
Results
KURTZ is highly expressed in the olfactory system
In order to determine the expression pattern of the krz gene product and to measure the requirement of krz in olfaction, we needed adult krz loss-of-function flies. Since the krz1 mutation results in lethality prior to pupa formation, we used a heat shock Gal4 driver to induce kurtz expression during development. After eclosion, the flies were transferred to 18° C for 4 days to reduce the hspGal4 driver activity and thereby turn off krz expression. Most of the krz1-rescued homozygotes (P{UASkrz}T12/+; krz1, P{hspGal4}/krz1) generated with this procedure have normal external sensory structures (Roman et al., 2000). Many broods, however, produce flies with a range of antennal structural defects (Figure 1). The flies with the most severe defects generally emerged much later than the homozygotes with normal antenna. These defects were never found in the heterozygotes, the b5.8T; krz1 genomic–rescued flies, or the c155; krz1 pan-neuronal–rescued flies, but similar defects were found in the krz1 homozygotes carrying either of the tested Or83bGal4 drivers. Thus, the antennal developmental defect stems from a neural requirement of krz, but expression driven by the OR83bGal4 driver is insufficient to rescue this phenotype.
Figure 1.

Rescued krz1 homozygous demonstrate a range of antennal structural defects. The rescued krz1 homozygous adults display variable penetrance and expressivity of antennal structural defects. (A) A w1118 adult with a wild-type antennal structure. (B) krz1 homozygote (P{UASkrz}T12/+; krz1, P{hspGal4}/krz1), normal antennal structure; this phenotype is the most common and is generally found in flies that are first to eclose (C) krz1 homozygote antenna with an arista that is bent and has a thicker base. This antenna also has a shortened bulbar third antennal segment. This phenotype is uncommon. (D) krz1 homozygote with no aristae and a third antennal segment missing many sensilla; this phenotype is rare and is typically found in flies that eclose several days to a week later than the flies that are first to emerge. (E) OR83B-rescued krz1 homozygote (P{UASkrz}T12/+; P{hspGal4}/krz1, P{Or83b-Gal4}), normal antennal structure; this phenotype is the most common. (F) OR83B-rescued krz1 homozygote with no aristae and a third antennal segment missing many sensilla; this phenotype is rare.
We next examined the levels of the kurtz gene product within the antennae of krz1-rescued homozygotes (P{UASkrz}T12/+; krz1, P{hspGal4}/krz1) and control genotypes. KRZ immunoreactivity was expressed throughout the sensory systems of the second and third antennal segments in wild-type flies (Figures 2A and 3). The second antennal segment contains Johnston’s organ, the primary auditory organ for adult Drosophila, while the third antennal segment is the primary olfactory organ of the adult fly (Miller, 1950). KRZ was present in all cells of the third antennal segment (see Figures 2A and 3C and compare to Figure 2C). In the ORNs, kurtz expression can be seen in the axonal tracks entering the lumen of the third antennal segment; KRZ was also found at low levels in the olfactory sensilla of wild-type antennae (Figures 2A and 3). KRZ immunoreactivity was rarely detected in the heat shock–rescued krz1 homozygotes, and when found, it was limited to very few cells; the expression of the elav nuclear neuronal marker remained unchanged, indicating that the neuron fate was not grossly altered (Figures 2B and 3D). The absence of detectable kurtz expression within the antenna indicates that these krz1 homozygous adults, which have been rescued through development by the induced expression of a kurtz cDNA, can be used as severe reduction-of-function mutants for behavioral analysis. Interestingly, the expression level of KRZ did not change 3 h after a 1-h heat shock in the rescued krz1 mutant antennae, indicating that the hspGal4 driver did not induce krz transcription in the mature antenna (data not shown). The c155 pan-neuronal–rescued krz1 homozygotes expressed high levels of KRZ within the neurons of the second and third antennal segments (Figure 2C). The P{Or83b-Gal4}-containing heat shock–rescued krz1 flies exhibited a strong KRZ immunoreactivity in the ORNs of the third antennal segment but not in the second antennal segment or within neurons of the central brain (Figure 2D; data not shown). KRZ immunoreactivity is more clearly seen in the ORN axonal tracks and cilia of both the c155-rescued krz1 homozygotes and the P{Or83b-Gal4}-containing heat shock–rescued krz1 flies than in the wild-type antenna.
Figure 2.

KURTZ is highly expressed in the olfactory system. KURTZ is shown to be expressed in the ORNs in the second and third antennal segments in the wild-type (A) (w1118), heat shock–rescued (B) (krz1), pan-neuron–rescued (C) (c155; krz1), and olfactory receptor–rescued homozygotes (D) (Or83bGal4, krz1). Affinity-purified α-KURTZ antibody was detected with goat anti-rabbit–Alexa488 and is shown in green. α-elav antibody is a pan-neuron nuclear marker shown in magenta and was detected with goat anti-mouse–Alexa594. Overlapping expression is shown in white. The genotypes of the flies are (A) w1118 = w1118; +; +; (B) krz1 = P{UASkrz}T12/+; krz1, P{hspGal4}/krz1; (C) c155; krz1 = c155/+; P{UASkrz}T12/+; krz1/krz1; and (D) Or83bGal4,krz1 = P{UASkrz}T12/+; krz1, P{hspGal4}/krz1, Or83bGal4 (III).
kurtz is required for the normal behavioral responsiveness to odors
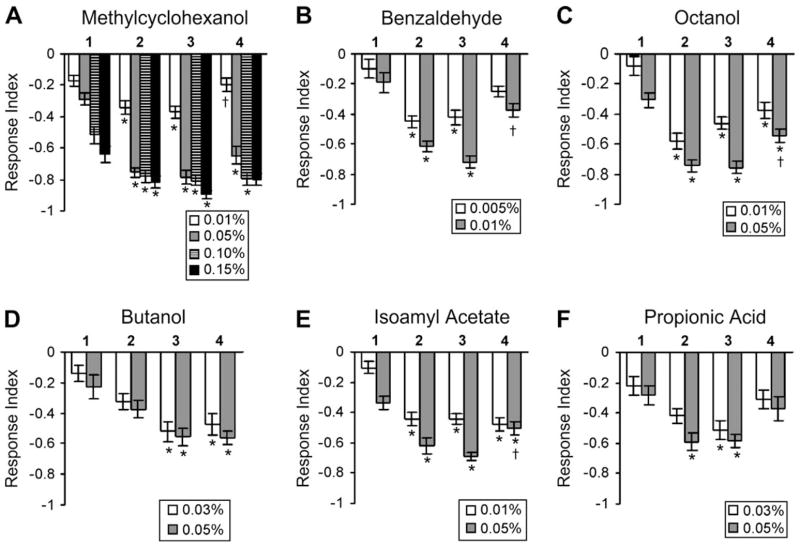
The expression of the krz gene product in the ORNs of the third antennal segment suggested that it may have a role in regulating olfactory responses. We tested this hypothesis by measuring the behavioral responses to six different odorants at both lower and higher concentrations. The four genotypes, krz1, krz1/+, hspGal4/+, and b5.8; krz1, were generated and tested under the same conditions. The heterozygous flies controlled for any possible dominant effects of the Gal4 and P{UASkrz} transgenes found within the krz1 homozygous mutants. The hspGal4/+ flies were used as the wild-type controls. The b5.8T4 (or T12); krz1 flies are genomic-transgene–rescued krz1 homozygotes; these genomic fragments rescue both the krz lethality and melanotic tumor phenotypes (Roman et al., 2000). All four genotypes exhibited aversion to MCH at 0.01%, 0.05%, 0.1%, and 0.15% (Figure 4A). The krz1 flies were significantly less sensitive to all odor concentrations than both hspGal4/+ and krz1/+ flies. The heterozygous controls were not statistically different from wild type, demonstrating the krz1 odor avoidance phenotype was recessive and therefore not due to effects of the rescuing P elements. In addition, the b5.8T12; krz1 flies had higher avoidance scores than the krz1 flies at every concentration, although significant differences were found only at 0.05% and 0.1% MCH. Similar results were obtained when we tested for avoidance to BEN, OCT, IA, and PA where at all concentrations the krz1 flies displayed a significantly lower avoidance compared to hspGal4/+ wild type (Figure 4). The krz genomic transgenes completely rescued the krz1 phenotype for OCT, butanol, and IA avoidance at both higher and lower concentrations. These genomic transgenes did not effectively rescue the avoidance to BEN and PA, perhaps due to the reduced or incomplete krz expression resulting from position effects of the rescuing transgene. No significant difference was found between the heterozygous flies and the hspGal4/+ wild-type control in these experiments.
Figure 4.
krz1 mutants demonstrate reduced olfactory avoidance. All genotypes were raised at the same conditions and examined for avoidance of different aversive odorants at the listed concentrations. A minimum of 16 groups were assayed for each data point. Significant effects of genotype were found for each odorant and concentration. In almost all cases, the krz1 exhibited a lower avoidance to the tested odorants than other groups. The b5.8; krz1-rescued flies had significantly higher avoidance scores than krz1 at most concentrations of each odorant tested, with the exception of 0.01% MCH, 0.005% BEN, and both concentrations of PA where no significant difference was found. The asterisk (*) indicates a significant difference with the hspGal4/+ control according to Bonferroni–Dunn post hoc analysis. The dagger (†) indicates where the b5.8; krz1-rescued flies are significantly different from the hspGal4/+ genotype. The b5.8; krz1-rescued flies were significantly different from the krz1/+ heterozygous flies at both concentrations of BEN but not for any other odor. The data demonstrate the olfactory deficit is due to the loss-of-kurtz function. The tested genotypes are as follows: 1. krz1 = P{UASkrz}T12/+; krz1, P{hspGal4}/krz1; 2. krz1/+ = P{UASkrz}T12/+; krz1/P{hspGal4}; 3. hspGal4/+ = P{hspGal4}/+; and 4. b5.8; krz1 = P{b5.8T12}; krz1 (in A, B, C) or P{b5.8T4}; krz1 (in D, E, F).
The rescued krz1 homozygotes were also tested for defects in odor attraction (Figure 5). EA is a relatively attractive odorant for flies at lower concentrations and becomes increasingly repellant at higher concentrations. The rescued krz1 homozygotes again displayed a shift toward reduced sensitivity. At 0.02% and 0.2% EA, the krz1 flies demonstrated attraction and avoidance, respectively, that was not significantly different than the control genotypes (Figure 5). However, at the lowest and highest concentrations of EA, the krz1 flies demonstrated significantly reduced responses to EA. This phenotype was not observed in the heterozygous flies or in the b5.8T4-rescued krz1 homozygotes, demonstrating that this behavioral defect is due to the loss of krz function. Thus, krz is required for normal sensitivity to both attractive and aversive odorants.
Figure 5.

krz1 mutants demonstrate reduced olfactory attraction. The krz1 homozygotes and control genotypes were tested for attraction and avoidance to different concentrations of EA. The control genotypes were attracted to lower concentrations of EA (0.01% and 0.02%) but avoided higher concentrations (0.2% and 0.4%). The krz1 mutants demonstrated both significantly less attraction to 0.01% EA and significantly less avoidance to 0.4% EA than the other genotypes. The asterisk (*) indicates a significant difference between krz1 and all other genotypes according to Bonferroni–Dunn post hoc analysis.
kurtz expression in ORNs is required for normal odor avoidance
By expressing the krz cDNA in different neurons of krz1 homozygotes, we have determined the cells responsible for the reduced sensitivity to odors. The lethality of krz1 can be rescued by the pan-neuronal expression of a krz cDNA (Roman et al., 2000). These flies (c155/+; P{UASkrz}T12/+; krz1/krz1) express KRZ throughout the nervous system, with no detectable expression outside of the nervous system (Figure 2C; data not shown). The neural expression in the c155; krz1 flies results in significantly greater avoidance to MCH and BEN than in the krz1 homozygotes (Figure 6). The c155; krz1 flies avoided both odors similarly to the c155; krz1/+ (c155/+; P{UASkrz}T12/+; krz1/+) heterozygous control flies. These data demonstrate a neural requirement for krz for normal olfactory sensitivity.
Figure 6.

Expression of kurtz in the nervous system rescues the deficit in olfactory avoidance. Flies were tested for avoidance to 0.005% BEN (white bars) and 0.05% MCH (gray bars). The c155; krz1 homozygotes expressing a krz cDNA within the nervous system have significantly higher avoidance scores than heat shock–rescued krz1 homozygotes. The level of KURTZ present within the c155-rescued antenna can be seen in Figure 2C. The minimum n for MCH = 9 and for BEN = 16. Significant effects of genotype were found for both odorants. The asterisk (*) indicates a significant difference from c155; krz1 according to Bonferroni–Dunn post hoc analysis. The exact genotypes used are as follows: krz1 = P{UASkrz}T12/+; krz1, P{hspGal4}/krz1; c155; krz1 = c155/+; P{UASkrz}T12/+; krz1/krz1; and c155; krz1/+ = c155/+; P{UASkrz}T12/+; krz1/+.
The neural focus of krz activity for olfaction was further defined with a pair of OR83b promoter-Gal4 drivers. These OR83bGal4 P elements drive the expression of krz specifically in approximately 70% of ORNs (Kalidas and Smith, 2002). These two Gal4 drivers were incorporated into the krz1 genotype (e.g., P{UASkrz}T12/+; krz1, P{hspGal4}/krz1, Or83bGal4), thereby providing KRZ expression within the ORNs, in an otherwise krz− nervous system (Figure 2D; data not shown). The resulting ORN-rescued krz1 flies displayed significantly greater olfactory avoidance to MCH than the krz1 homozygotes and is not significantly different from the control genotypes (Figure 7). Interestingly, both of these krz1, ORN-rescued genotypes displayed the same range of antennal defects seen with the krz1 homozygotes (Figure 1). The ability of the OR83bGal4 drivers to rescue the behavioral deficit, but not the developmental defect, led us to ask when, during development, this driver activates transcription within the ORNs. Both of these drivers did not begin to activate green fluorescent protein (GFP) reporter expression until approximately 82 h after puparium formation (APF) in almost mature ORNs (Figure 8). This is consistent with the OR83b gene, in which message becomes detectable within the ORNs at 80 h APF; a time lag between the transcription of Gal4 in the OR83bGal4 drivers and the activation of transcription of the UAS responder would be expected (Larsson et al., 2004). Therefore, krz expression within essentially mature ORNs is sufficient for normal avoidance of MCH in the krz1 mutants, indicating that for wild-type behavioral responses to odors the requirement for krz may entirely lie within the ORNs.
Figure 7.
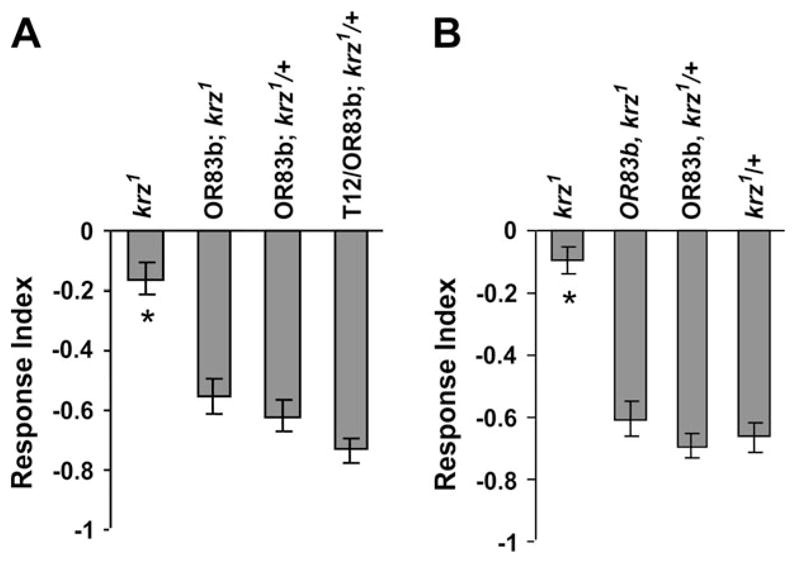
kurtz is required in ORNs for normal olfaction. The avoidance to 0.05% MCH of ORN-rescued krz1 homozygotes and control genotypes is shown. Both the chromosome 2 (A) and chromosome 3 (B) P{Or83b-Gal4} P elements were used independently to drive krz expression within the ORNs of the krz1 homozygotes. The level of KURTZ present within P{Or83b-Gal4} (third chromosome)–rescued antenna can be seen in Figure 2D; results were similar for the second chromosome P{Or83b-Gal4} P element. The P{Or83b-Gal4} driver first begins activating transcription. The P{Or83b-Gal4}–rescued krz1 homozygotes have significantly higher avoidance scores than heat shock–rescued krz1 homozygotes. The minimum n = 16 for panel (A) and 18 for panel (B). Significant effects of genotype were found in both experiments. The asterisk (*) indicates a significant difference of krz1 from all the other genotypes according to Bonferroni–Dunn post hoc analysis. These data indicate that KURTZ is required in ORNs for normal MCH sensitivity. The complete genotypes were as follows: krz1 = P{UASkrz}T12/+; krz1, P{hspGal4}/krz1. In panel (A): Or83b; krz1 = P{UASkrz}T12/P{Or83b-Gal4}; krz1, P{hspGal4}/krz1; Or83b; krz1/+ = P{Or83b-Gal4}/+; krz1/+; T12/Or83b; krz1/+ = P{UASkrz}T12/P{Or83b-Gal4}; krz1, P{hspGal4}/+. In panel (B): OR83b, krz1 = P{UASkrz}T12/+; krz1, P{hspGal4}/krz1, Or83bGal4 (III); OR83b, krz1/+ = P{UASkrz}T12/+; P{hspGal4}/krz1, P{Or83b-Gal4}; krz1/+ = P{UASkrz}T12/+; krz1/P{hspGal4}.
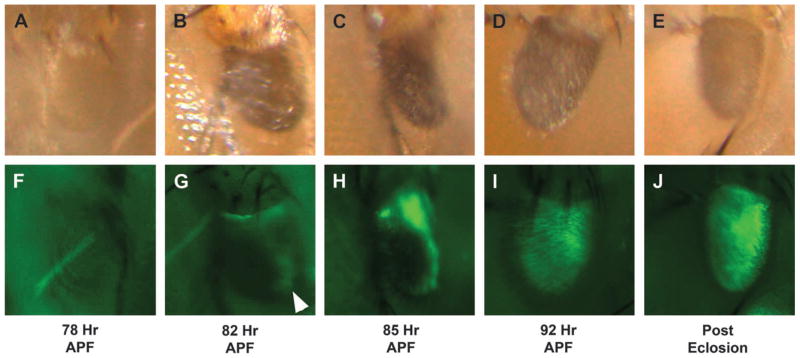
Figure 8.
P{Or83b-Gal4} transcriptional activation begins shortly before eclosion. P{Or83b-Gal4} (third chromosome)/UAS-EGFP transheterozygous antennae are shown at the designated times APF. Panels (A) through (E) display the antennal structures, while panels (F) through (J) show expression of enhanced green fluorescent protein (EGFP). At 78 h APF, the head bristles were fully pigmented, pigmentation was beginning in the abdominal bristles, and GFP expression was not yet visible in the third antennal segment (A, F). The first hints of GFP expression were found at 82 h APF (arrowhead; B, G). At 85 h APF, GFP expression is beginning to spread, but it is not yet established throughout the third antennal segment (C, D). By 92 h APF, GFP is detected widely throughout the third antennal segment (D) and (I). GFP expression was even more pronounced after eclosion E and (J).
kurtz is required for normal electrophysiological responses to odors
The localization of the krz olfactory defect to the ORNs suggested that these cells may have defects in the perception and initial transduction of olfactory information. To address this question, we have measured the initial odor-induced electrophysiological responses in krz1 homozygotes with EAGs (Figure 9). The krz1 homozygotes displayed significantly lower EAG amplitudes to both MCH and OCT than the control hspGal4/+ line. Like the krz1 behavioral defect, the reduced EAG amplitude was recessive and reversed by the targeted expression of the krz cDNA within the ORNs (Figure 9). Interestingly, there is a 40% reduction in EAG amplitude found in the krz1 homozygotes for 0.01% MCH, while the behavioral avoidance to this concentration results in a 49% reduction in the avoidance index of krz1 homozygotes avoiding the odor (Figure 3A). Even so, direct comparisons between the strength of a behavioral avoidance of an odorant and the electrophysiological response to that odor found in an EAG should be interpreted carefully as the context for these different responses are very different and they may also have different detection thresholds or nonlinear components that can be saturated. In summary, the loss-of-kurtz function reduces the summed receptor potentials elicited by odors; this defect in the early stages in olfactory transduction can, at least partially, account for the behavioral defects of the krz1 homozygotes.
Figure 9.

krz1 homozygotes display reduced receptor potentials. The EAG amplitude of krz1 homozygotes and control genotype are shown for 0.0001% OCT (A) and for 0.01% MCH (B). The genotypes are as follows: krz1 = (P{UASkrz}T12/+; krz1, P{hspGal4}/krz1); krz1, OR83b = (P{UASkrz}T12/+; krz1, P{hspGal4}/krz1, Or83bGal4); krz1, OR83b/+ = (P{UASkrz}T12/+; P{hspGal4}/krz1, P{Or83b-Gal4}); and hspGal4/+ = P{hspGal4}/+. (A) OCT: a significant effect of genotype was found in this experiment (F = 9.557, P < 0.0001). The OCT-induced EAG amplitude of krz1 homozygotes was significantly lower than that of the three control genotypes (Bonferroni–Dunn, P < 0.002). The three control genotypes were not significantly different from each other. n = 12 for each genotype. (B) MCH: a significant effect of genotype was found in this experiment (F = 28.1, P < 0.0001). The MCH-induced EAG amplitude of krz1 homozygotes was significantly lower than that of the other three genotypes (Bonferroni–Dunn, P < 0.0007). The EAG amplitude of the OR83b, krz1 heterozygous but not OR83b, and krz1 homozygous flies was also significantly lower than that of the hspGal4/+ wild-type control flies (Bonferroni–Dunn, P < 0.0001). n = 8 females for each control genotype and n = 14 for the krz1 homozygotes.
We next wanted to see if krz mutants experienced deficits in olfactory adaptation. Olfactory adaptation is a short-term reduction in olfactory sensitivity brought about by an exposure to high concentrations of odorants. In Drosophila, the exposure to high concentrations of odorants brings about a significant reduction in olfactory responsiveness as measured by EAGs, which lasts for several minutes after the initial exposure (Stortkuhl et al., 1999). In Caenorhabditus elegans, the behavioral adaptation to high odor concentrations is controlled by the nonvisual arrestin (Palmitessa et al., 2005). In newts, the primary molecular event that controls olfactory adaptation acts downstream of cyclic adenosine 3′ :5′ monophosphate (cAMP) production and therefore after OR activation (Kurahashi and Menini, 1997). In these vertebrates, olfactory adaptation appears to occur through the negative feedback of Ca++ on the affinity of the cyclic nucleotide-gated Ca++ channel. If krz is responsible for the desensitization of activated ORs within the cilia, then the loss of krz activity would increase the magnitude and duration of OR activation. From this we can make two predictions regarding olfactory adaptation. If adaptation in Drosophila results from events downstream of the OR activation similar to vertebrates, then krz1 mutant antenna should show increases in the magnitude of adaptation, that is, the loss of krz activity would resemble stronger odor stimuli, leading to greater adaptation. Alternatively, if adaptation is dependent on the agonist-dependent desensitization similar to C. elegans, then the krz1 mutants will fail to display adaptation. We tested these two predictions by examining the reduction and recovery of receptor potentials after a prolonged stimulus in the krz1 homozygotes and hspGal4/+ flies (Figure 10). In these experiments, a 30-s stimulus of odorant was applied to induce adaptation. Following this stimulus, EAGs elicited by a 1-s pulse of the same odorant were recorded at defined intervals (Figure 10). Even though the krz1 homozygotes were less sensitive to odorants in general, they still displayed adaptation of the EAG responses. In these experiments, we found significant differences between the krz1 homozygotes and hspGal4/+ flies with both OCT and MCH. However, the differences were limited and not consistent between odorants. For example, 5 s after the adapting stimulus, the krz1 homozygotes were significantly less sensitive to OCT, but they were more sensitive to MCH than the control flies. These data suggest that, while krz has a dramatic effect on maintaining olfactory sensitivity, this gene does not have a major role in regulating a decreased sensitivity and recovery rate after strong and persistent exposure to odors.
Figure 10.

Olfactory adaptation and recovery in krz1 homozygotes. The EAGs of krz1 homozygotes (P{UASkrz}T12/+; krz1, P{hspGal4}/krz1) and hspGal4/+ flies were measured prior to and following a 30-s adapting stimulus of either (A) OCTor (B) MCH. The percentage of EAG recovery is shown as the amplitude elicited by 1-s pulses of odor at the indicated times following the adapting stimulus. There were significant effects of both time and genotype for OCT and MCH. The asterisk (*) indicates significant difference by Bonferroni–Dunn post hoc analysis. Independent flies were used for the two odors. n = 8 females of each genotype in each experiment.
Discussion
Herein we have demonstrated that there are at least two requirements in olfaction for the krz nonvisual arrestin: one developmental and the other postdevelopmental. The adult rescued loss-of-function krz mutations have an incompletely penetrant structural defect in the antenna. This recessive phenotype is rescued by the expression of a krz cDNA in the nervous system but not by the late expression of krz within the ORNs. The krz1 mutants without the visible structural defects have a blunted behavioral responsiveness to odorants. This latter phenotype is a general shift toward reduced sensitivity at several concentrations and affects both aversive and attractive odorants. The defect in olfactory sensitivity was shown to be recessive and was mapped to the krz locus both by a genomic transgene and through the targeted expression of a krz cDNA within the ORNs. The behavioral defect is also accompanied by a reduced odor-induced receptor potential within the third antennal segment. The reduced EAG amplitude was also recessive and can be rescued by late expression of the krz cDNA within the ORNs. Thus, krz—the only nonvisual arrestin in Drosophila—is required essentially postdevelopmentally for generating normal receptor potentials after olfactory stimulation, and this reduced responsiveness to odors may account for most of the reduced sensitivity in olfactory behavioral responses.
An important question arising from this study is when krz is required for these two requirements. The krz gene is broadly expressed in the late third instar antennal imaginal discs, consistent with a role for this gene during development of this organ (Roman et al., 2000). The variable penetrance and expressivity of the structural antennal defect in the rescued krz1 homozygotes are most likely due to differences in the induced expression of krz between individuals during development, which may relate to how old they are when krz induction ceases. The induction of krz activity in wandering third instar homozygotes results in a very low rate of antennal defects (Roman et al., 2000; data not shown). However, in our preparation of krz mutants, some homozygotes will receive the final heat shock induction of krz activity as a late L2 to an early L3 instar and, as a consequence, may have less krz gene product available during early to mid metamorphosis, when the antenna structure develops, than the older flies. This hypothesis is also consistent with our observation that the later a fly ecloses, the more severe is the antennal phenotype. During metamorphosis, most of the rescued krz homozygotes die shortly after the heat shock and none will eclose (Roman et al., 2000). Therefore, a more thorough dissection of the timing of this krz requirement is difficult. Nevertheless, since the pan-neuronal (c155)–rescued krz homozygotes have completely normal antenna, the foci for this developmental requirement is neuronal. The activation of krz activity in the ORN with the OR83bGal4 drivers is too late to rescue the developmental defect. It is also not clear whether the ORNs are responsible for the krz-defined developmental requirement.
The krz gene product is present in adult ORNs, suggesting a continued physiological requirement for this gene. The rescue of both the olfactory avoidance and the reduced receptor potential phenotypes with the OR83bGal4 driver places the earliest time point for a krz requirement for these responses at just before eclosion. By this time (~85 h APF), the ORNs have all differentiated, the antennal nerve has stopped expanding, ORN axons have found their targets in the antennal lobe, and at least some of the ORs have begun to accumulate in the cell bodies awaiting transfer to the cilia (Stocker et al., 1995; Clyne et al., 1999; Elmore and Smith, 2001; Jefferis et al., 2004). The OR83b gene is required to shuttle most ORs into the cilia, allowing for proper subcellular expression of these receptors (Larsson et al., 2004). The krz gene product may function in the maturation of the dendritic component of the ORN, and it may have a function that would be persistently required for odor sensitivity. However, since the krz1 mutants can respond to odors, this gene is not essential for the odor response, indicating that either there are redundant functions for this gene or krz acts as a modifier of the olfaction-signaling pathway.
The earliest step in odor-induced signal transduction within the Drosophila ORNs is thought to involve the activation of the ORs within the cilia membranes. These ORs are members of a GPCR family, although it is not known if they, in fact, couple to G proteins. In Xenopus oocytes, however, the OR43a protein was capable of coupling with a human Gα 15-containing G protein, suggesting that these receptors may also couple to heterotrimeric G proteins in vivo (Wetzel et al., 2001). The vertebrate nonvisual arrestins regulate the activity of GPCRs in two ways: first, by binding to phosphorylated, agonist-bound receptors and thereby inhibiting further interactions of the activated GPCR with heterotrimeric G proteins and, second, by drawing the receptor into the endocyotic machinery where the GPCR is dephosphorylated and can be recycled back to the plasma membrane. Nonvisual arrestins promote the endocytosis of activated GPCRs through the clathrin-binding domain and an adaptor protein (AP)-2–binding domain. The interaction of nonvisual arrestins with the β 2-adaptin subunit of the AP-2 appears to be the rate-limiting step in bringing the GPCR–arrestin complex into clathrin-coated pits (Kirchhausen et al., 1997). Most nonvisual arrestin–GPCR complexes dissociate at the coated pit, but in a few cases, the complex persists through internalization (Pitcher et al., 1995; Krueger et al., 1997; Oakley et al., 1999). The activity of nonvisual arrestins is not limited, however, to members of the GPCR superfamily but includes the ability to bind to and internalize a larger group of membrane receptors (Lefkowitz and Shenoy, 2005).
Since the primary step in olfactory signal transduction is the activation of ORs within the cilia of the ORNs, an evident hypothesis is that nonvisual arrestins have the specific role of regulating the responsiveness of these neurons through agonist-dependent desensitization of these receptors. In rat olfactory membranes, pretreatment with neutralizing antibodies against GRK3 or β arrestin-2 lead to dramatic increases in odor-induced cAMP production, which indicates that the GRK3 and β arrestin-2 are most probably desensitizing the OR within these sensory neurons (Dawson et al., 1993; Schleicher et al., 1993). In contrast, the loss of GRK activity in mutants results in a diminished sensitivity to odorants. The deletion of the GRK3 gene results in mice that have reduced odor-induced cAMP production and hence most probably reduced olfactory sensitivity (Peppel et al., 1997). Mutants of the grk-2 gene in C. elegans also have decreased behavioral and physiological responses to odorants (Fukuto et al., 2004). The reduced sensitivity to odorants in these mutants would suggest that the chronic loss of GRK activity leads to a compensatory mechanism that downregulates early events in olfactory signal transduction. It is not presently clear what this mechanism may be. The phenotypes of the mouse and C. elegans GRKs are analogous to those that we report here for the krz gene of Drosophila. It is interesting that loss-of-function mutants in the only arrestin in C. elegans displaying wild-type behavioral sensitivity to odorants, suggesting that the grk-2 gene product can regulate ORs in an arrestin-independent manner (Fukuto et al., 2004; Palmitessa et al., 2005).
Visual arrestins differ from nonvisual arrestins both in their patterns of expression and structurally (Craft and Whitmore, 1995). The vertebrate visual arrestins are specialists; they are located almost exclusively in photoreceptor cells and function primarily to desensitize the opsins (Wilden et al., 1986). The vertebrate β Arr1 and β Arr2 nonvisual arrestins are generalists; they are expressed in most tissues and can desensitize a large number of GPCRs and non-GPCRs (Parruti et al., 1993; Sterne-Marr et al., 1993; Gurevich et al., 1995; Lefkowtiz and Shenoy, 2005). The vertebrate and Drosophila visual arrestins do not have obvious clathrin-binding domains or AP-2–binding domains similar to those located near the carboxyl end of the non-visual arrestins (Krupnick et al., 1997; Laporte et al., 1999). These two arrestin classes are similar in how they bind to activated GPCRs but differ in the events subsequent to binding (Palczewski et al., 1989; Gurevich et al., 1995; Pitcher et al., 1995). The visual arrestins are released from rhodopsin after this receptor is dephosphorylated on the disc membrane of the rod outer segment (Palczewski et al., 1989; King et al., 1994).
In Drosophila, there are two visual arrestins: arr1 and arr2 (Merrill et al., 2002, 2004). A third Drosophila gene, CG32683, is identified as an arrestin by significant sequence similarity in both the amino- and carboxy-terminal domains, though it is about twice the size of the other arrestins; this atypical arrestin also lacks both the clathrin-binding and AP-2 adaptor–binding domains that structurally and functionally differentiate the nonvisual arrestins from the visual arrestins, suggesting that it may operate as a third visual arrestin (Krupnick et al., 1997; Laporte et al., 1999). The arr1 and arr2 genes are expressed in the antenna and maxillary palps in addition to the photoreceptor neurons, while the expression of CG32683 is presently uncharacterized. Loss-of-function mutations in either of the two characterized visual arrestins have variably reduced EAG amplitudes (Merrill et al., 2002, 2005). The individual arr1 and arr2 mutants displayed different electrophysiological responses to different odors and odor concentrations; these responses were frequently more severe in the arr1, arr2 double mutant, suggesting a functional redundancy between the two genes. Additionally, these mutants had minor differences in olfactory adaptation as measured by EAGs (Merrill et al., 2005). Together, these results indicate a role for these two visual arrestins in modulating olfactory responsiveness.
Although our data demonstrate a role for krz in regulating the early events in olfactory signal transduction, it is not clear whether krz affects ORs through the canonical desensitization–resensitization cycle of vertebrate nonvisual arrestins. Even though KRZ is found, albeit at low levels, within the sensilla and therefore may directly interact with the activated ORs, we did not find that krz had a considerable effect on olfactory adaptation to OCT or MCH; the kinetics of desensitization and resensitization of the receptor potentials in the krz1 homozygotes were similar to wild-type controls. The reduced agonist-dependent desensitization of ORs predicted in krz1 homozygotes would be expected to lead to a prolonged activation of downstream effectors. The resulting increase in secondary messengers should lead to increased adaptation (Kurahashi and Menini, 1997). The absence of a prominent and general effect on olfactory adaptation in the krz1 homozygotes could be reconciled with the krz-dependent desensitization of ORs model either if there is a functional redundancy with the visual arrestins or if a separate compensating mechanism may mask this effect. The visual arrestins are also expressed in the ORNs and may provide functional redundancy. Moreover, as stated above, the differences between the effects of short-term inhibition of GRK3 and the GRK3 mutations suggest the presence of such a compensating mechanism in the rodent ORNs, which may also exist in the Drosophila ORNS (Dawson et al., 1993; Schleicher et al., 1993; Peppel et al., 1997; Merrill et al., 2002). It also remains possible that krz is not required for the agonist-dependent desensitization of ORs but has a different role in regulating early events in olfactory signal transduction.
It is not known if Drosophila ORs are internalized after odor presentation. In catfish, agonist-bound metabotropic glutamate receptors are internalized into the dendrites and cell bodies of ORNs through a clathrin- and dynamin-dependent pathway, indicating that the machinery for internalization of ORs is present in these cells (Rankin et al., 1999). Nevertheless, the internalization and resensitization of desensitized ORs seems unlikely to occur within the olfactory cilia through the clathrin-dependent internalization pathway. The Drosophila olfactory cilia are long, branched processes that are as thin as 50 nm in diameter within the large basiconic sensilla (Shanbhag et al., 1999). Clathrin-coated vesicles appear to come in two sizes: 60 or 100 nm in diameter (Stoorvogel et al., 1996). Thus, even if a single clathrin-coated vesicle was internalized within a basiconic cilium, it would likely occlude protein trafficking and perhaps inhibit signaling from distal parts of the dendrite. It seems unlikely therefore that arrestin-dependent internalization of ORs would take place within the cilia. In both frog photoreceptor neurons and in the olfactory neurons of C. elegans, specialized areas of clathrin-mediated vesicle sorting are located at the base of the rod outer segment and olfactory cilia, respectively (Deretic and Papermaster, 1991; Dwyer et al., 2001). A similar region of specialization may exist in Drosophila ORNs, where coated vesicles have been found forming at the base of the olfactory cilia (Shanbhag et al., 2000). It will be interesting to see if KRZ is involved in the removal of ORs from the cilia membrane or the recycling of ORs back to the cilia membranes at these sites and whether this function can account for the reduced olfactory sensitivity found in the krz1 mutants.
Understanding how organisms perceive and respond to the environment is a fundamental problem for behavioral neuroscience. GPCRs are sentries for much of the information that an organism perceives. We need a better understanding of the mechanisms that regulate the fidelity of these sensory receptors before we can fully comprehend how organisms react to and interact with external stimuli. Nonvisual arrestins are among the most important regulators of the activities of GPCRs. We have shown for the first time that a mutant of a nonvisual arrestin has reduced, but not eliminated, olfactory sensitivity. Thus, this molecule is required for the preservation of the full range of olfactory responsiveness in Drosophila. The further characterization of the role of krz in the ORNs should provide us with a detailed understanding of how nonvisual arrestins maintain the sensitivity of early events in olfactory signal transduction.
Acknowledgments
We thank Stuart Dryer, in whose laboratory several experiments were performed. We are indebted to Dean Smith and Leslie Vosshall for supplying us with antibodies and Gal4 drivers. We also thank Curtis Wilson and Jacob Ferris for expert technical assistance. Additional fly stocks were kindly provided by the Bloomington Stock Center. This work was funded by National Institutes of Health grants NS42185 and DC04857.
References
- Buck L, Axel R. A novel multigene family may encode odorant receptors: a molecular basis for odor recognition. Cell. 1991;65:175–187. doi: 10.1016/0092-8674(91)90418-x. [DOI] [PubMed] [Google Scholar]
- Clyne PJ, Warr CG, Freeman MR, Lessing D, Kim JH, Carlson JR. A novel family of divergent seven-transmembrane proteins: candidate odorant receptors in Drosophila. Neuron. 1999;22:327–338. doi: 10.1016/s0896-6273(00)81093-4. [DOI] [PubMed] [Google Scholar]
- Craft CM, Whitmore DH. The arrestin superfamily: cone arrestins are a fourth family. FEBS Lett. 1995;362:247–255. doi: 10.1016/0014-5793(95)00213-s. [DOI] [PubMed] [Google Scholar]
- Dawson TM, Arriza JL, Jaworsky DE, Borisy FF, Attramadal H, Lefkowitz RJ, Ronnett GV. Beta-adrenergic receptor kinase-2 and beta-arrestin-2 as mediators of odorant-induced desensitization. Science. 1993;259:825–829. doi: 10.1126/science.8381559. [DOI] [PubMed] [Google Scholar]
- Deretic D, Papermaster DS. Polarized sorting of rhodopsin on post-Golgi membranes in frog retinal photoreceptor cells. J Cell Biol. 1991;113:1281–1293. doi: 10.1083/jcb.113.6.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devaud J, Acebes A, Ferrus A. Odor exposure causes central adaptation and morphological changes in selected olfactory glomeruli in Drosophila. J Neurosci. 2001;21:6274–6282. doi: 10.1523/JNEUROSCI.21-16-06274.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwyer ND, Adler CE, Crump JG, L’Etoile ND, Bargmann CI. Polarized dendritic transport and the AP-1 mu1 clathrin adaptor UNC-101 localize odorant receptors to olfactory cilia. Neuron. 2001;31:277–287. doi: 10.1016/s0896-6273(01)00361-0. [DOI] [PubMed] [Google Scholar]
- Elmore T, Smith DP. Putative Drosophila odor receptor OR43b localizes to dendrites of olfactory neurons. Insect Biochem Mol Biol. 2001;31:791–798. doi: 10.1016/s0965-1748(00)00184-3. [DOI] [PubMed] [Google Scholar]
- Ferguson SS, Barak LS, Zhang J, Caron MG. G-protein-coupled receptor regulation: role of G-protein-coupled receptor kinases and arrestins. Can J Physiol Pharmacol. 1996;74:1095–1110. doi: 10.1139/cjpp-74-10-1095. [DOI] [PubMed] [Google Scholar]
- Fukuto HS, Ferkey DM, Apicella AJ, Lans H, Sharmeen T, Chen W, Lefkowitz RJ, Jansen G, Schafer WR, Hart AC. G protein-coupled receptor kinase function is essential for chemo-sensation in C. elegans. Neuron. 2004;42:581–593. doi: 10.1016/s0896-6273(04)00252-1. [DOI] [PubMed] [Google Scholar]
- Gao Q, Chess A. Identification of candidate Drosophila olfactory receptors from genomic DNA sequence. Genomics. 1999;60:31–39. doi: 10.1006/geno.1999.5894. [DOI] [PubMed] [Google Scholar]
- Goldman AL, van Naters WVDG, Lessing D, Warr CG, Carlson JR. Coexpression of two functional odor receptors in one neuron. Neuron. 2005;45:661–666. doi: 10.1016/j.neuron.2005.01.025. [DOI] [PubMed] [Google Scholar]
- Gurevich VV, Dion SB, Onorato JJ, Ptasienski J, Kim CM, Sterne-Marr R, Hosey MM, Benovic JL. Arrestin interactions with G protein-coupled receptors. Direct binding studies of wild type and mutant arrestins with rhodopsin, beta 2-adrenergic, and m2 muscarinic cholinergic receptors. J Biol Chem. 1995;270:720–731. doi: 10.1074/jbc.270.2.720. [DOI] [PubMed] [Google Scholar]
- Jefferis GS, Vyas RM, Berdnik D, Ramaekers A, Stocker RF, Tanaka NK, Ito K, Luo L. Developmental origin of wiring specificity in the olfactory system of Drosophila. Development. 2004;131:117–130. doi: 10.1242/dev.00896. [DOI] [PubMed] [Google Scholar]
- Kalidas S, Smith DP. Novel genomic cDNA hybrids produce effective RNA interference in adult Drosophila. Neuron. 2002;33:177–184. doi: 10.1016/s0896-6273(02)00560-3. [DOI] [PubMed] [Google Scholar]
- King AJ, Andjelkovic N, Hemmings BA, Akhtar M. The phospho-opsin phosphatase from bovine rod outer segments. An insight into the mechanism of stimulation of type-2A protein phosphatase activity by protamine. Eur J Biochem. 1994;225:383–394. doi: 10.1111/j.1432-1033.1994.00383.x. [DOI] [PubMed] [Google Scholar]
- Kirchhausen T, Bonifacino JS, Riezman H. Linking cargo to vesicle formation: receptor tail interactions with coat proteins. Curr Opin Cell Biol. 1997;9:488–495. doi: 10.1016/s0955-0674(97)80024-5. [DOI] [PubMed] [Google Scholar]
- Krishnan B, Dryer SE, Hardin PE. Circadian rhythms in olfactory responses of Drosophila melanogaster. Nature. 1999;400:375–378. doi: 10.1038/22566. [DOI] [PubMed] [Google Scholar]
- Krueger KM, Daaka Y, Pitcher JA, Lefkowitz RF. The role of sequestration in G-protein coupled receptor resensitization: regulation of β2-adrenergic receptor dephosphorylation by vesicular acidification. J Biol Chem. 1997;272:5–8. doi: 10.1074/jbc.272.1.5. [DOI] [PubMed] [Google Scholar]
- Krupnick JG, Benovic JL. The role of receptor kinases and arrestins in G protein-coupled receptor regulation. Annu Rev Pharmacol Toxicol. 1998;38:289–319. doi: 10.1146/annurev.pharmtox.38.1.289. [DOI] [PubMed] [Google Scholar]
- Krupnick JG, Goodman OB, Jr, Keen JH, Benovic JL. Arrestin/clathrin interaction. Localization of the clathrin binding domain of nonvisual arrestins to the carboxy terminus. J Biol Chem. 1997;272:15011–15016. doi: 10.1074/jbc.272.23.15011. [DOI] [PubMed] [Google Scholar]
- Kurahashi T, Menini A. Mechanism of odorant adaptation in the olfactory receptor cell. Nature. 1997;385:725–733. doi: 10.1038/385725a0. [DOI] [PubMed] [Google Scholar]
- Lamb TD. Gain and kinetics of activation in the G-protein cascade of phototransduction. Proc Natl Acad Sci USA. 1996;93:566–570. doi: 10.1073/pnas.93.2.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laporte SA, Oakley RH, Zhang J, Holt JA, Ferguson SS, Caron MG, Barak LS. The beta2-adrenergic receptor/beta-arrestin complex recruits the clathrin adaptor AP-2 during endocytosis. Proc Natl Acad Sci USA. 1999;96:3712–3717. doi: 10.1073/pnas.96.7.3712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson MC, Domingos AI, Jones WD, Chiappe ME, Amrein H, Vosshall LB. Or83b encodes a broadly expressed odorant receptor essential for Drosophila olfaction. Neuron. 2004;43:703–714. doi: 10.1016/j.neuron.2004.08.019. [DOI] [PubMed] [Google Scholar]
- Lefkowitz RJ, Shenoy SK. Transduction of receptor signals by beta-arrestins. Science. 2005;308:512–517. doi: 10.1126/science.1109237. [DOI] [PubMed] [Google Scholar]
- Menini A, Picco C, Firestein S. Quantal-like current fluctuations induced by odorants in olfactory receptor cells. Nature. 1995;373:435–437. doi: 10.1038/373435a0. [DOI] [PubMed] [Google Scholar]
- Merrill CE, Riesgo-Escovar J, Pitts RJ, Kafatos FC, Carlson JR, Zwiebel LJ. Visual arrestins in olfactory pathways of Drosophila and the malaria vector mosquito Anopheles gambiae. Proc Natl Acad Sci USA. 2002;99:1633–1638. doi: 10.1073/pnas.022505499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrill EC, Sherertz TM, Walker WB, Zwiebel LJ. Odorant-specific requirements for arrestin function in Drosophila olfaction. J Neurobiol. 2005;63:15–28. doi: 10.1002/neu.20113. [DOI] [PubMed] [Google Scholar]
- Miller A. The internal anatomy and histology of the imago of Drosophila melanogaster. In: Demerec M, editor. Biology of Drosophila. CSHL Press; New York: 1950. pp. 420–534. [Google Scholar]
- Oakley RH, Laporte SA, Holt JA, Barak LS, Caron MG. Association of beta-arrestin with G protein-coupled receptors during clathrin-mediated endocytosis dictates the profile of receptor resensitization. J Biol Chem. 1999;274:32248–32257. doi: 10.1074/jbc.274.45.32248. [DOI] [PubMed] [Google Scholar]
- Palmitessa A, Hess H, Bany IA, Kim YM, Koelle MR, Benovic JL. Caenorhabditus elegans arrestin regulates neural G protein signaling and olfactory adaptation and recovery. J Biol Chem. 2005;280:24649–24662. doi: 10.1074/jbc.M502637200. [DOI] [PubMed] [Google Scholar]
- Parruti G, Peracchia F, Sallese M, Ambrosini G, Masini M, Rotilio D, De Blasi A. Molecular analysis of human beta-arrestin-1: cloning, tissue distribution, and regulation of expression. Identification of two isoforms generated by alternative splicing. J Biol Chem. 1993;268:9753–9761. [PubMed] [Google Scholar]
- Peppel K, Boekhoff I, McDonald P, Breer H, Caron MG, Lefkowitz RJ. G protein-coupled receptor kinase 3 (GRK3) gene disruption leads to loss of odorant receptor desensitization. J Biol Chem. 1997;272:25425–25428. doi: 10.1074/jbc.272.41.25425. [DOI] [PubMed] [Google Scholar]
- Pitcher JA, Payne ES, Csortos C, DePaoli-Roach AA, Lefkowitz RJ. The G-protein coupled receptor phosphatase: a protein phosphatase type 2A with a distinct subcellular distribution and substrate specificity. Proc Natl Acad Sci USA. 1995;92:8343–8347. doi: 10.1073/pnas.92.18.8343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palczewski K, McDowell JH, Jakes S, Ingebritsen TS, Hargrave PA. Regulation of rhodopsin dephosphorylation by arrestin. J Biol Chem. 1989;264:15770–15773. [PubMed] [Google Scholar]
- Rankin ML, Alvania RS, Gleason EL, Bruch RC. Internalization of G protein-coupled receptors in single olfactory receptor neurons. J Neurochem. 1999;72:541–548. doi: 10.1046/j.1471-4159.1999.0720541.x. [DOI] [PubMed] [Google Scholar]
- Roman G, He J, Davis RL. kurtz, a novel non-visual arrestin, is an essential neural gene in Drosophila. Genetics. 2000;155:1281–1295. doi: 10.1093/genetics/155.3.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roman G, Meller V, Wu KH, Davis RL. The opt1 gene of Drosophila melanogaster encodes a proton-dependent dipeptide transporter. Am J Physiol. 1998;275:C857–C869. doi: 10.1152/ajpcell.1998.275.3.C857. [DOI] [PubMed] [Google Scholar]
- Schleicher S, Boekhoff I, Arriza J, Lefkowitz RJ, Breer H. A beta-adrenergic receptor kinase-like enzyme is involved in olfactory signal termination. Proc Natl Acad Sci USA. 1993;90:1420–1424. doi: 10.1073/pnas.90.4.1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanbhag SR, Müller B, Steinbrecht RA. Atlas of olfactory organs of Drosophila melanogaster. 1 Types, external organization, innervation, and distribution of olfactory sensilla. Int J Insect Morphol Embryol. 1999;28:377–397. [Google Scholar]
- Shanbhag SR, Müller B, Steinbrecht RA. Atlas of olfactory organs of Drosophila melanogaster. 2 Internal organization and cellular architecture of olfactory sensilla. Arthropod Struct Dev. 2000;29:211–229. doi: 10.1016/s1467-8039(00)00028-1. [DOI] [PubMed] [Google Scholar]
- Smith DE, Fisher PA. Identification, developmental regulation, and response to heat shock of two antigenically related forms of a major nuclear envelope protein in Drosophila embryos: application of an improved method for affinity purification of antibodies using polypeptides immobilized on nitrocellulose blots. J Cell Biol. 1984;99:20–28. doi: 10.1083/jcb.99.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterne-Marr R, Gurevich VV, Goldsmith P, Bodine RC, Sanders C, Donoso LA, Benovic JL. Polypeptide variants of beta-arrestin and arrestin3. J Biol Chem. 1993;268:15640–15648. [PubMed] [Google Scholar]
- Stocker RF. The organization of the chemosensory system in Drosophila melanogaster: a review. Cell Tissue Res. 1994;275:3–26. doi: 10.1007/BF00305372. [DOI] [PubMed] [Google Scholar]
- Stocker RF, Tissot M, Gendre N. Morphogenesis and cellular proliferation pattern in the developing antennal lobe in Drosophila melanogaster. Roux’s Arch Dev Biol. 1995;205:62–72. doi: 10.1007/BF00188844. [DOI] [PubMed] [Google Scholar]
- Stoorvogel W, Oorschot V, Geuze HJ. A novel class of clathrin-coated vesicles budding from endosomes. J Cell Biol. 1996;132:21–33. doi: 10.1083/jcb.132.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stortkuhl KF, Hovemann BT, Carlson JR. Olfactory adaptation depends on the Trp Ca2+ channel in Drosophila. J Neurosci. 1999;19:4839–4846. doi: 10.1523/JNEUROSCI.19-12-04839.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanoue S, Krishnan P, Krishnan B, Dryer SE, Hardin PE. Circadian clocks in antennal neurons are necessary and sufficient for olfactory rhythms in Drosophila. Curr Biol. 2004;14:638–649. doi: 10.1016/j.cub.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Tully T, Quinn W. Classical conditioning and retention in normal and mutant Drosophila melanogaster. J Comp Physiol A Sens Neural Behav Physiol. 1985;157:263–277. doi: 10.1007/BF01350033. [DOI] [PubMed] [Google Scholar]
- Vosshall LB, Amrein H, Morozov PS, Rzhetsky A, Axel R. A spatial map of olfactory receptor expression in the Drosophila antenna. Cell. 1999;96:725–736. doi: 10.1016/s0092-8674(00)80582-6. [DOI] [PubMed] [Google Scholar]
- Wetzel CH, Behrendt HJ, Gisselmann G, Stortkuhl KF, Hovemann B, Hatt H. Functional expression and characterization of a Drosophila odorant receptor in a heterologous cell system. Proc Natl Acad Sci USA. 2001;98:9377–9380. doi: 10.1073/pnas.151103998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilden U, Hall SH, Kuhn H. Phosphodiesterase activation by photoexcited rhodopsin is quenched when rhodopsin is phosphorylated and binds the intrinsic 48-kDa protein of rod outer segments. Proc Natl Acad Sci USA. 1986;83:1174–1178. doi: 10.1073/pnas.83.5.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]