Abstract
Background
Diet-induced obesity results from increased ingestion of energy dense food and sedentary lifestyle in genetically susceptible individuals. An environmental factor that may have shaped our energy homeostasis throughout evolution is parasitic nematode infection.
Methods
To test the hypothesis that a metabolically “thrifty phenotype” is advantageous during intestinal nematode infection, we compared the responses to Heligmosomoides polygyrus infection between two mouse strains: obesity-prone C57Bl/6J versus obesity-resistant SWR/J. Metabolic phenotyping was performed using indirect calorimetry, DEXA and MRI scanning. Gene expression was assessed by quantitative RT-PCR and immunohistochemistry.
Results
Body weight was maintained in both strains during nematode infection via different mechanisms. There was no apparent change in energy expenditure between the strains; however, SWR/J mice exhibited a marked hyperphagia (calorie intake 60% higher than C57Bl/6J) in order to maintain body weight. The importance of hyperphagia was confirmed by severe weight loss in a group of infected SWR/J mice whose food intake was restricted to that of naïve mice. Furthermore, SWR/J mice expelled nematodes more rapidly than C57Bl/6J mice, an effect related to a Th2 immune response.
Conclusions
C57Bl/6J mice are more energy efficient during parasitic nematode infection, which may explain their ability to tolerate the infection. SWR/J mice, on the other hand, require an increase in food intake to maintain energy stores during nematode infection. In addition, a strong TH2-mediated immune response that facilitates a prompt clearance of nematode infection in SWR/J mice may have evolved to conserve energy in this strain.
Introduction
Obesity and associated co-morbidities such as diabetes and cardiovascular disease are increasing rapidly worldwide1. Although excess food consumption and sedentary lifestyle are often blamed for the obesity epidemic, it is clear that certain populations are genetically predisposed to the development of obesity and associated diseases 2, 3. From an evolutionary standpoint, as descendents of hunter-gatherer societies, the most cogent explanation for the development of obesity is the concept of the “thrifty genotype” associated with efficient use of energy substrates in the setting of a limited food supply4. Efficient energy storage mainly in the form of triglycerides and adipose tissue occurs during nutritional abundance and is readily available for use when food supply is limited5. Conceptually, this provides an evolutionarily-conserved survival advantage to maintain energy homeostasis. Unfortunately, the mechanisms that evolved to conserve energy stores now predispose individuals to obesity in modern societies where food is abundant and physical activity is minimal4, 5. Despite the extensive citation of the “thrifty gene hypothesis”, to date no studies have been reported that support this concept in a physiologically relevant model system.
Here, we provide a model in which a “thrifty metabolic phenotype” is linked to the immune response to parasitic intestinal nematode infection. The World Health Organization estimates suggest that more than 3.5 billion people harbor intestinal helminth infection6. The four most common human gastrointestinal nematodes, Ascaris lumbricoides, Trichuris trichuria, Necator Americana, and Ancylostoma duodenale, are responsible for over 4 millions years of life lost per annum due to premature death or disability7. In addition, malnutrition and intestinal parasitism share a similar geographical distribution, with the same individuals often experiencing both disease states simultaneously8. In developing countries, children are most commonly infected, with the prevalence of infection approaching 100% by the age of ten in endemic areas9 where there are clear associations between malnutrition and growth retardation in the setting of chronic parasitic infection10. This current immense burden of parasitic nematode infections underscores the likelihood that, throughout the evolution of modern humans originating in Africa about 100,000 years ago11, 12, these infections may have been a significant environmental factor in shaping the human metabolic genotype.
Murine parasitic nematode infections, which have been used as models of human disease, demonstrate that certain inbred strains of mice are susceptible to chronic infection while others exhibit a resistant phenotype leading to rapid expulsion of the parasite. This process is mediated by a strong Th2 immune response typified by high levels of IL-4 and IL-1313. We hypothesized that the immune response to parasitic nematode infection is linked to energy homeostasis, such that mice susceptible to obesity would tolerate infection better than lean mice. This idea was tested by infecting obesity-prone C57Bl/6J and obesity-resistant SWR/J mice with the small intestine-specific nematode Heligmosomoides polygurus. As predicted, infected C56Bl/6J mice maintained body weight despite consuming a similar amount of food as naïve mice, indicating efficient energy utilization. In contrast, SWR/J developed hyperphagia in an attempt to maintain body weight, in parallel to a robust immune response to rapidly expel the nematodes.
Materials and Methods
Animals
Male SWR/J and C57Bl/6/J mice ages 10–12 weeks (Jackson Laboratories) were maintained on standard chow (LabDiet 5001). The mice had free access to chow and water except for the H. polygyrus infected SWR/J pair-fed mice. The latter were fed daily with the amount of food consumed by age-matched naïve SWR/J mice. All experimental protocols were approved by the IACUC committee at the University of Pennsylvania in accordance with the Guide for Care and Use of Laboratory Animals, Institute of Laboratory Animal Resources, National Research Council.
Heligmosomoides Polygyrus Parasites
H. polygyrus was propagated in C57Bl/10 mice (Taconic Laboratories) and infective L3 larvae were isolated using previously described methods14. The infection protocols in this study utilized primary H. polygyrus infections with 300 L3 larvae inoculated by oral gavage. After incubation of expressed intestinal contents and tissue from first 4 cm of longitudinally transected intestine in PBS, worm burden was assessed by quantification using a dissecting microscope.
Indirect Calorimetry
Energy expenditure was measured by open-circuit calorimetry (Oxymax system, Columbus Instruments, Columbus OH) and locomotor activity was measured simultaneously by infrared beam interruption (Optovarimax System, Columbus Instruments, OH). Mice were housed individually in calorimetry cages at 22° C. Room air was pumped at a rate of 0.52 liters/min and exhaust air was sampled at 15 min intervals for 5 hours. Oxygen consumption (VO2) and carbon dioxide production (VCO2) were measured using electrochemical and spectrophotometric sensors, respectively. Respiratory quotient (RQ), a measure of fuel use, was calculated as the ratio of oxygen consumption to carbon dioxide production. A decrease in RQ indicates fatty acid oxidation. Total energy expenditure (heat) = Calorific value (CV) × VO2, where CV = 3.815 + 1.232 × RQ. Core body temperature was measured at room temperature following calorimetry using a thermistor (YSI Model 4600, YSI Temperature, Dayton, OH).
Assessment of Body Composition
Mice were injected intraperitoneally with sodium pentobarbital (40mg/kg) and dual energy x-ray absorptiometry was performed (DEXA; Lunar PIXImus2; General Electric Medical systems, Madison, WI) on day 26 of infection. Body composition was measured using nuclear magnetic resonance on day 27 of infection (EchoMRI 3-in-1™; Echo Medical Systems, Houston, TX). BrdU labeling reagent (Zymed laboratories, San Francisco, CA) was administered by intraperitoneal injection (1ml/100g) 2 hours prior to harvest. The mice were euthanized using CO2, livers were excised and weighed, and samples were snap frozen in liquid nitrogen for triglyceride measurement, or fixed in neutral buffered formalin for histology. Duodenal (first 4 cm distal to the pylorus), jejunal (4 cm distal from the duodenum) and terminal ileal tissue (first 4 cm proximal to the cecum) sections were excised. Transverse sections were cut from the most proximal end of excised sections and fixed overnight in 10% neutral buffered formalin, and processed for paraffin embedding and sectioning. The remainder of the excised sections were split longitudinally and snap frozen for mRNA isolation.
Immunohistochemistry
Liver and intestinal samples were embedded in paraffin, and sections were cut at 6 μm, cleared in xylene and stained with hematoxylin and eosin. BrdU staining was performed in intestinal sections using a sheep anti-BrdU mAb (US Biological, Swampscott, MA) and an avidin-biotin-peroxidase conjugate (Vectastain ABC kit; Vector Laboratories, Burlingame, CA). Sections were counterstained with hematoxylin (Gill’s formulation). Quantification of crypt depth, villus height and number of BrdU positive cells per crypt were performed on samples of the SWR/J terminal ileum under 200 X magnification using IPLab image analysis software (Scanalytics, BD Biosciences, Rockville, MD). Neutral lipid was stained with Oil Red O (Poly Scientific, Bay Shore, NY) on frozen hepatic sections.
Liver and Stool Triglyceride quanitification
600μl of alcoholic KOH (60ml EtOH:30ml 30% KOH) was added to each sample of 100mg tissue or each 100mg sample of stool which had been dried overnight. Samples were incubated at 60°C for 5–6 hours until the digestion was complete. 500 μl of digested sample was added to 540 μl of 1 M MgCl2, mixed well and incubated on ice for 10 minutes. Following centrifugation of the samples for 30 minutes, the supernatant was aspirated and analyzed using an enzymatic triglyceride kit (Stanbio, Boeme, Texas N0.2150-101).
RNA isolation and Quantitative reverse transcription-polymerase chain reaction (RT-PCR)
RNA was isolated using Trizol (Invitrogen, Carlsbad, California, USA) per the manufacture’s instructions. Reverse transcription utilizing SuperScript II First Strand Synthesis Kit (Invitrogen, Carlsbad, California, USA) was followed by Syber Green quantitative PCR performed on an ABI Prism 7000 Sequence Detector System (Applied Biosystems Inc., Foster City, California, USA). Primers were designed using Primer Express software (Applied Biosystems Inc.). Primers used in these studies were: mGAPDH: forward 5′-GGT GGT CTC CTC TGA CTT CAA CA-3′, reverse 5′-GTT GCT GTA GCC AAA TTC GTT GT-3′; IL-4: forward 5′-CTC ATG GAG CTG CAG AGA CTC TT -3′, reverse 5′-CAT TCA TGG TGC AGC TTA TCG A-3′; IL-13 forward 5′-GAG CTG AGC AAC ATC ACA CAA GA -3′, reverse 5′-GCG GCC ACC TCC ACA CT-3′; IGF-1: forward 5′-TGC CCA GCG CCA CAC T-3′, reverse 5′-CCT GCA CTT CCT CTA CTT GTG TTC-3′ PCR conditions used were 50°C for two minutes, 95°C for 10 minutes, and then 40 cycles at 95°C for 15 seconds followed by 60°C for one minute. A dissociation curve was run with each PCR to ensure that primer-dimer formation did not occur. PCR results were analysed using the ΔCt analysis (User Bulletin #2; Applied Biosystems, Inc) with GAPDH as the housekeeping gene.
Statistical Analysis
A student’s t-test, performed using GraphPad Prism software (San Diego, CA) was utilized to determine statistical significance between two variables. Throughout the analyses, a p<0.05 was be used as the level of significance and all data was presented as the mean ± SE.
Results
C57Bl/6J and SWR/J maintain body weight during H. polygyrus infection through different mechanisms
We compared the effects of intestinal nematode infection in two mouse strains with divergent energy homeostatic phenotypes15. C57Bl/6J mice are highly energy efficient and susceptible to obesity, diabetes and hyperlipidemia. In contrast, SWR/J mice are resistant to obesity and diabetes due in part to a high rate of lipid catabolism. We chose H. polygyrus as the most appropriate model system for these studies since this pathogen is specific for the small intestine and infections persist for several weeks, thus allowing the long term measurements of energy balance and body composition.
Both mouse strains maintained their weight after 3 weeks of H. polygyrus infection (Figure 1A). Food intake was similar between infected and naïve C57Bl/6J mice (Figure 1B). In contrast, infected SWR/J consumed 60% more food than naïve controls (Figure 1B). We performed indirect calorimetry and measured locomotor activity using photobeams over 24 hours to determine if this increase in food intake was due to an increase in energy expenditure. As expected in rodents, oxygen consumption (VO2) and locomotor activity were higher at night than during the day in the naïve state in SWR/J mice (Figures 1C, D). There was a trend towards an increase in nocturnal VO2 upon infection in SWR/J mice, however, the VO2 over the 24h period was not affected (Figure 1C). There was no significant change in either VO2 or locomotor activity in C57Bl/6J mice with nematode infection (data not shown). Body temperature and stool triglyceride levels did not change in either strain upon infection (data not shown). Together, these results demonstrate that the increase in food intake induced by nematode infection in SWR/J mice is not a compensatory response to an alteration in energy expenditure or nutrient malabsorption.
Figure 1.
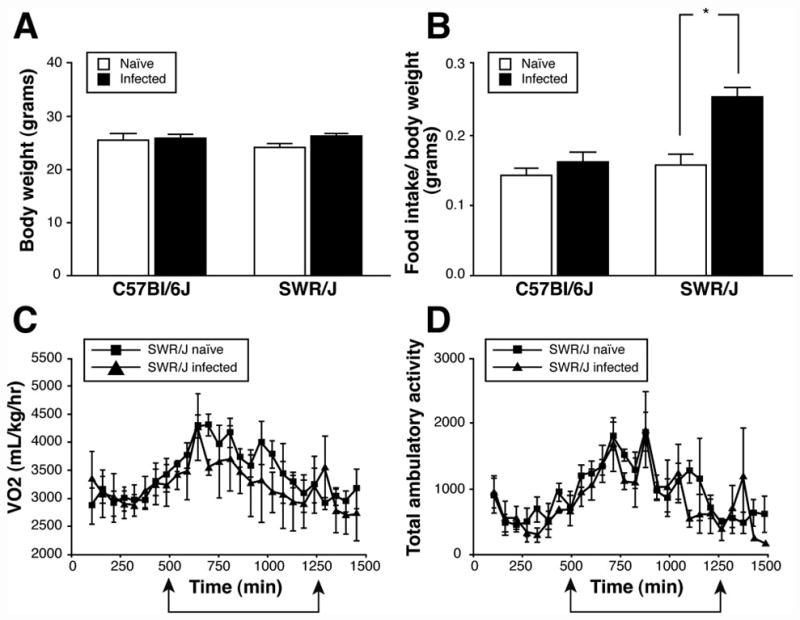
Metabolic adaptation of C57Bl/6J and SWR/J mice to infection with H. polygyrus. A) Body weights of naïve mice compared to those infected with H. polygyrus for 21 days, Mean ± SEM, N=5–8 in each group; B) Average food intake per 24 hours in naïve vs. 21 day H. polygyrus infected mice, Mean ± SEM, N=5–8 mice per group, *p=0.001; C) A comparison of energy expenditure in naïve vs. H. polygyrus infected SWR/J mice over 24 hours measured by indirect calorimetry, arrows indicate dark cycle period, N=4 mice per group; D) Total ambulatory activity in naïve vs. H. polygyrus infected SWR/J mice over 24 hours, arrows indicate dark cycle period, N=4 mice per group.
Body composition is regulated differently by H. polygyrus infection in C57Bl/6J and SWR/J mice
To determine if an alteration in energy partitioning could account for the difference in energy consumption observed in SWR/J infected mice but not C57Bl/6J, we evaluated body composition by DEXA scan as well as through the determination of organ weights and composition. DEXA scanning revealed that, in the naïve state, C57Bl/6J mice had higher fat mass than SWR/J mice (Figures 2A, B). Although H. polygyrus infection increased abdominal lean mass in both C57Bl/6J and SWR/J mice, it drastically reduced abdominal fat mass only in C57Bl/6J mice (Figures 2A, B). We examined intra-abdominal organs to relate the effects of H. polygyrus infection to the changes observed on DEXA scanning. Liver triglyceride content was higher in naïve C57Bl/6J than SWR/J mice (Figure 2C). H. polygyrus infection decreased hepatic triglyceride level in C57Bl/6J but not SWR/J mice (Figures 2C and D). Perigonadal fat was higher in C57Bl/6J than SWR/J mice, and decreased in the former in response to infection (Figure 3A, B). Small intestine and spleen weights as well as small intestinal length increased in both strains in response to infection (Figure 3A–C). In contrast, liver weight increased following infection in SWR/J but not C57Bl/6J mice (Figure 3B). There was no increase in the weight of the colon in either strain (data not shown). Together, these results suggest that the energy required for the increase in intra-abdominal lean mass in C57Bl/6J mice, induced by nematode infection, is provided by stored energy in the form of intra-abdominal fat. By contrast, since SWR/J mice, which have a lean phenotype at baseline, do not utilize intra-abdominal fat stores in the same fashion, they demonstrate a remarkable increase in food intake (Figure 1B) to compensate for the increase in intra-abdominal lean mass induced by nematode infection.
Figure 2.
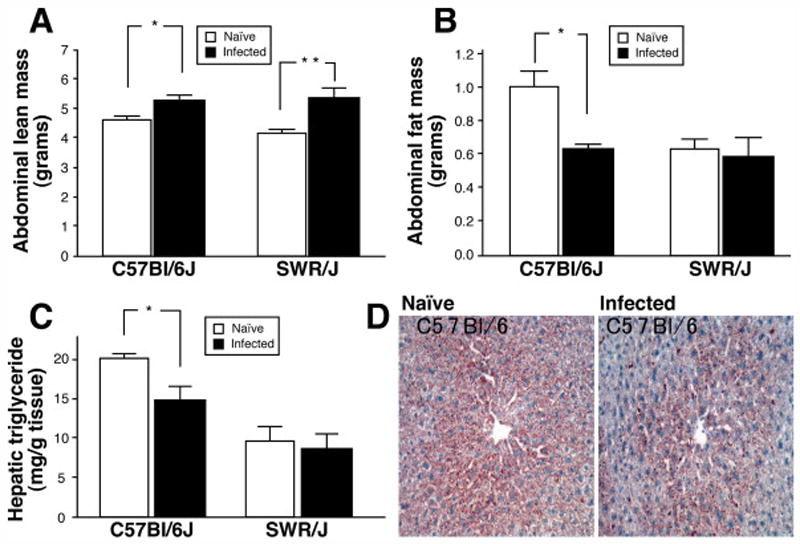
Effect of H. polygyrus infection on body composition of C57Bl/6J and SWR/J mice. A) Comparison of abdominal lean mass in naïve and H. polygyrus infected mice measured by DEXA scan, Mean ± SEM, N=4–8 mice per group, *p=0.01 and **p=0.03; B) Comparison of abdominal fat mass in naïve and H. polygyrus infected mice measured by DEXA scan, Mean ± SEM, N=4–8 mice per group, *p=0.01; C) Hepatic triglyceride content in naïve and H. polygyrus infected mice, Mean ± SEM, N=4–8 mice per group, *p=0.03; D) Representative photomicrographs of liver from naïve and H. polygyrus infected C57Bl/6J mice stained with Oil red O, 200 X Mag.
Figure 3.
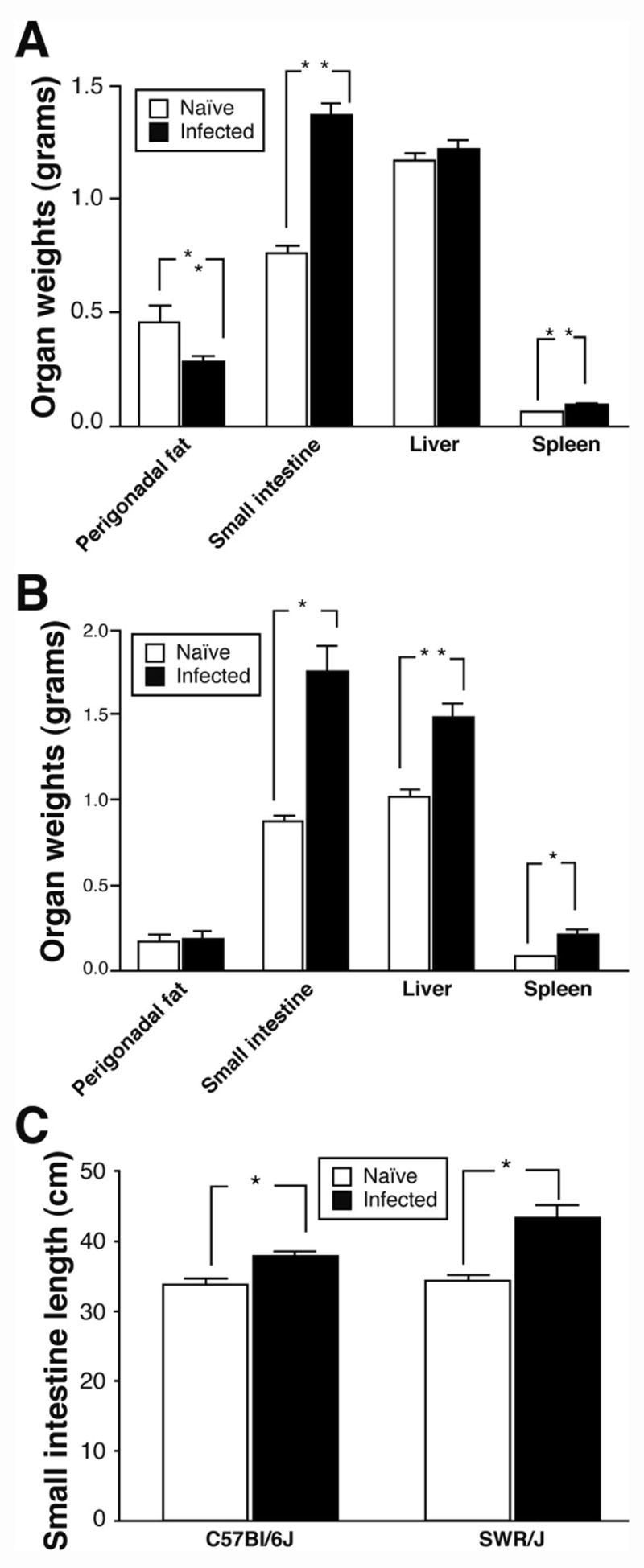
Morphometric analysis of intra-abdominal organs. A) Organ weights in naïve vs. H. polygyrus infected C57Bl/6J mice, Mean ± SEM, N=5–8 mice per group, *p<0.01 and **p<0.001; B) Organ weights in naïve vs. H. polygyrus infected SWR/J mice, Mean ± SEM, N=4–5 mice per group, *p<0.001 and **p<0.01; C) Comparison of small intestinal length in naïve vs. H. poygyrus infected C57Bl/6J and SWR/J mice, N=4–8 mice per group, *p<0.001.
SWR/J mice demonstrate significant weight loss when food intake is restricted during H. polygyrus infection
We determined whether the hyperphagia manifested in infected SWR/J mice played a crucial role in their ability to maintain body weight. A group of SWR/J mice were infected with H. polygyrus and their daily food intake limited to that of naïve SWR/J mice. H. polygyrus infection in this pair-feeding paradigm led to a significant weight loss in SWR/J mice (Figure 4A) due to a reduction in both total fat and lean mass, as assessed by MRI (Figure 4B). Body temperature fell in pair-fed infected mice (Table 1). Although there was a trend for H. polygyrus infection to decrease both ambulatory activity and oxygen consumption, these differences did not reach statistical significance (Table 1). Perigonadal fat pad weight was lower in the infected pair-fed SWR/J mice (Figure 4C), consistent with the reduction of total body fat mass measured by MRI (Figure 4B). Remarkably, however, despite a significant loss in total body weight, the weight and length of the small intestinal increased in the infected pair-fed SWR/J mice (Figures 4C and D).
Figure 4.
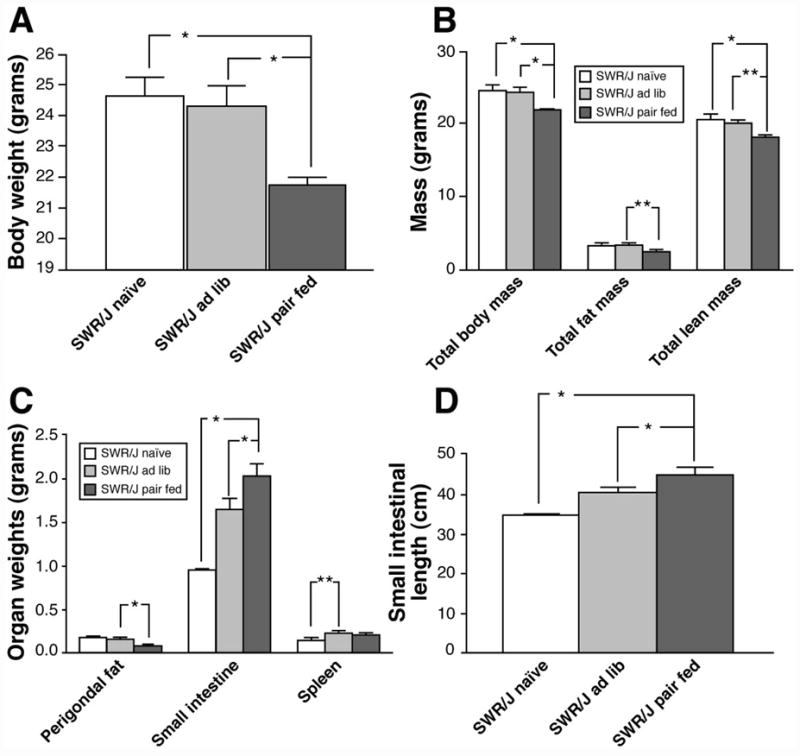
Effect of calorie restriction on body composition in H. polygyrus infected SWR/J mice by pair feeding. A) Body weights of naïve and H. polygyrus infected SWR/J mice after 28 days, Mean ± SEM, N=3–6 animals per group, *p<0.01; B) Body composition, assessed by MRI, in naïve and H. polygyrus infected SWR/J mice after 27 days, Mean ± SEM, N=3–6 animals per group, *p<0.01 and **p<0.05; C) Organ weights in naïve and H. polygyrus infected SWR/J mice after 28 days, Mean ± SEM, N=3–6 animals per group, *p≤0.01 and **p<0.05; D) Small intestinal length in naïve and H. polygyrus infected SWR/J mice after 28 days, Mean ± SEM, N=3–6 animals per group, *p<0.01.
Table 1. Energy expenditure in SWR/J mice infected with H. polygyrus.
H. polygyrus infection of SWR/J mice lead to a trend toward a decrease in energy expenditure revealed by oxygen consumption (VO2), ambulatory activity, and core body temperature.
| Naïve SWR | Infected SWR (Ad lib fed) | Infected SWR (Pair fed) | |
|---|---|---|---|
| VO2 (ml/kg/hour) | 2454.3±242.5 | 2160±31.5 | 1936.9±119.8 |
| Total Ambulatory Activity (Movement/hr) | 2281±343 | 1797.5±432.5 | 1484±153.9 |
| Core Body Temperature (°C) | 38±0.45 | 38.5±0.22 | 37.4±0.34* |
p< 0.05 compared to ad lib fed infected SWR/J mice.
H. polygyrus infection induces mucosal growth and IGF-1 in the small intestinal tract
We performed morphometric analysis to further characterize the trophic effect of H. polygyrus infection on the small intestine. Although H. polygyrus was only found in the proximal third of the small intestine, as previously reported16, segmental mass increased by the same proportion throughout the entire length of the small intestine of SWR/J mice (Figure 5A). Indeed, even the terminal ileum of infected mice exhibited crypt hypertrophy with an increase in BrdU labeled cells as well as an increase in villus height (Figures 5B, C and E). Insulin-like growth factor (IGF)-1 has been previously shown to induce both mucosal hypertrophy and increase small intestinal length16, 17. Serum levels of IGF-1 were not altered by H. polygyrus infection (data not shown); however, there was a dramatic induction of IGF-1 in the small intestine of both C57Bl/6/J and SWR/J mice (Figure 5D).
Figure 5.
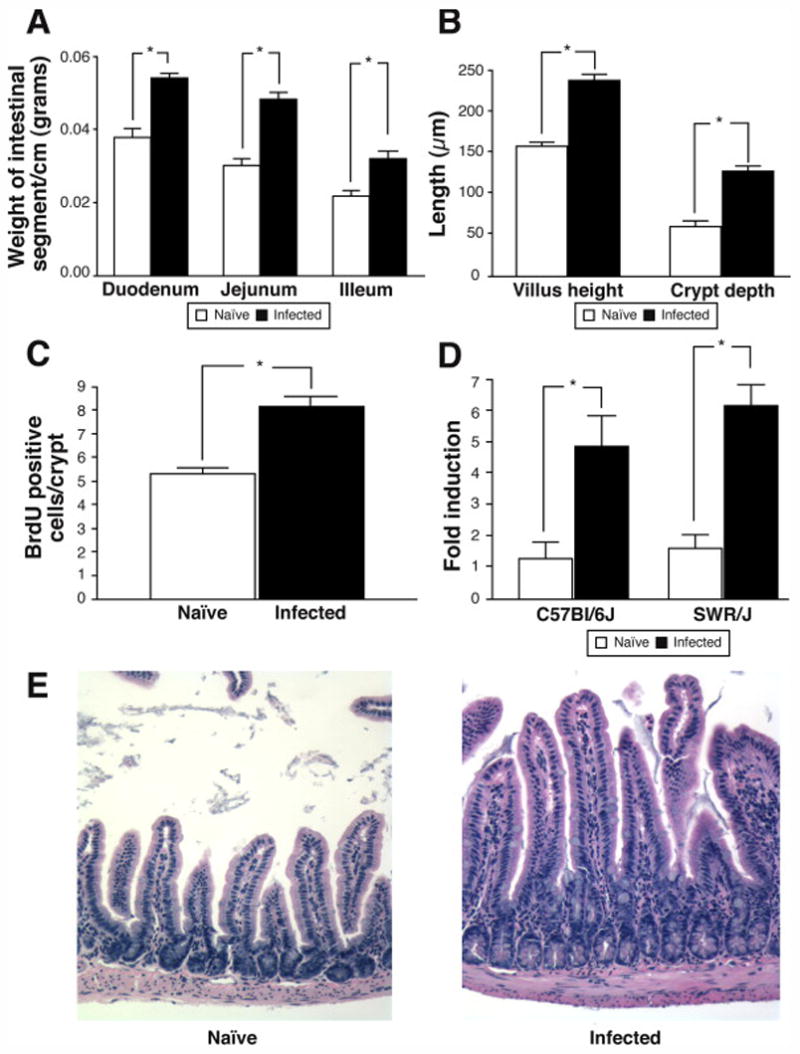
Trophic effects of H. polygyrus infection on the small intestine of SWR/J mice. A) Mass of small intestinal segments in naïve and H. polygyrus infected SWR/J mice, Mean ± SEM, N=8, *p<0.01; B) Quantification of villus height and crypt depth in the terminal ileum of naïve and H. polygyrus infected SWR/J mice. Mean ± SEM, N=5, *p<0.001. C) Quantification of BrdU positive epithelial cells in the terminal ileum of naïve and H. polygyrus infected SWR/J, Mean ± SEM, N=4, *p<0.001; D) IGF1 mRNA expression in the small intestine of naïve and H. polygyrus infected SWR/J and C57Bl/6J mice, Mean ± SEM, N=3–4, *p<0.05; E) Representative photomicrographs of H&E stained sections of the terminal ileum from naïve and H. polygyrus infected SWR/J mice, 200X mag.
Th2 immune response to H. polygyrus infection is related to worm burden in obesity-prone C57Bl/6J and obesity-resistant SWR/J mice
We verified the Th2 immune profile in response to H. polygyrus infection in C57Bl/6J and SWR/J mice, by performing worm counts and assessing intestinal cytokine expression. Primary infection with H. polygyrus induced a much stronger intestinal Th2 immune response, as assessed by the expression of IL-4 and IL-13 mRNA, in SWR/J compared to infected C57Bl/6J mice (Figures 6A and B). As a result of this strong Th2 immune response, there was a dramatic reduction of the nematode burden in SWR/J mice (Figure 6C). By contrast to the robust induction of a Th2 immune response in SWR/J mice upon infection, there was only a modest activation of a Th1 response typified by a six-fold induction of jejunal IFN-γ mRNA expression in SWR/J mice whereas no induction was observed in C57Bl/6J mice (data not shown).
Figure 6.
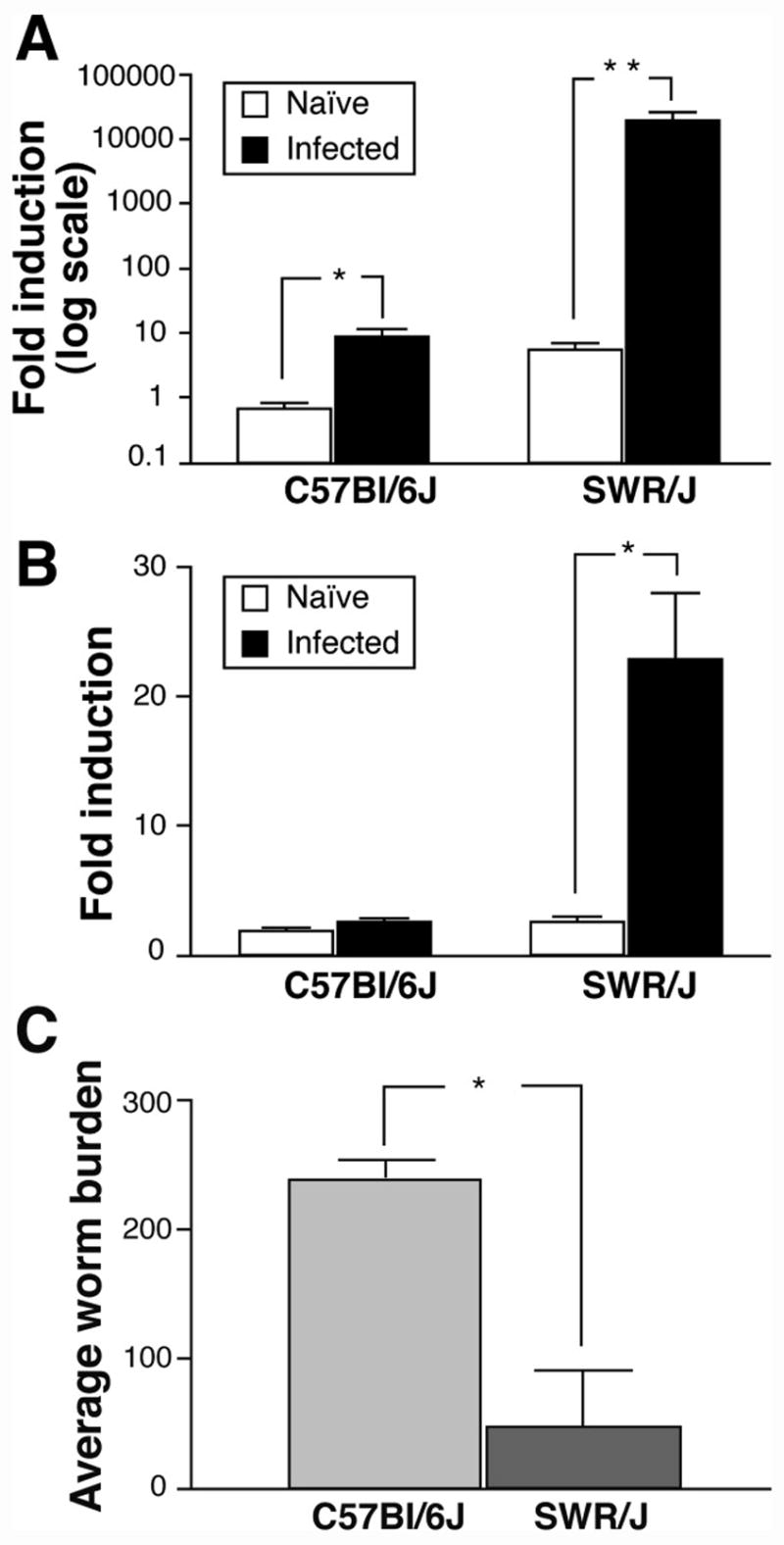
Immune responses of C57Bl/6J and SWR/J mice to infection with H. polygyrus. A) Intestinal mRNA expression for IL-13 in C57Bl/6J and SWR/J mice, Mean ± SEM, N=4–8 animals per group, *p<0.05 and **p=0.01; B) Intestinal mRNA expression for IL-4 in C57Bl/6J and SWR/J mice, Mean ± SEM, N=4–8 animals per group, *p<0.05 and **p=0.01; C) H. polygyrus worm burdens after 28 days of infection, Mean ± SEM, N=4–8 animals per group, *p<0.001.
Discussion
In this study, we provide the first evidence for the relative “fitness” of the energy efficient obese-prone phenotype, compared to the lean phenotype, in a physiologically relevant model of metabolic stress. These results provide a model system supporting the notion of the energy efficient phenotype as an evolutionarily conserved, adaptive response to an environmental challenge to survival. The dramatic increase in the prevalence of diet-induced obesity in response to very recent environmental changes with the abundance of calorically-rich food, along with technological advances that promote a sedentary lifestyle, suggest that the energy efficient phenotype is the result of strong genetic influences acquired throughout the millennia. Interestingly, many individuals maintain a lean phenotype despite these environmental influences, pointing to existence of “thrifty genes” as proposed by Neel4, 15. Indeed, twin and adoption studies support the existence of such genes2.
Similar to humans, inbred strains of mice also show differences in susceptibility to the development of diet-induced obesity15 (Table 2). In our study, we chose to examine the C57B6/J strain to represent the obese-prone phenotype since it is the most widely studied strain in this regard. At the other end of the spectrum, SWR/J mice remain lean even when fed a high fat diet15. Although the precise mechanism(s) by which SWR/J mice exhibit a lean phenotype remain to be identified, it is likely that several factors play an important role such as hypothalamic function18, the regulation of thermogenesis19, and response to leptin20.
Table 2. The relationship between the metabolic phenotype of inbred strains of mice and the immunologic response to intestinal parasitic nematode infections.
Mice that are prone to the development of diet-induced obesity on a high fat diet are susceptible to chronic infection by intestinal parasitic nematodes. By contrast, mouse strains that exhibit a lean phenotype expel intestinal parasitic nematodes rapidly by Th2-mediated immune responses and are, therefore resistant to chronic infection.
| Parasite | Slow Responder (Susceptible) | Intermediate Responder | Rapid Responder (Resistant) |
|---|---|---|---|
| Heligmosomoides polygyrus | C3H | BALB/c | SWR, SJL |
| Trichinella spiralis | C3H | C57Bl/6 | SWR, BALB/c |
| Trichuris muris | AKR | C57Bl/6 | BALB/c |
| Obese-Prone Strains | Lean Strains | ||
| AKR, C57Bl/6, C3H | SWR,BALB/c, SJL | ||
In a separate body of literature, investigators examining the immunobiology of several murine models of intestinal roundworm infections, Heligmosomoides polygyrus, Trichinella spiralis, and Trichuris muris, have shown that certain inbred strains of mice are susceptible to chronic infection with these nematodes whereas others are capable of rapidly expelling these parasites and are, therefore, resistant to chronic infection13, 21, 22. In Table 2, the classification of inbred mouse strains by their level of resistance to chronic infections with these various parasitic nematodes reveals a remarkably consistent association with mouse strains prone to the development of diet-induced obesity and susceptibility to chronic infection. Conversely, mouse strains capable of rapid nematode expulsion with high level IL-4 and IL-13 expression (resistant phenotype) exhibit a lean phenotype. Although further investigation is needed, this association suggests that there may be a mechanistic link between leanness and Th2-polarized immune responses. Indeed, there is growing evidence that obesity is associated with elevated systemic levels of pro-inflammatory cytokines of a predominately Th1 profile, produced by adipose tissue, that have been shown to play a role in the development of insulin resistance, the hallmark of type 2 diabetes mellitus23. More recent studies have shown that a primary source for these inflammatory mediators are macrophages located in the stroma of adipose tissue24. In this regard, adipose tissue macrophages in lean mice exhibit a Th2-biased alternatively activated phenotype whereas, in obese mice, adipose tissue macrophages exhibit a classically activated phenotype expressing Th1 cytokines such as TNF-α24. Ultimately, additional studies will be required to determine if the differences in metabolic host responses to parasitic nematode infections reported herein can be generalized to other strains of obese-prone and lean mice.
While both C57B/6J and SWR/J mice exhibit a trophic effect on intestinal mass and length in response to nematode infection, obese-prone C57B/6J mice do not consume additional food presumably because they are capable of utilizing fat stores to compensate for this increase in abdominal lean mass (Figures 2 and 3). By contrast, SWR/J mice become hyperphagic to maintain body weight when infected (Figure 2B). Our results in C57Bl/6J mice agree with those of Kristen et al. who have shown a similar effect of H. polygyrus on body composition in outbred Swiss Webster mice25. The trophic response of the intestinal tract appears to be a highly conserved response that occurs in all strains of mice thus far tested. Remarkably, we show that this response is maintained even at the expense of total body fat and lean mass in pair-fed SWR/J mice (Figure 4). Previous studies have shown that H. polygyrus infection decreases nutrient absorption in the proximal small intestine25, 26 and increases intestinal muscle contractility27, physiologic alterations known to result in malabsorptive states in humans. Nevertheless, by measuring fecal triglyceride levels, we observed that infection of either C57Bl/6J or SWR/J mice does not result in malabsorption (data not shown) suggesting that the intestinal trophic response may be a critical adaptation to increase mucosal surface area in order to maintain nutrient absorption. In support of this notion, we show, in Figure 5, that the intestinal trophic effect of H. polygyrus infection occurs throughout the small intestine including the terminal ileum, a region of the small bowel distant from the duodenum where H. polygyrus resides21. Indeed, H. polygyrus infection enhances the ability of the distal small intestine to absorb glucose25.
It is likely that the induction IGF1 by H. polygyrus infection (Figure 5D) plays an important role in this growth response. Indeed, previous studies have shown that the activation of the growth hormone/IGF1 axis, specifically in the intestinal tract, can result in both mucosal hypertrophy as well as an increase in the length of the small intestine16, 17, 28. Identification of the mechanism(s) by which intestinal parasitic nematode infections induce IGF1 expression specifically in the small intestine may have particular relevance to disease processes, such as short gut syndrome where small bowel trophic responses are an essential adaptive response29.
Mice in the wild are normally calorie-restricted30. Therefore, the metabolic alterations in H. polygyrus-infected SWR/J mice, where food intake increases by 60% compared to naïve controls in the setting of unrestricted access to food (Figure 1B), may not accurately reflect the metabolic stress associated with a lean phenotype. To determine if the SWR/J metabolic response to nematode infection is maladaptive in an environment that more accurately reflects the challenges to energy balance in the wild, a pair-feeding study was performed whereby the food intake of SWR/J mice infected with H. polygyrus was matched to that of naïve SWR/J mice. Under these conditions, nematode infection led to a significant degree of weight loss in the infected SWR/J pair-fed mice (Figure 4A), demonstrating the metabolic consequences of H. polygyrus infection when hyperphagia is not permitted. In part, this may be due to the metabolic inefficiency of a lean mouse that is not able to effectively store energy as adipose tissue (Figures 2A and 6). Presumably, weight loss would have continued in the pair-fed cohort, ultimately leading to severe malnutrition, if it were not for the rapid expulsion of H. polygyrus induced by the strong Th2 immune response in this strain of mice (Figure 6). Severe malnutrition not only prolongs infection with the parasitic nematodes31, 32, but also increases the susceptibility of the host to co-infections with other pathogens32. From a mechanistic standpoint, one critical factor that links adaptive immune function and host metabolism is leptin, a hormone produced primarily by adipose tissue that regulates feeding behavior as well as CD4+ T cell immune responses33. Thus, the robust Th2 immune response may be a protective host immune mechanism to prevent long term infection in a lean host that is not well adapted to compensate for the metabolic challenges imposed by the parasite (Table 2).
As a disease process resulting in significant metabolic stress leading to malnutrition of large populations of humans throughout evolution, intestinal parasitic nematode infections may have had a significant impact on evolutionarily conserved metabolic adaptive responses. Herein, we provide direct evidence to support this notion in a murine model system. There is growing evidence demonstrating that the epidemic of diet-induced obesity and type 2 diabetes mellitus are influenced by alterations in systemic responses associated with both innate and adaptive immunity. Ultimately, as “thrifty genes” are identified that predispose individuals to the development of diet-induced obesity, examination of their impact in models such as parasitic nematode infections of the intestine may provide additional insights into mechanisms by which immune-mediated responses influence metabolic homeostasis.
Acknowledgments
This work was supported by grants NIH AI39368 (GDW and MH), DK062348 (RSA), AI14490 (JAA), T32 RR0759 (SMT), the Molecular Biology, Morphology and the Mouse Phenotyping, Physiology and Metabolism Cores of NIH/NIDDK Center Grants P30-DK50306 and P30-DK19525. TMW was supported by T32 DK007066. The authors also acknowledge the technical assistance of Dr. Laila D. McVay with the H. polygyrus model system.
Footnotes
The authors confirm that no conflicts of interest exist with respect to the information contained in this manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ogden CL, Yanovski SZ, Carroll MD, Flegal KM. The epidemiology of obesity. Gastroenterology. 2007;132:2087–102. doi: 10.1053/j.gastro.2007.03.052. [DOI] [PubMed] [Google Scholar]
- 2.Maes HH, Neale MC, Eaves LJ. Genetic and environmental factors in relative body weight and human adiposity. Behav Genet. 1997;27:325–51. doi: 10.1023/a:1025635913927. [DOI] [PubMed] [Google Scholar]
- 3.Farooqi S, O’Rahilly S. Genetics of obesity in humans. Endocr Rev. 2006;27:710–18. doi: 10.1210/er.2006-0040. [DOI] [PubMed] [Google Scholar]
- 4.Neel JV. Diabetes mellitus: a “thrifty” genotype rendered detrimental by “progress”? Am J Hum Genet. 1962;14:353–62. [PMC free article] [PubMed] [Google Scholar]
- 5.Spiegelman BM, Flier JS. Obesity and the regulation of energy balance. Cell. 2001;104:531–43. doi: 10.1016/s0092-8674(01)00240-9. [DOI] [PubMed] [Google Scholar]
- 6.Cancrini G. Human infections due to nematode helminths nowadays: epidemiology and diagnostic tools. Parassitologia. 2006;48:53–6. [PubMed] [Google Scholar]
- 7.WHO. The World Health Report 1999: Making a Difference. Geneva: 1999. p. 122. [Google Scholar]
- 8.Pelletier DL. The potentiating effects of malnutrition on child mortality: epidemiologic evidence and policy implications. Nutr Rev. 1994;52:409–15. doi: 10.1111/j.1753-4887.1994.tb01376.x. [DOI] [PubMed] [Google Scholar]
- 9.Kightlinger LK, Seed JR, Kightlinger MB. The epidemiology of Ascaris lumbricoides, Trichuris trichiura, and hookworm in children in the Ranomafana rainforest, Madagascar. J Parasitol. 1995;81:159–69. [PubMed] [Google Scholar]
- 10.Quihui-Cota L, Valencia ME, Crompton DW, Phillips S, Hagan P, Diaz-Camacho SP, Triana Tejas A. Prevalence and intensity of intestinal parasitic infections in relation to nutritional status in Mexican schoolchildren. Trans R Soc Trop Med Hyg. 2004;98:653–9. doi: 10.1016/j.trstmh.2003.12.017. [DOI] [PubMed] [Google Scholar]
- 11.Cann RL, Stoneking M, Wilson AC. Mitochondrial DNA and human evolution. Nature. 1987;325:31–6. doi: 10.1038/325031a0. [DOI] [PubMed] [Google Scholar]
- 12.Stringer CB, Andrews P. Genetic and fossil evidence for the origin of modern humans. Science. 1988;239:1263–8. doi: 10.1126/science.3125610. [DOI] [PubMed] [Google Scholar]
- 13.Finkelman FD, Shea-Donohue T, Goldhill J, Sullivan CA, Morris SC, Madden KB, Gause WC, Urban JF., Jr Cytokine regulation of host defense against parasitic gastrointestinal nematodes: lessons from studies with rodent models. Annu Rev Immunol. 1997;15:505–33. doi: 10.1146/annurev.immunol.15.1.505. [DOI] [PubMed] [Google Scholar]
- 14.Bell RG, McGregor DD. Requirement for two discrete stimuli for induction of the intestinal rapid expulsion response against Trichinella spiralis in rats. Infect Immun. 1980;29:186–93. doi: 10.1128/iai.29.1.186-193.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.West DB, Boozer CN, Moody DL, Atkinson RL. Dietary obesity in nine inbred mouse strains. Am J Physiol. 1992;262:R1025–32. doi: 10.1152/ajpregu.1992.262.6.R1025. [DOI] [PubMed] [Google Scholar]
- 16.Theiss AL, Fruchtman S, Lund PK. Growth factors in inflammatory bowel disease: the actions and interactions of growth hormone and insulin-like growth factor-I. Inflamm Bowel Dis. 2004;10:871–80. doi: 10.1097/00054725-200411000-00021. [DOI] [PubMed] [Google Scholar]
- 17.Knott AW, Juno RJ, Jarboe MD, Profitt SA, Erwin CR, Smith EP, Fagin JA, Warner BW. Smooth muscle overexpression of IGF-I induces a novel adaptive response to small bowel resection. Am J Physiol Gastrointest Liver Physiol. 2004;287:G562–70. doi: 10.1152/ajpgi.00438.2003. [DOI] [PubMed] [Google Scholar]
- 18.Leibowitz SF, Alexander J, Dourmashkin JT, Hill JO, Gayles EC, Chang GQ. Phenotypic profile of SWR/J and A/J mice compared to control strains: possible mechanisms underlying resistance to obesity on a high-fat diet. Brain Res. 2005;1047:137–47. doi: 10.1016/j.brainres.2005.03.047. [DOI] [PubMed] [Google Scholar]
- 19.Prpic V, Watson PM, Frampton IC, Sabol MA, Jezek GE, Gettys TW. Adaptive changes in adipocyte gene expression differ in AKR/J and SWR/J mice during diet-induced obesity. J Nutr. 2002;132:3325–32. doi: 10.1093/jn/132.11.3325. [DOI] [PubMed] [Google Scholar]
- 20.Takahashi N, Patel HR, Qi Y, Dushay J, Ahima RS. Divergent effects of leptin in mice susceptible or resistant to obesity. Horm Metab Res. 2002;34:691–7. doi: 10.1055/s-2002-38251. [DOI] [PubMed] [Google Scholar]
- 21.Ben-Smith A, Lammas DA, Behnke JM. The relative involvement of Th1 and Th2 associated immune responses in the expulsion of a primary infection of Heligmosomoides polygyrus in mice of differing response phenotype. J Helminthol. 2003;77:133–46. doi: 10.1079/JOH2003173. [DOI] [PubMed] [Google Scholar]
- 22.Wassom DL, Brooks BO, Cypess RH, David CS. A survey of susceptibility to infection with Trichinella spiralis of inbred mouse strains sharing common H-2 alleles but different genetic backgrounds. J Parasitol. 1983;69:1033–7. [PubMed] [Google Scholar]
- 23.Matarese G, La Cava A. The intricate interface between immune system and metabolism. Trends Immunol. 2004;25:193–200. doi: 10.1016/j.it.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 24.Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007;117:175–84. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kristan DM, Hammond KA. Parasite infection and caloric restriction induce physiological and morphological plasticity. Am J Physiol Regul Integr Comp Physiol. 2001;281:R502–10. doi: 10.1152/ajpregu.2001.281.2.R502. [DOI] [PubMed] [Google Scholar]
- 26.Madden KB, Yeung KA, Zhao A, Gause WC, Finkelman FD, Katona IM, Urban JF, Jr, Shea-Donohue T. Enteric nematodes induce stereotypic STAT6-dependent alterations in intestinal epithelial cell function. J Immunol. 2004;172:5616–21. doi: 10.4049/jimmunol.172.9.5616. [DOI] [PubMed] [Google Scholar]
- 27.Vallance BA, Blennerhassett PA, Collins SM. Increased intestinal muscle contractility and worm expulsion in nematode-infected mice. Am J Physiol. 1997;272:G321–7. doi: 10.1152/ajpgi.1997.272.2.G321. [DOI] [PubMed] [Google Scholar]
- 28.Williams KL, Fuller CR, Fagin J, Lund PK. Mesenchymal IGF-I overexpression: paracrine effects in the intestine, distinct from endocrine actions. Am J Physiol Gastrointest Liver Physiol. 2002;283:G875–85. doi: 10.1152/ajpgi.00089.2002. [DOI] [PubMed] [Google Scholar]
- 29.Buchman AL. Etiology and initial management of short bowel syndrome. Gastroenterology. 2006;130:S5–S15. doi: 10.1053/j.gastro.2005.07.063. [DOI] [PubMed] [Google Scholar]
- 30.Austad SN, Kristan DM. Are mice calorically restricted in nature? Aging Cell. 2003;2:201–7. doi: 10.1046/j.1474-9728.2003.00053.x. [DOI] [PubMed] [Google Scholar]
- 31.Ing R, Su Z, Scott ME, Koski KG. Suppressed T helper 2 immunity and prolonged survival of a nematode parasite in protein-malnourished mice. Proc Natl Acad Sci U S A. 2000;97:7078–83. doi: 10.1073/pnas.97.13.7078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koski KG, Scott ME. Gastrointestinal nematodes, nutrition and immunity: breaking the negative spiral. Annu Rev Nutr. 2001;21:297–321. doi: 10.1146/annurev.nutr.21.1.297. [DOI] [PubMed] [Google Scholar]
- 33.Fantuzzi G, Faggioni R. Leptin in the regulation of immunity, inflammation, and hematopoiesis. J Leukoc Biol. 2000;68:437–46. [PubMed] [Google Scholar]


