Abstract
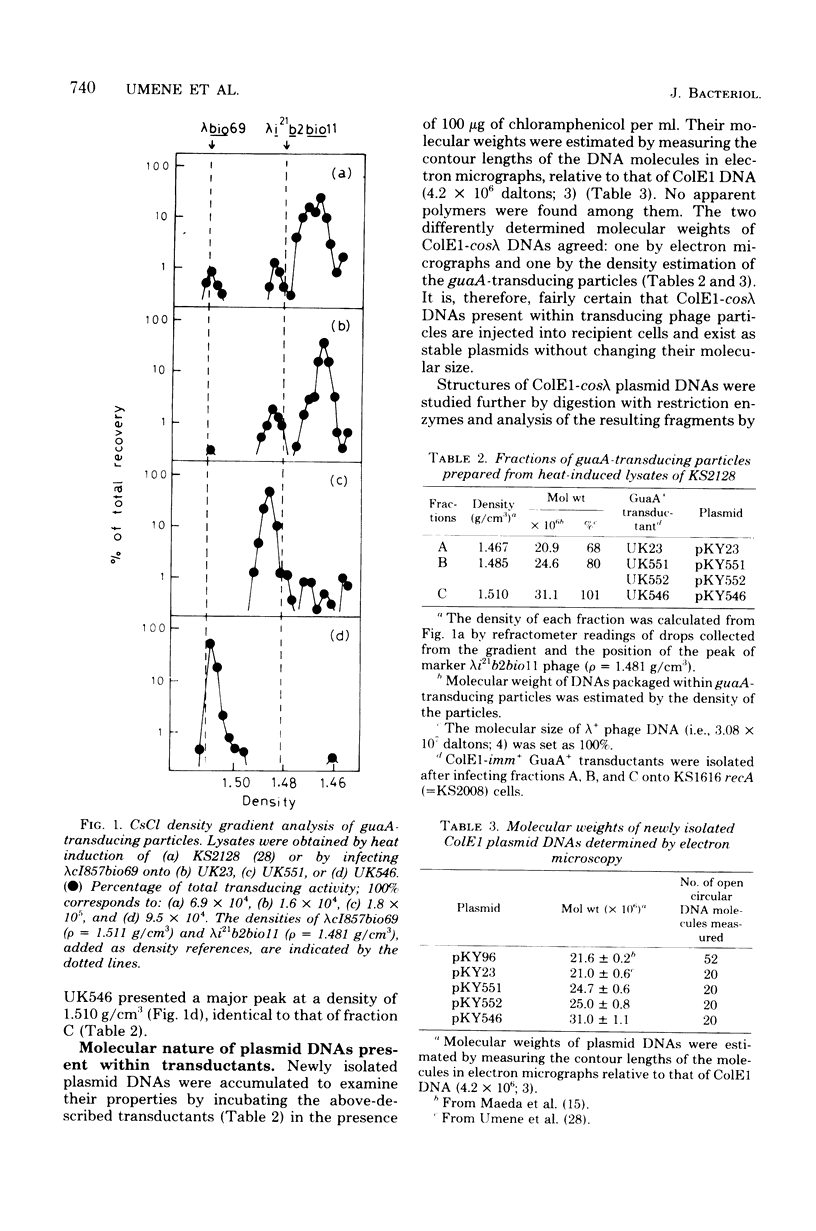
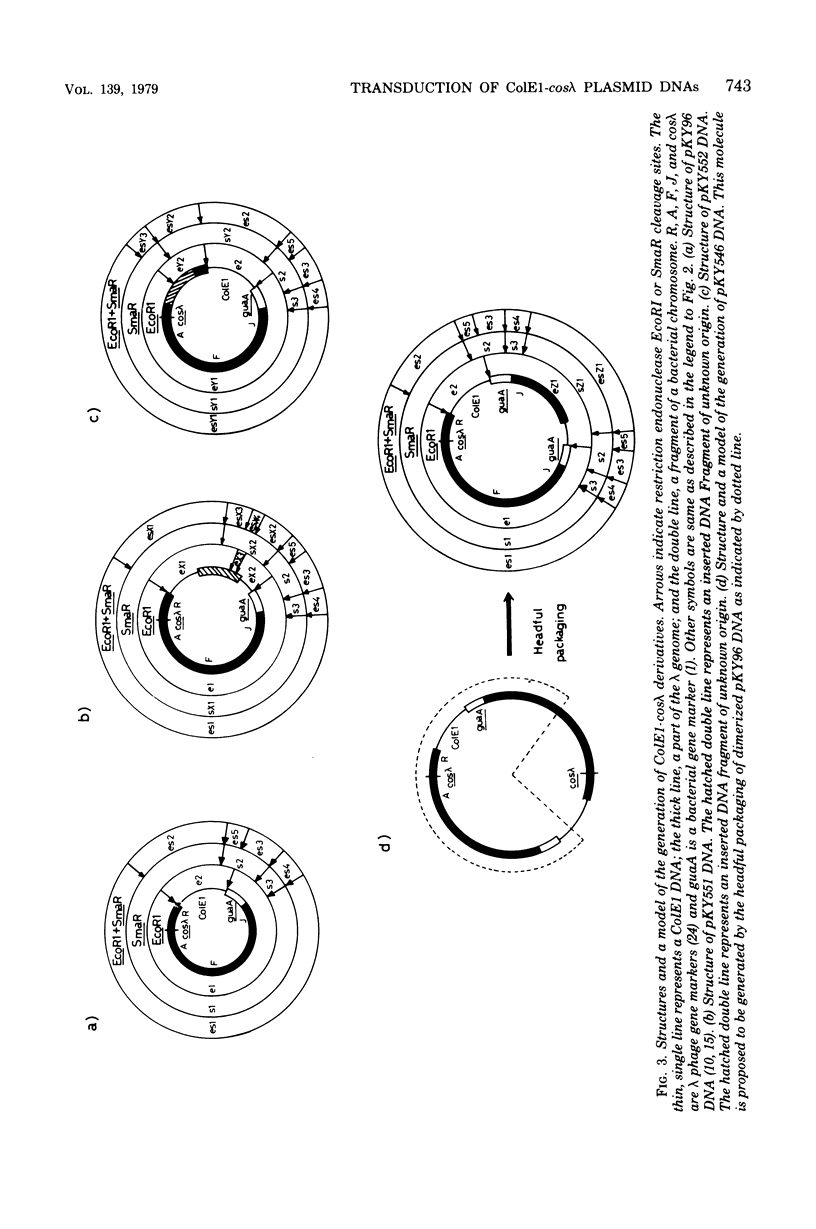
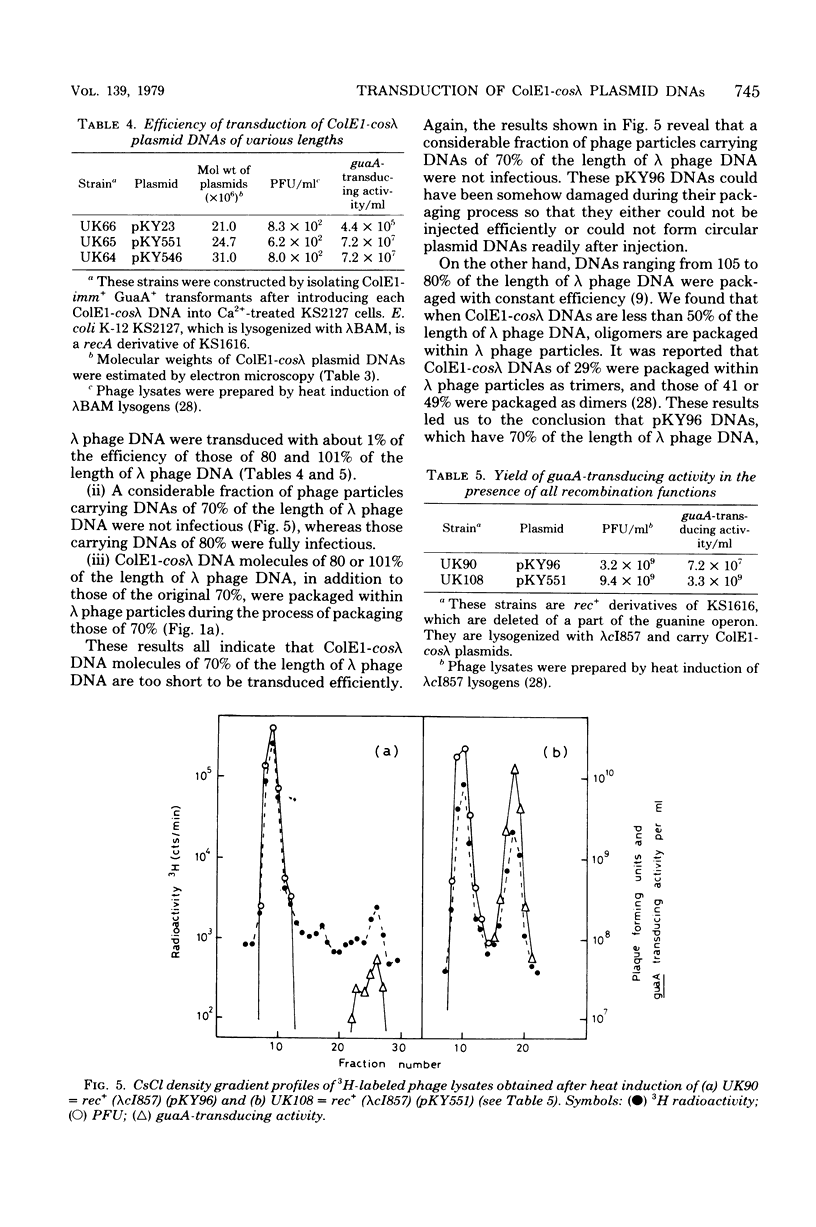
An in vitro recombinant ColE1-cos lambda deoxyribonucleic acid (DNA) molecule, pKY96, has 70% of the length of lambda phage DNA. The process of lambda phage-mediated transduction of pKY96 generated a small amount of transducing phage particles containing ColE1-cos lambda DNA molecules of 80 or 101% of the length of lambda phage DNA, in addition to those containing original pKY96 DNA molecules. The newly isolated larger plasmid DNAs were transduced 100 times more efficiently than pKY96 DNA. Their structures were compared with that of a prototype pKY96 DNA, and the mechanism of the formation of these molecules is discussed.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachmann B. J., Low K. B., Taylor A. L. Recalibrated linkage map of Escherichia coli K-12. Bacteriol Rev. 1976 Mar;40(1):116–167. doi: 10.1128/br.40.1.116-167.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. R. Supercoiled circular DNA-protein complex in Escherichia coli: purification and induced conversion to an opern circular DNA form. Proc Natl Acad Sci U S A. 1969 Apr;62(4):1159–1166. doi: 10.1073/pnas.62.4.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmons S. W. Bacteriophage lambda derivatives carrying two copies of the cohesive end site. J Mol Biol. 1974 Mar 15;83(4):511–525. doi: 10.1016/0022-2836(74)90511-7. [DOI] [PubMed] [Google Scholar]
- Enquist L. W., Skalka A. Replication of bacteriophage lambda DNA dependent on the function of host and viral genes. I. Interaction of red, gam and rec. J Mol Biol. 1973 Apr 5;75(2):185–212. doi: 10.1016/0022-2836(73)90016-8. [DOI] [PubMed] [Google Scholar]
- Enquist L. W., Weisberg R. A. A genetic analysis of the att-int-xis region of coliphage lambda. J Mol Biol. 1977 Apr;111(2):97–120. doi: 10.1016/s0022-2836(77)80117-4. [DOI] [PubMed] [Google Scholar]
- Feiss M., Campbell A. Duplication of the bacteriophage lambda cohesive end site: genetic studies. J Mol Biol. 1974 Mar 15;83(4):527–540. doi: 10.1016/0022-2836(74)90512-9. [DOI] [PubMed] [Google Scholar]
- Feiss M., Fisher R. A., Crayton M. A., Egner C. Packaging of the bacteriophage lambda chromosome: effect of chromosome length. Virology. 1977 Mar;77(1):281–293. doi: 10.1016/0042-6822(77)90425-1. [DOI] [PubMed] [Google Scholar]
- Fukumaki Y., Shimada K., Takagi Y. Specialized transduction of colicin E1 DNA in Escherichia coli K-12. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3238–3242. doi: 10.1073/pnas.73.9.3238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohn B. DNA as substrate for packaging into bacteriophage lambda, in vitro. J Mol Biol. 1975 Oct 15;98(1):93–106. doi: 10.1016/s0022-2836(75)80103-3. [DOI] [PubMed] [Google Scholar]
- Hradecna Z., Szybalski W. Electron micrographic maps of deletions and substitutions in the genomes of transducing coliphages lambda dg and lambda bio. Virology. 1969 Jul;38(3):473–477. doi: 10.1016/0042-6822(69)90160-3. [DOI] [PubMed] [Google Scholar]
- Jordan E., Saedler H., Starlinger P. O0 and strong-polar mutations in the gal operon are insertions. Mol Gen Genet. 1968;102(4):353–363. doi: 10.1007/BF00433726. [DOI] [PubMed] [Google Scholar]
- Lederberg E. M., Cohen S. N. Transformation of Salmonella typhimurium by plasmid deoxyribonucleic acid. J Bacteriol. 1974 Sep;119(3):1072–1074. doi: 10.1128/jb.119.3.1072-1074.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda S., Shimada K., Takagi Y. Molecular nature of an in vitro recombinant molecule: colicin El factor carrying genes for synthesis of guanine. Biochem Biophys Res Commun. 1976 Oct 4;72(3):1129–1136. doi: 10.1016/s0006-291x(76)80249-5. [DOI] [PubMed] [Google Scholar]
- Matsubara K., Takagi Y., Mukai T. In vitro construction of different oligomeric forms of lambdadv DNA and studies of their transforming activities. J Virol. 1975 Sep;16(3):479–485. doi: 10.1128/jvi.16.3.479-485.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada K., Weisberg R. A., Gottesman M. E. Prophage lambda at unusual chromosomal locations. I. Location of the secondary attachment sites and the properties of the lysogens. J Mol Biol. 1972 Feb 14;63(3):483–503. doi: 10.1016/0022-2836(72)90443-3. [DOI] [PubMed] [Google Scholar]
- Syvanen M. Processing of bacteriophage lambda DNA during its assembly into heads. J Mol Biol. 1975 Jan 15;91(2):165–174. doi: 10.1016/0022-2836(75)90157-6. [DOI] [PubMed] [Google Scholar]
- Tanaka T., Weisblum B. Construction of a colicin E1-R factor composite plasmid in vitro: means for amplification of deoxyribonucleic acid. J Bacteriol. 1975 Jan;121(1):354–362. doi: 10.1128/jb.121.1.354-362.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas M., Cameron J. R., Davis R. W. Viable molecular hybrids of bacteriophage lambda and eukaryotic DNA. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4579–4583. doi: 10.1073/pnas.71.11.4579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas M., Davis R. W. Studies on the cleavage of bacteriophage lambda DNA with EcoRI Restriction endonuclease. J Mol Biol. 1975 Jan 25;91(3):315–328. doi: 10.1016/0022-2836(75)90383-6. [DOI] [PubMed] [Google Scholar]
- Umene K., Shimada K., Takagi Y. Packaging of ColE1 DNA having a lambda phage cohesive end site. Mol Gen Genet. 1978 Feb 7;159(1):39–45. doi: 10.1007/BF00401746. [DOI] [PubMed] [Google Scholar]
- Yamagishi H., Inokuchi H., Ozeki H. Excision and duplication of su3+-transducing fragments carried by bacteriophage phi 80. I. Novel structure of phi 80sus2psu3+ DNA molecule. J Virol. 1976 Jun;18(3):1016–1023. doi: 10.1128/jvi.18.3.1016-1023.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]





