Abstract
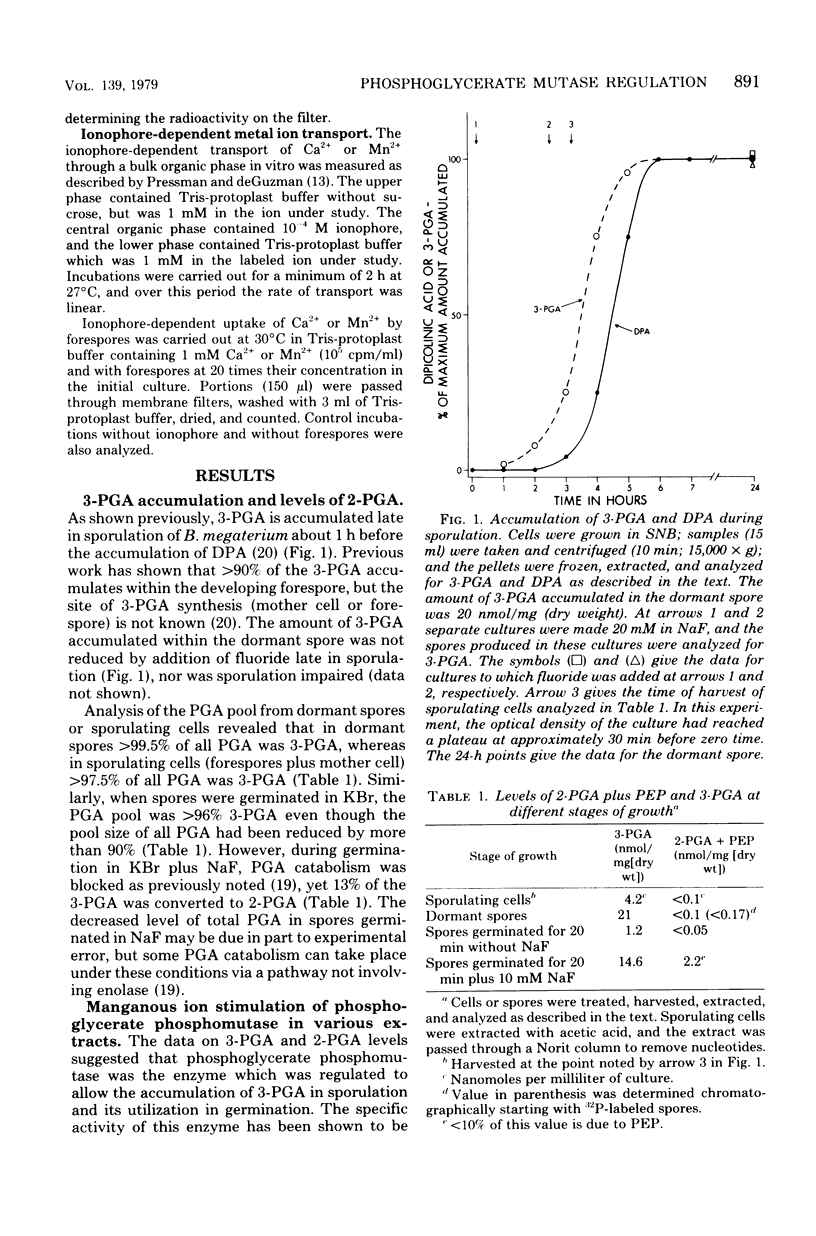
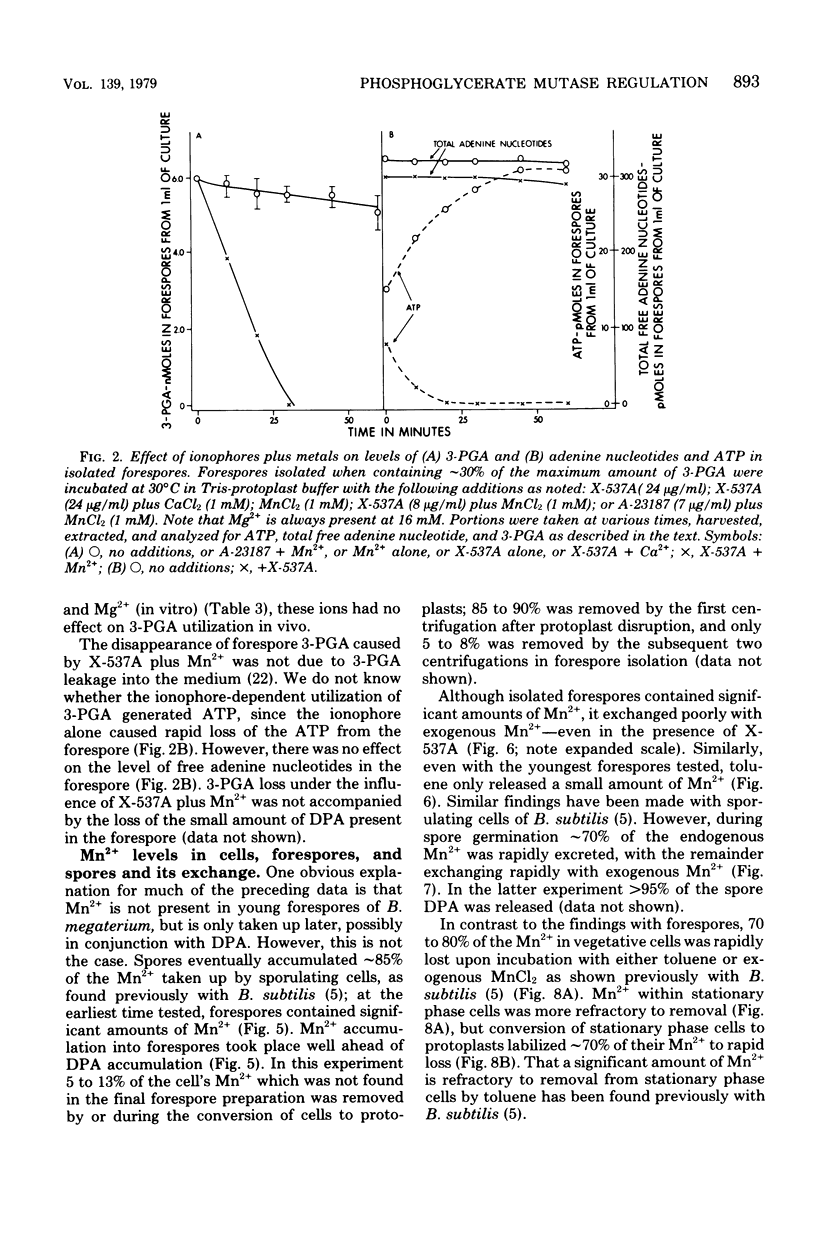
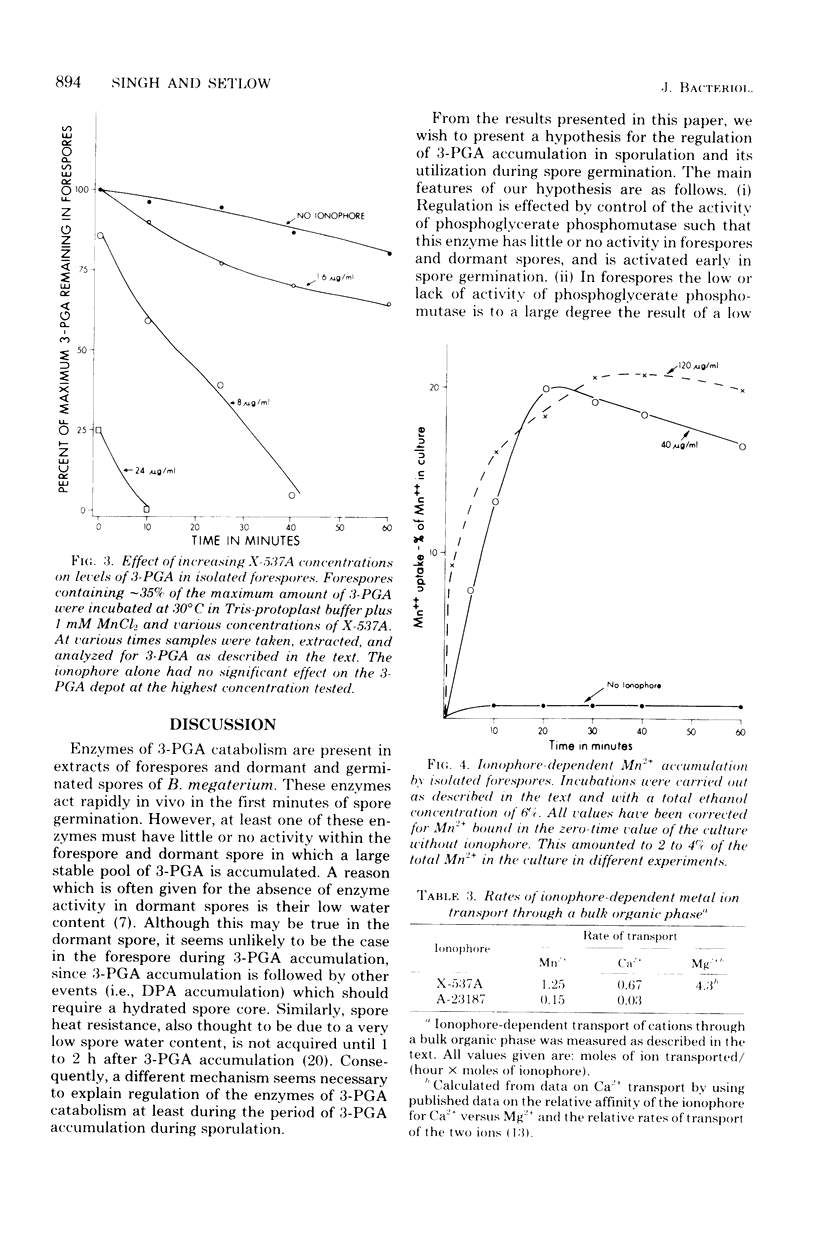
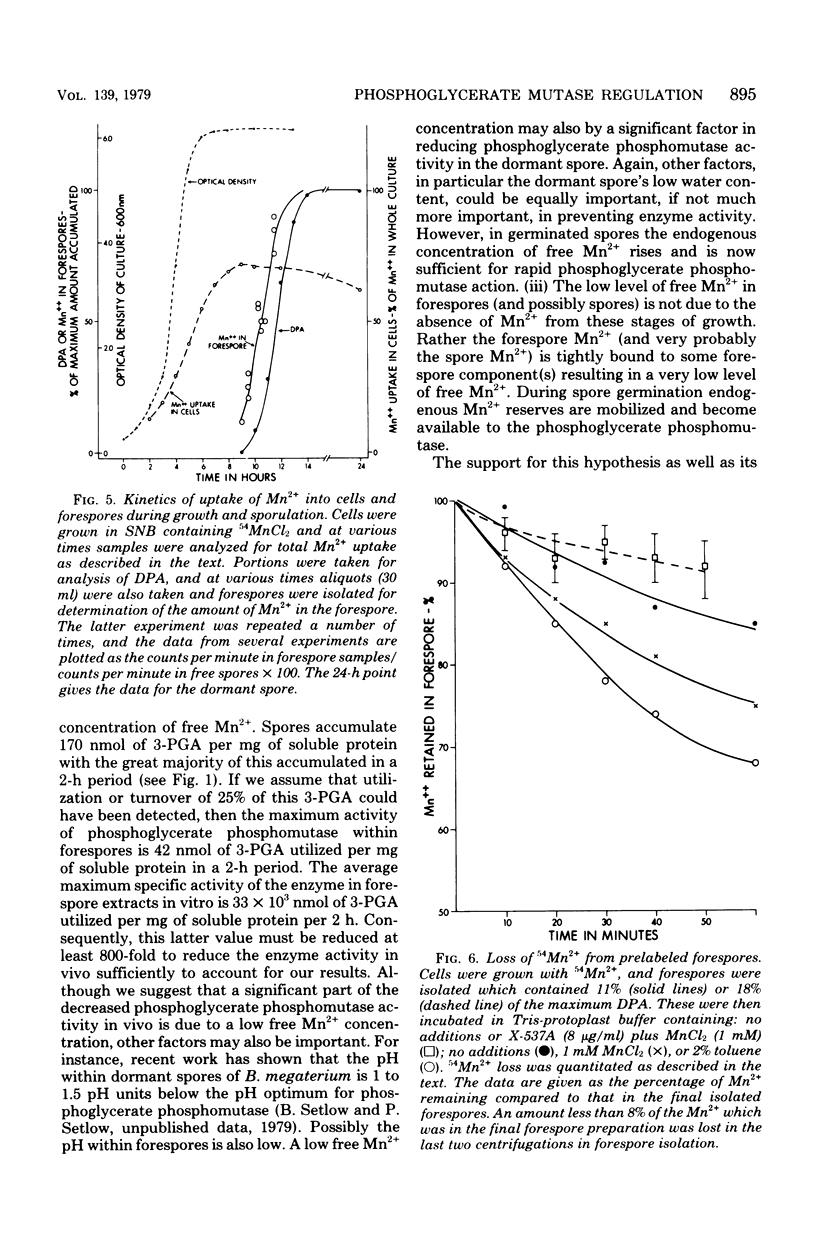
The large depot of phosphoglyceric acid (PGA) which is accumulated within spores of Bacillus megaterium is greater than 99% 3-phosphoglyceric acid (3-PGA). The 3-PGA depot is stable in forespores and dormant spores, but is utilized rapidly during spore germination. When spores were germinated in KBr plus NaF, the PGA depot was not utilized, but 13% of the 3-PGA was converted to 2-PGA. These data suggest phosphoglycerate phosphomutase as the enzyme which is regulated to allow 3-PGA accumulation during sporulation. Young isolated forespores, in which 3-PGA was normally stable, utilized their 3-PGA rapidly when incubated with Mn2+ plus the divalent cation ionophore X-537A; Mn2+ or ionophore alone or Mg2+ or Ca2+ plus ionophore was without effect. Young forespores contained significant amounts of Mn2+. However, forespore Mn2+ exchanged slowly with exogenous Mn2+ and was removed poorly by toluene treatment. This suggests that much of the forespore Mn2+ is tightly bound to some forespore component. Since phosphoglycerate phosphomutase from B. megaterium has an absolute and specific requirement for Mn2+, these data suggest that the activity of this enzyme in vivo may be regulated to a large degree by the level of free Mn2+. Indeed, the activity of this enzyme in forespore or dormant spore extracts was stimulated greater than 25-fold by Mn2+, whereas comparable extracts from cells or germinated spores were stimulated only two- to fourfold.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BIELESKI R. L. THE PROBLEM OF HALTING ENZYME ACTION WHEN EXTRACTING PLANT TISSUES. Anal Biochem. 1964 Dec;9:431–442. doi: 10.1016/0003-2697(64)90204-0. [DOI] [PubMed] [Google Scholar]
- COWGILL R. W. Paper chromatographic separation of 2-phosphoglyceric acid and 3-phosphoglyceric acid. Biochim Biophys Acta. 1955 Apr;16(4):614–614. doi: 10.1016/0006-3002(55)90300-0. [DOI] [PubMed] [Google Scholar]
- Chambers E. L., Pressman B. C., Rose B. The activation of sea urchin eggs by the divalent ionophores A23187 and X-537A. Biochem Biophys Res Commun. 1974 Sep 9;60(1):126–132. doi: 10.1016/0006-291x(74)90181-8. [DOI] [PubMed] [Google Scholar]
- Eisenstadt E., Fisher S., Der C. L., Silver S. Manganese transport in Bacillus subtilis W23 during growth and sporulation. J Bacteriol. 1973 Mar;113(3):1363–1372. doi: 10.1128/jb.113.3.1363-1372.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epel D. The program of fertilization. Sci Am. 1977 Nov;237(5):128–138. doi: 10.1038/scientificamerican1177-128. [DOI] [PubMed] [Google Scholar]
- Gould G. W., Dring G. J. Heat resistance of bacterial endospores and concept of an expanded osmoregulatory cortex. Nature. 1975 Dec 4;258(5534):402–405. doi: 10.1038/258402a0. [DOI] [PubMed] [Google Scholar]
- HANES C. S., ISHERWOOD F. A. Separation of the phosphoric esters on the filter paper chromatogram. Nature. 1949 Dec 31;164(4183):1107-12, illust. doi: 10.1038/1641107a0. [DOI] [PubMed] [Google Scholar]
- Hanson R. S., Curry M. V., Garner J. V., Halvorson H. O. Mutants of Bacillus cereus strain T that produce thermoresistant spores lacking dipicolinate and have low levels of calcium. Can J Microbiol. 1972 Jul;18(7):1139–1143. doi: 10.1139/m72-175. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Nelson D. L., Kornberg A. Biochemical studies of bacterial sporulation and germination. XIX. Phosphate metabolism during sporulation. J Biol Chem. 1970 Mar 10;245(5):1137–1145. [PubMed] [Google Scholar]
- Oh Y. K., Freese E. Manganese requirement of phosphoglycerate phosphomutase and its consequences for growth and sporulation of Bacillus subtilis. J Bacteriol. 1976 Aug;127(2):739–746. doi: 10.1128/jb.127.2.739-746.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pressman B. C., deGuzman N. T. Biological applications of ionophores: theory and practice. Ann N Y Acad Sci. 1975 Dec 30;264:373–386. doi: 10.1111/j.1749-6632.1975.tb31497.x. [DOI] [PubMed] [Google Scholar]
- Reed P. W., Lardy H. A. A23187: a divalent cation ionophore. J Biol Chem. 1972 Nov 10;247(21):6970–6977. [PubMed] [Google Scholar]
- Rotman Y., Fields M. L. A modified reagent for dipicolinic acid analysis. Anal Biochem. 1968 Jan;22(1):168–168. doi: 10.1016/0003-2697(68)90272-8. [DOI] [PubMed] [Google Scholar]
- SACKS L. E., BAILEY G. F. DRY RUPTURE OF BACTERIAL SPORES. J Bacteriol. 1963 Mar;85:720–721. doi: 10.1128/jb.85.3.720-721.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow P., Kornberg A. Biochemical studies of bacterial sporulation and germination. XVII. Sulfhydryl and disulfide levels in dormancy and germination. J Bacteriol. 1969 Dec;100(3):1155–1160. doi: 10.1128/jb.100.3.1155-1160.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow P., Kornberg A. Biochemical studies of bacterial sporulation and germination. XXII. Energy metabolism in early stages of germination of Bacillus megaterium spores. J Biol Chem. 1970 Jul 25;245(14):3637–3644. [PubMed] [Google Scholar]
- Singh R. P., Setlow B., Setlow P. Levels of small molecules and enzymes in the mother cell compartment and the forespore of sporulating Bacillus megaterium. J Bacteriol. 1977 Jun;130(3):1130–1138. doi: 10.1128/jb.130.3.1130-1138.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh R. P., Setlow P. Enolase from spores and cells of Bacillus megaterium: two-step purification of the enzyme and some of its properties. J Bacteriol. 1978 Apr;134(1):353–355. doi: 10.1128/jb.134.1.353-355.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh R. P., Setlow P. Phosphoglycerate mutase in developing forespores of Bacillus megaterium may be regulated by the intrasporal level of free manganous ion. Biochem Biophys Res Commun. 1978 May 15;82(1):1–5. doi: 10.1016/0006-291x(78)90567-3. [DOI] [PubMed] [Google Scholar]
- Singh R. P., Setlow P. Purification and properties of phosphoglycerate phosphomutase from spores and cells of Bacillus megaterium. J Bacteriol. 1979 Feb;137(2):1024–1027. doi: 10.1128/jb.137.2.1024-1027.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhardt R. A., Epel D., Carroll E. J., Jr, Yanagimachi R. Is calcium ionophore a universal activator for unfertilised eggs? Nature. 1974 Nov 1;252(5478):41–43. doi: 10.1038/252041a0. [DOI] [PubMed] [Google Scholar]
- WINDLE J. J., SACKS L. E. Electron paramagnetic resonance of managanese(II) and copper(II) in spores. Biochim Biophys Acta. 1963 Mar 19;66:173–179. doi: 10.1016/0006-3002(63)91183-1. [DOI] [PubMed] [Google Scholar]
- Watabe K., Freese E. Purification and properties of the manganese-dependent phosphoglycerate mutase of Bacillus subtilis. J Bacteriol. 1979 Feb;137(2):773–778. doi: 10.1128/jb.137.2.773-778.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]



