Abstract
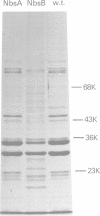
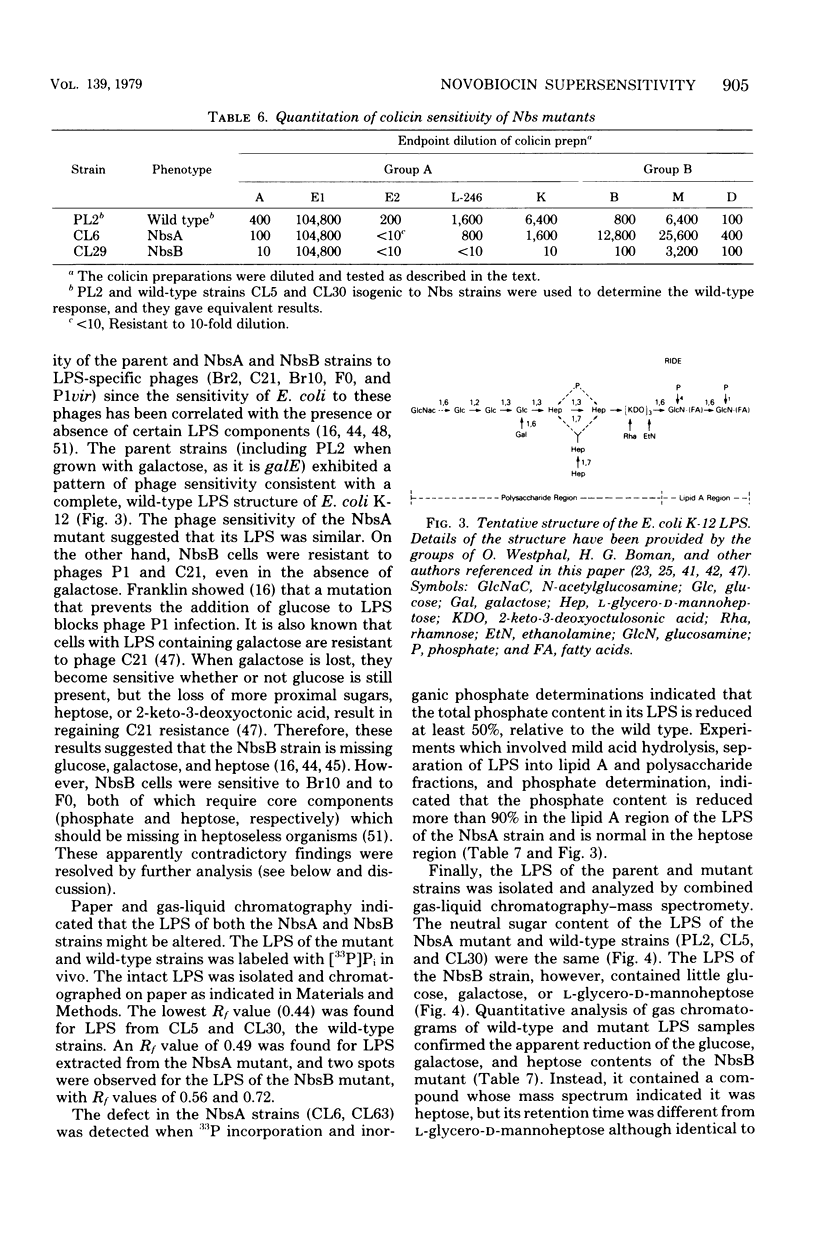
Two genetically distinct classes of novobiocin-supersensitive mutants were isolated from Escherichia coli K-12. One class, given the phenotypic name NbsA, lies at 10 min on the E. coli chromosome. The order of the genes in this region, based on transductional analyses, is proC NbsA plsA purE. The second, NbsB, lies at 80 min. The order of the genes in this region, based on transduction analyses, is xyl cysE NbsB pyrE. Both classes of mutants show increased sensitivity to hydrophobic drugs but are different: NbsA cells tend to be more sensitive to cationic agents, whereas NbsB cells show the opposite tendency. The sole detectable biochemical alteration in NbsA strain is greater than 90% reduction in the phosphate content of the lipid A region of the lipopolysaccharide. The NbsB mutation results in lipopolysaccharide that contains primarily the stereoisomer D-glycero-D-mannoheptose, rather than L-glycero-D-mannoheptose, and which contains very little of the distal sugars. Since NbsA strains have apparently normal outer membrane proteins and total cellular phospholipids, changes solely in lipopolysaccharide can increase permeability to certain hydrophobic antibiotics. Complementation studies indicate that the NbsA marker is probably allelic with acrA. In addition, the NbsB marker is genetically and phenotypically similar to the rfaD locus of Salmonella typhimurium. For this reason, the phenotypic designations NbsA and NbsB have been changed to the genotypic designations acrA and rfaD, respectively.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams G. A., Quadling C., Perry M. B. D-glycero-D-manno-heptose as a component of lipopolysaccharides from Gram-negative bacteria. Can J Microbiol. 1967 Dec;13(12):1605–1613. doi: 10.1139/m67-210. [DOI] [PubMed] [Google Scholar]
- Ames B. N., Lee F. D., Durston W. E. An improved bacterial test system for the detection and classification of mutagens and carcinogens. Proc Natl Acad Sci U S A. 1973 Mar;70(3):782–786. doi: 10.1073/pnas.70.3.782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ames G. F. Lipids of Salmonella typhimurium and Escherichia coli: structure and metabolism. J Bacteriol. 1968 Mar;95(3):833–843. doi: 10.1128/jb.95.3.833-843.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ames G. F. Resolution of bacterial proteins by polyacrylamide gel electrophoresis on slabs. Membrane, soluble, and periplasmic fractions. J Biol Chem. 1974 Jan 25;249(2):634–644. [PubMed] [Google Scholar]
- Ames G. F., Spudich E. N., Nikaido H. Protein composition of the outer membrane of Salmonella typhimurium: effect of lipopolysaccharide mutations. J Bacteriol. 1974 Feb;117(2):406–416. doi: 10.1128/jb.117.2.406-416.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann B. J., Low K. B., Taylor A. L. Recalibrated linkage map of Escherichia coli K-12. Bacteriol Rev. 1976 Mar;40(1):116–167. doi: 10.1128/br.40.1.116-167.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boman H. G., Monner D. A. Characterization of lipopolysaccharides from Escherichia coli K-12 mutants. J Bacteriol. 1975 Feb;121(2):455–464. doi: 10.1128/jb.121.2.455-464.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman W. G., Jr, Goebel P. J., Leive L. Genetic analysis of Escherichia coli O111:B4, a strain of medical and biochemical interest. J Bacteriol. 1977 May;130(2):656–660. doi: 10.1128/jb.130.2.656-660.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. D., MINGIOLI E. S. Mutants of Escherichia coli requiring methionine or vitamin B12. J Bacteriol. 1950 Jul;60(1):17–28. doi: 10.1128/jb.60.1.17-28.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies J. K., Reeves P. Genetics of resistance to colicins in Escherichia coli K-12: cross-resistance among colicins of group A. J Bacteriol. 1975 Jul;123(1):102–117. doi: 10.1128/jb.123.1.102-117.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies J. K., Reeves P. Genetics of resistance to colicins in Escherichia coli K-12: cross-resistance among colicins of group B. J Bacteriol. 1975 Jul;123(1):96–101. doi: 10.1128/jb.123.1.96-101.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eidels L., Osborn M. J. Lipopolysaccharide and aldoheptose biosynthesis in transketolase mutants of Salmonella typhimurium. Proc Natl Acad Sci U S A. 1971 Aug;68(8):1673–1677. doi: 10.1073/pnas.68.8.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ennis H. L. Mutants of Escherichia coli sensitive to antibiotics. J Bacteriol. 1971 Aug;107(2):486–490. doi: 10.1128/jb.107.2.486-490.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin N. C. Mutation in gal U gene of E. coli blocks phage P1 infection. Virology. 1969 May;38(1):189–191. doi: 10.1016/0042-6822(69)90144-5. [DOI] [PubMed] [Google Scholar]
- GINSBURG V., O'BRIEN P. J., HALL C. W. Guanosine diphosphate D-glycero-D-manno-heptose. J Biol Chem. 1962 Feb;237:497–499. [PubMed] [Google Scholar]
- Galanos C., Lüderitz O., Westphal O. A new method for the extraction of R lipopolysaccharides. Eur J Biochem. 1969 Jun;9(2):245–249. doi: 10.1111/j.1432-1033.1969.tb00601.x. [DOI] [PubMed] [Google Scholar]
- Gellert M., O'Dea M. H., Itoh T., Tomizawa J. Novobiocin and coumermycin inhibit DNA supercoiling catalyzed by DNA gyrase. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4474–4478. doi: 10.1073/pnas.73.12.4474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock R. E., Reeves P. Bacteriophage resistance in Escherichia coli K-12: general pattern of resistance. J Bacteriol. 1975 Mar;121(3):983–993. doi: 10.1128/jb.121.3.983-993.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havekes L. M., Lugtenberg B. J., Hoekstra W. P. Conjugation deficient E. coli K12 F- mutants with heptose-less lipopolysaccharide. Mol Gen Genet. 1976 Jul 5;146(1):43–50. doi: 10.1007/BF00267981. [DOI] [PubMed] [Google Scholar]
- Holland I. B., Threlfall E. J., Holland E. M., Darby V., Samson A. C. Mutants of Escherichia coli with altered surface properties which are refractory to colicin E2, sensitive to ultraviolet light and which can also show recombination deficiency, abortive growth of bacteriophage lambda and filament formation. J Gen Microbiol. 1970 Aug;62(3):371–382. doi: 10.1099/00221287-62-3-371. [DOI] [PubMed] [Google Scholar]
- Imae Y. Mitomycin C-sensitive mutant of Eschericha coli K-12. J Bacteriol. 1968 Mar;95(3):1191–1192. doi: 10.1128/jb.95.3.1191-1192.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadner R. J., Winkler H. H. Isolation and characterization of mutations affecting the transport of hexose phosphates in Escherichia coli. J Bacteriol. 1973 Feb;113(2):895–900. doi: 10.1128/jb.113.2.895-900.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koplow J., Goldfine H. Alterations in the outer membrane of the cell envelope of heptose-deficient mutants of Escherichia coli. J Bacteriol. 1974 Feb;117(2):527–543. doi: 10.1128/jb.117.2.527-543.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo T. T., Stocker B. A. Mapping of rfa Genes in Salmonella typhimurium by ES18 and P22 Transduction and by Conjugation. J Bacteriol. 1972 Oct;112(1):48–57. doi: 10.1128/jb.112.1.48-57.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LENNOX E. S. Transduction of linked genetic characters of the host by bacteriophage P1. Virology. 1955 Jul;1(2):190–206. doi: 10.1016/0042-6822(55)90016-7. [DOI] [PubMed] [Google Scholar]
- LIN E. C., LERNER S. A., JORGENSEN S. E. A method for isolating constitutive mutants for carbohydrate-catabolizing enzymes. Biochim Biophys Acta. 1962 Jul 2;60:422–424. doi: 10.1016/0006-3002(62)90423-7. [DOI] [PubMed] [Google Scholar]
- LURIA S. E., BURROUS J. W. Hybridization between Escherichia coli and Shigella. J Bacteriol. 1957 Oct;74(4):461–476. doi: 10.1128/jb.74.4.461-476.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann V., Hämmerling G., Nurminen M., Minner I., Ruschmann E., Lüderitz O., Kuo T. T., Stocker B. A. A new class of heptose-defective mutant of Salmonella typhimurium. Eur J Biochem. 1973 Jan 15;32(2):268–275. doi: 10.1111/j.1432-1033.1973.tb02607.x. [DOI] [PubMed] [Google Scholar]
- Leive L. Release of lipopolysaccharide by EDTA treatment of E. coli. Biochem Biophys Res Commun. 1965 Nov 22;21(4):290–296. doi: 10.1016/0006-291x(65)90191-9. [DOI] [PubMed] [Google Scholar]
- Leive L. The barrier function of the gram-negative envelope. Ann N Y Acad Sci. 1974 May 10;235(0):109–129. doi: 10.1111/j.1749-6632.1974.tb43261.x. [DOI] [PubMed] [Google Scholar]
- Low B. Rapid mapping of conditional and auxotrophic mutations in Escherichia coli K-12. J Bacteriol. 1973 Feb;113(2):798–812. doi: 10.1128/jb.113.2.798-812.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monner D. A., Jonsson S., Boman H. G. Ampicillin-resistant mutants of Escherichia coli K-12 with lipopolysaccharide alterations affecting mating ability and susceptibility to sex-specific bacteriophages. J Bacteriol. 1971 Aug;107(2):420–432. doi: 10.1128/jb.107.2.420-432.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakae T., Nikaido H. Outer membrane as a diffusion barrier in Salmonella typhimurium. Penetration of oligo- and polysaccharides into isolated outer membrane vesicles and cells with degraded peptidoglycan layer. J Biol Chem. 1975 Sep 25;250(18):7359–7365. [PubMed] [Google Scholar]
- Nakae T. Outer membrane of Salmonella typhimurium: reconstitution of sucrose-permeable membrane vesicles. Biochem Biophys Res Commun. 1975 Jun 16;64(4):1224–1230. doi: 10.1016/0006-291x(75)90823-2. [DOI] [PubMed] [Google Scholar]
- Nakae T. Outer membrane of Salmonella. Isolation of protein complex that produces transmembrane channels. J Biol Chem. 1976 Apr 10;251(7):2176–2178. [PubMed] [Google Scholar]
- Nikaido H. Outer membrane of Salmonella typhimurium. Transmembrane diffusion of some hydrophobic substances. Biochim Biophys Acta. 1976 Apr 16;433(1):118–132. doi: 10.1016/0005-2736(76)90182-6. [DOI] [PubMed] [Google Scholar]
- Payne J. W., Gilvarg C. Size restriction on peptide utilization in Escherichia coli. J Biol Chem. 1968 Dec 10;243(23):6291–6299. [PubMed] [Google Scholar]
- Prehm P., Stirm S., Jann B., Jann K., Boman H. G. Cell-wall lipopolysaccharides of ampicillin-resistant mutants of Escherichia coli K-12. Eur J Biochem. 1976 Jul 1;66(2):369–377. doi: 10.1111/j.1432-1033.1976.tb10526.x. [DOI] [PubMed] [Google Scholar]
- Rapin A. M., Kalckar H. M., Alberico L. The metabolic basis for masking of receptor-sites on E. coli K-12 for C21, a lipopolysaccharide core-specific phage. Arch Biochem Biophys. 1968 Oct;128(1):95–105. doi: 10.1016/0003-9861(68)90011-8. [DOI] [PubMed] [Google Scholar]
- Rooney S. A., Goldfine H. Isolation and characterization of 2-keto-3-deoxyoctonate-lipid A from a heptose-deficient mutant of Escherichia coli. J Bacteriol. 1972 Aug;111(2):531–541. doi: 10.1128/jb.111.2.531-541.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosner J. L. Formation, induction, and curing of bacteriophage P1 lysogens. Virology. 1972 Jun;48(3):679–689. doi: 10.1016/0042-6822(72)90152-3. [DOI] [PubMed] [Google Scholar]
- Sanderson K. E., Van Wyngaarden J., Lüderitz O., Stocker B. A. Rough mutants of Salmonella typhimurium with defects in the heptose region of the lipopolysaccharide core. Can J Microbiol. 1974 Aug;20(8):1127–1134. doi: 10.1139/m74-175. [DOI] [PubMed] [Google Scholar]
- Schlecht S., Westphal O. Untersuchungen zur Typisierung von Salmonella-R-Formen. 4. Typisierung von S. minnesota-R-Mutanten mittels Antibiotica. Zentralbl Bakteriol Orig. 1970 Apr;213(3):356–380. [PubMed] [Google Scholar]
- Schmidt G., Fromme I., Mayer H. Immunochemical studies on core lipopolysaccharides of Enterobacteriaceae of different genera. Eur J Biochem. 1970 Jun;14(2):357–366. doi: 10.1111/j.1432-1033.1970.tb00297.x. [DOI] [PubMed] [Google Scholar]
- Schmidt G., Jann B., Jann K. Immunochemistry of R lipopolysaccharides of Escherichia coli. Studies on R mutants with an incomplete core, derived from E. coli O8:K27. Eur J Biochem. 1970 Oct;16(2):382–392. doi: 10.1111/j.1432-1033.1970.tb01092.x. [DOI] [PubMed] [Google Scholar]
- Schnaitman C. A. Solubilization of the cytoplasmic membrane of Escherichia coli by Triton X-100. J Bacteriol. 1971 Oct;108(1):545–552. doi: 10.1128/jb.108.1.545-552.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studier F. W. Analysis of bacteriophage T7 early RNAs and proteins on slab gels. J Mol Biol. 1973 Sep 15;79(2):237–248. doi: 10.1016/0022-2836(73)90003-x. [DOI] [PubMed] [Google Scholar]
- Tamaki S., Sato T., Matsuhashi M. Role of lipopolysaccharides in antibiotic resistance and bacteriophage adsorption of Escherichia coli K-12. J Bacteriol. 1971 Mar;105(3):968–975. doi: 10.1128/jb.105.3.968-975.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEISSBACH A., HURWITZ J. The formation of 2-keto-3-deoxyheptonic acid in extracts of Escherichia coli B. I. Identification. J Biol Chem. 1959 Apr;234(4):705–709. [PubMed] [Google Scholar]