Abstract
In recent years, ice-free cryopreservation by vitrification has been demonstrated to provide superior preservation of tissues compared with conventional freezing methods. To date, this has been accomplished almost exclusively for small model systems, whereas cryopreservation of large tissue samples—of a clinically useful size—continues to be hampered by thermomechanical effects that compromise the structure and function of the tissue. Reduction of mechanical stress is an integral condition of successful cryopreservation of large specimens. The current study focuses on the impact of sample size on both the physical events, observed by cryomacroscopy, and on the outcome on tissue function. To this end, the current study sought to address the question of functional recovery of vitrified carotid artery segments, processed as either artery rings (3–4 mm long) or segments (25 mm long) as selected models; the latter model represents a significant increase in sample size for evaluating the effects of vitrification. Tissue vitrification using an 8.4 M cryoprotectant cocktail solution (VS55) was achieved in 1-ml samples by imposing either a high (50–70 °C/min) or a low (2–3 °C/min) cooling rate, between −40°C and −100°C, and a high rewarming rate between −100°C and −40°C. Following cryoprotectant removal, the artery segments were cut into 3 to 4-mm rings for function testing on a contractility apparatus by measuring isometric responses to four agonist and antagonists (norepinephrine, phenylepinephrine, calcium ionophore, and sodium nitroprusside). In addition, nonspecific metabolic function of the vessel rings was determined using the REDOX indicator alamarBlue. Contractile function in response to the agonists norepinephrine and phenylepinephrine was maintained at the same level (350%) for the segments as for the rings, when compared with noncryopreserved control samples. Relaxation in response to the antagonists calcium ionophore and sodium nitroprusside was maintained at between 75% and 100% of control levels, irrespective of cooling rate or sample size. No evidence of macroscopic crystallization or fractures was observed by cryomacroscopy at the above rates in any of the samples. In conclusion, this study verifies that the rate of cooling and warming can be reduced from our baseline vitrification technique such that the function of larger tissue samples is not significantly different from that of smaller blood vessel rings. This represents a step toward the goal of achieving vitreous cryopreservation of large tissue samples without the destructive effect of thermal stresses.
INTRODUCTION
In recent years, techniques have been developed to preserve native tissues, such as blood vessels and articular cartilage, in an essentially ice-free condition.1–4 However, although vitreous cryopreservation has been demonstrated to provide superior preservation, compared with conventional freezing methods in small model systems, cryopreservation of large tissue samples continues to be hampered by thermomechanical effects.5 These include problems arising from the limits of heat and mass transfer in bulky systems and damage induced by mechanical stress, with fractures as its most dramatic outcome.4,6,7 It is our opinion that in some circumstances even a single major fracture may prevent the tissue from recovery or its effective clinical use after cryogenic storage. Reduction of mechanical stress, and thereby prevention of fracture formation, is a necessary integral condition for successful cryopreservation of large specimens. For example, immersion of frozen human valves directly into liquid nitrogen, for as little as 5 min, may result in tissue fractures.8 This problem came to light when a hospital-based frozen valve storage system overfilled during an automatic refill cycle. Valves from this accident were discovered to have numerous full thickness fractures of the valve conduit, following normal thawing procedures in the operating room.9 Adams et al.8 reproduced this phenomenon experimentally.
Fractures are attributed to stresses that arise either due to nonuniform thermal expansion, resulting from nonuniform temperature distribution in larger tissues, or to volume changes associated with phase transition; frequently, it is the combination of both that leads to structural damage of the tissue. Whereas pure vitrification eliminates phase transition effects all together, the higher cooling rates necessary to facilitate vitrification typically lead to a higher nonuniformity in thermal expansion distribution, resulting in higher mechanical stresses. The competing needs of rapid cooling to facilitate vitrification and minimization of mechanical damage demand a greater understanding of both vitrification and stress development. Although mechanical stress has long been recognized as an important mechanism of tissue destruction, it has received very little attention in the context of cryobiology until recent years.
Kroener and Luyet10 suggested that fracturing in aqueous glycerol solutions is associated with abrupt changes in the thermal expansion of the vitrifying tissue matrix around its glass transition temperature. Subsequently, they reported11 that the formation and the disappearance of cracks depend on the interaction of several factors, in particular the mechanical properties of the material, the concentration of solute, the temperature gradients, the overall temperature, and the rate of temperature change. Although the thermal expansion has been recognized as the driving mechanism of thermal stress,12 this physical property has been measured only recently for crystallized biomaterials,13 and for vitrifying cryoprotectants in the absence14,15 and presence16,17 of biological material.
Mathematical models have been offered to predict the level of stress in crystallized18,19 and vitrified20,21 biomaterials. The key physical properties to be used in these models, such as elastic modulus, viscosity, and fracture strength are only at the beginning of their exploration.20,21 Nevertheless, it has been demonstrated that mathematical modeling can explain various effects associated with thermal stress. For example, it has been demonstrated why fractures often appear at the beginning or rewarming, rather than during cooling of a crystallized material.19 In another study, it has been demonstrated why differential thermal expansion is responsible for fracturing in a vitrified material.20
As part of an ongoing integrated research program, a new imaging device has been developed recently, called a “cryomacroscope,”22 to study macroscopic events and physical changes in samples of vitrified tissues. The principal objectives for this development were to identify thermophysical events, such as devitrification22 and fracturing21 in vitrified samples of blood vessels, and to correlate these events with the functional recovery of the tissue, which is the subject matter of the current report. Furthermore, this study was designed to evaluate the impact of sample size on both the physical events, observed during cooling and rewarming, and on the outcome on tissue function. To this end, the current study sought to address the question of functional recovery of vitrified carotid artery segments, processed as either artery rings (3–4 mm long) or segments (25 mm long) as selected models; the latter model represents a significant increase in sample size for evaluating the effects of vitrification. This is the next step in scale-up following our demonstration that blood vessel rings can be successfully vitrified in small volumes (1 ml) with a high degree of functional recovery.1,4 The thermal protocol applied in the current study has been developed to ensure vitrification and prevent fracture formation, using the same cryomacroscope setup.
METHODS
For the present study, 13 New Zealand white rabbits (male, average weight 3.5 kg, Myrtle’s Rabbitry Inc, Thompson Station, TN) were used as tissue donors. Two carotid arteries per rabbit were recovered under anesthesia, using a standard “no-touch” technique,1,23 and the vessels were immersed in cold Dulbecco’s modified eagle’s medium (DMEM) for transportation to the research laboratory. One artery was immediately ice-free cryopreserved while the other was refrigerated overnight in DMEM. The overnight hypothermic storage did not affect tissue viability and functionality as determined by preliminary testing (not shown). The vessels were vitrified either as 3 to 4 mm-long rings or as 2.5-cm-long artery segments. The vitrification of arteries as rings or segments was alternated between being immediately cryopreserved or refrigerated overnight.
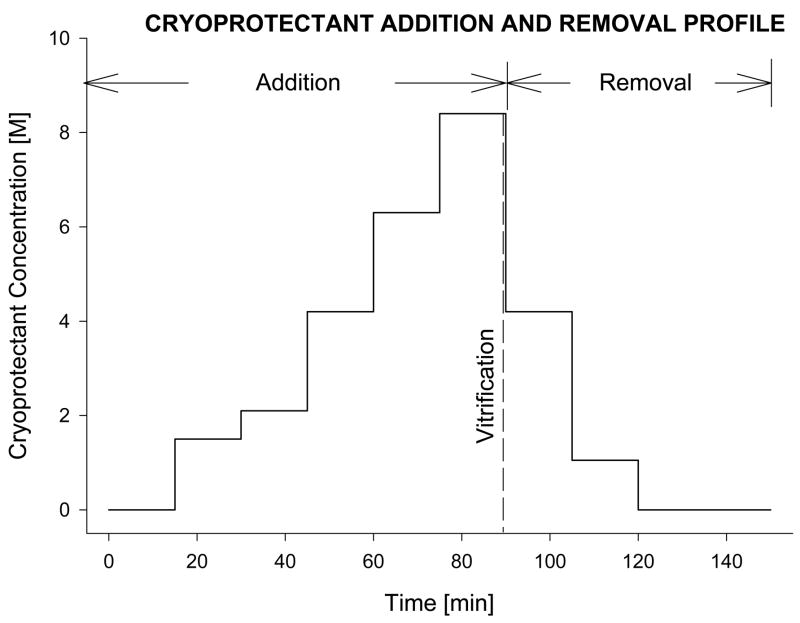
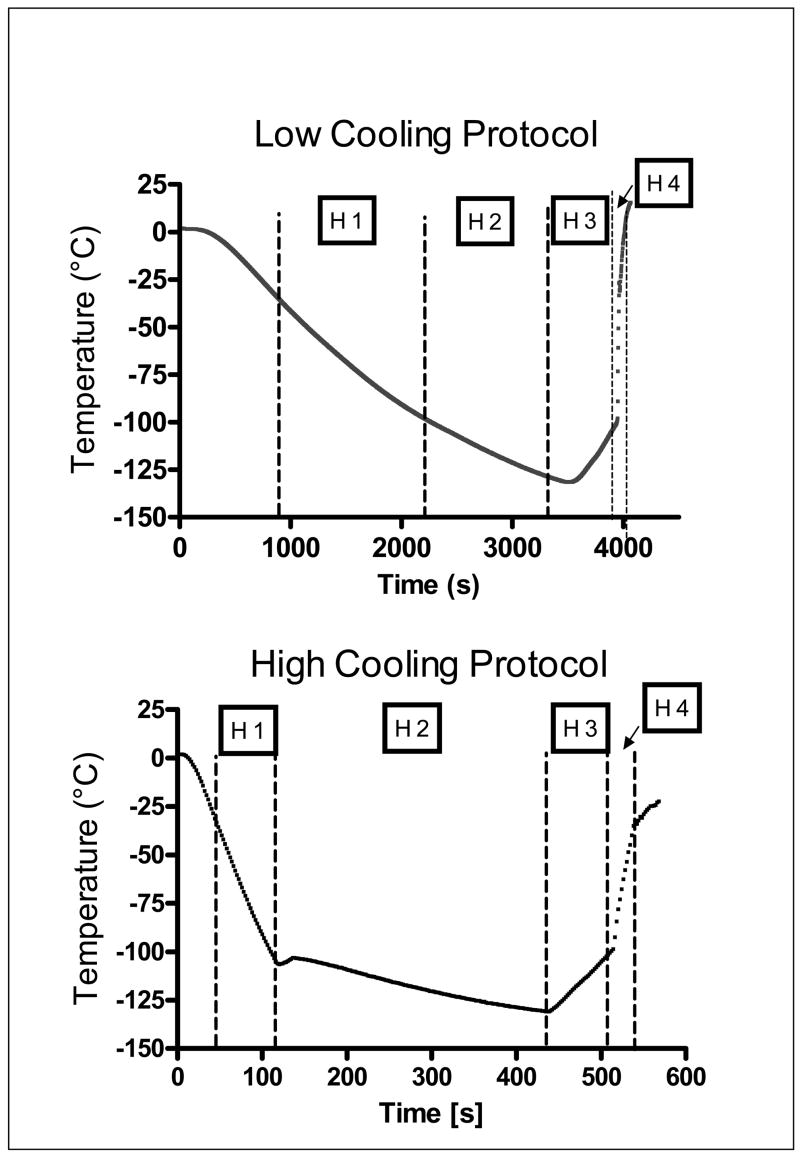
An 8.4 M cryoprotectant vitrification solution (VS55; 3.1 M DMSO, 2.2 M 1,2-propanediol, and 3.1 M formamide) was used.24 Tissue vitrification was performed by imposing either a high (50–70°C/min) or a low (2–3°C/min) cooling rate between −40°C and −100°C, and a high re-warming rate between −100°C and −40°C. For VS55, the temperature range between −100°C and −40°C is considered the hazardous range for crystal formation and growth during both cooling and rewarming. Table 1 summarizes the cryopreservation cooling rates as measured and averaged over 13 rabbits and expressed as mean ± standard error of the mean (SEM). The tissue was loaded and unloaded with cryoprotectant using a stepwise addition and removal protocol at 0°C, as shown in Fig. 1. The loading and unloading of 3 the cryoprotectant was performed by either passive diffusion or gravity-driven perfusion, for the rings and vessel segments, respectively. The addition and removal of the cryoprotectant cocktails in a stepwise manner by perfusion of carotid segments was accomplished using the method described and illustrated previously for vein segments.25 Following cryoprotectant loading, the tissue was placed in 1 mL of precooled (0°C) VS55 in a 20-mL (25-mm diameter × 60 mm high) glass scintillation vial. The sample vial was mounted on the cryomacroscope, comprising a borescope-video camera connected to a computer data acquisition system that allowed for real-time visualization and recording of the cooling and rewarming phases, as described previously.22 Preliminary experiments without tissue were carried out to determine the conditions and set-up necessary to replicate on the cryomacroscope those conditions previously shown to yield high survival of vein rings after vitrification. To achieve the desired cooling and rewarming rates, the experimental conditions summarized in Table 1 were developed and applied. Temperature control was achieved passively by introducing the sample to a convective cryogenic environment, as previously described.22 For these conditions, predetermined constant-volume baths of liquid nitrogen (cooling) and a 30% di-methylsulfoxide (DMSO)–water mixture (warming) were used. The former was employed to cool the air (inside of a 600-mL glass beaker) surrounding the tissue sample vial. Moving from one condition to another was rapidly performed, without interrupting the normal course of temperature and cryopreservation process.
Table 1.
Experimental Cooling Rates, Rewarming Rates, and Thermal Conditions
| Coolinga |
Warminga |
|||
|---|---|---|---|---|
| H1 (−40°C to −100°C) | H2 (−100°C to −130°C) | H3 (−130°C to −100°C) | H4 (−100°C to −40°C) | |
| High cooling protocol (°C/min) | 63.50 ± 1.84 | 10.22 ± 3.96 | 26.8 ± 0.60 | 175.05 ± 9.58 |
| Experimental conditions | Sample vial with sleeve (5-mm wall thickness) in liquid nitrogen | Sample vial in air at liquid nitrogen boiling temperature | Sample vial in ambient air | Sample vial in 30% DMSO–water bath at room temperature |
| Low cooling protocol (°C/min) | 2.53 ± 0.08 | 1.33 ± 0.08 | 4.73 ± 0.10 | 172.9 ± 12.47 |
| Experimental conditions | Sample vial with sleeve (15-mm wall thickness) in air at liquid nitrogen boiling temperature | Sample vial with sleeve (15-mm wall thickness) in air at liquid nitrogen boiling temperature | Sample vial with sleeve (15-mm wall thickness) in ambient air | Sample vial in30% DMSO–water bath at room temperature |
The experimental set up used to achieve these conditions has been described in more detail elsewhere.22
Figure 1.
Schematic diagram of the sequential incubation steps, adapted from a previously reported technique for adding and removing the vitrification cocktail, VS55 [24]. For artery rings, the loading and unloading of the cryoprotectant mixture was accomplished by immersing of the rings in the pre-cooled solutions (passive diffusion) for 15mins at each concentration step. For vessel segments, the process was facilitated by gravity perfusion of the solutions through the lumen of the artery segment [25]. Similarly, after vitrification and rewarming, the tissue samples were returned to physiological medium by eluting the cryoprotectants sequentially, using stepped changes. The cryoprotectant solution used at each step in the dilution was supplemented with 300mM mannitol as an osmotic buffer, to prevent excess cell swelling.Schematic diagram of the sequential incubation steps, adapted from a previously reported technique for adding and removing the vitrification cocktail, VS55.
Following cryoprotectant removal (Fig. 1), the vitrified segments were cut in 3 to 4-mm-long rings for function testing on a contractility apparatus as previously described.1,23 Each ring sample, either vitrified or unpreserved, was mounted between two stainless steel wire hooks, suspended in a physiological temperature tissue organ bath (Radnoti Glass Technology, Monrovia, CA), which contains 5 mL of continuously gassed (95% O2-5% CO2) Krebs–Henseleit (KH) solution. After 1 h of equilibration, the baseline pretension of each artery ring was adjusted, in the range of 0.25 and 0.75 g-force, and the isometric response of the rings to a selection of four agonist and antagonists—norephinephrine (N), phenylephrine (P), calcium ionophore (Ca-I), and sodium-nitro-prusside (SNP)—was tested to assess the endothelium-independent and dependent smooth muscle cell mechanical function. Changes in ring tension relative to their baseline, in response to various doses of these pharmacological agents, were recorded by a digital data acquisition and analysis system (Gould Instruments Systems, Valley View, OH); results are expressed in percent relative to the fresh-unpreserved mechanical response of control rings from the same artery.
The viability of cryopreserved and fresh control rings was determined using alamarBlue (Trek Diagnostics Systems, Cleveland, OH), which is a noninvasive, nonspecific cell metabolic activity indicator. It incorporates a water-soluble oxidation-reduction (REDOX) indicator that fluoresces and changes color in response to a cell’s innate metabolic activity. After 3 h of incubation (37°C, 5% CO2) of the rings with 10% alamarBlue working solution, 100-μL aliquots of the supernatant medium were removed, placed in a 96-well plate, and read on a microtiter plate spectrofluorometer at 590 nm. The relative fluorescence intensity was normalized to the dry weight of the tissue, and expressed in percent normalized fluorescence intensity of untreated controls.
RESULTS AND DISCUSSION
For small cryopreserved samples, it is well established that increasing the cooling rate leads to two desirable outcomes: the likelihood of complete vitrification increases, and the toxicity potential of the cryoprotectant decreases; the toxicity effect is known to be more significant at higher temperatures. In large samples however, even if the cooling rate at the sample’s outer surface is extremely high, the cooling rate distribution within the sample is dominated by a mechanism of heat transfer, and by the thermal properties of the tissue. It is the cooling rate at the center of the sample that must exceed the conditions for vitrification, to ensure complete vitrification of the sample; at the center, the temperature is the highest and the cooling rate is lowest. The larger the sample is, the slower the cooling rate becomes at the center of the sample, which defines a sample-size effect on vitrification. The sample size also affects the likelihood of structural damage, such as fracture, as reviewed in the introduction; the likelihood of fracturing increases with the increasing sample size.
In a previous study,22 the minimum cooling rate to achieve vitrification and the minimum rewarming rate to prevent devitrification (crystal nucleation during rewarming) or recrystal-lization (crystal growth around existing nuclei during rewarming) were established, with the objective of exceeding these threshold values in scale-up experiments. Those previous studies were performed on a 1-mL cryoprotectant sample in a 20-mL vial. Furthermore, the maximum allowable cooling rate and the lowest allowable temperature to prevent fracture formation were established, using the exact same sample volume and vial size.21
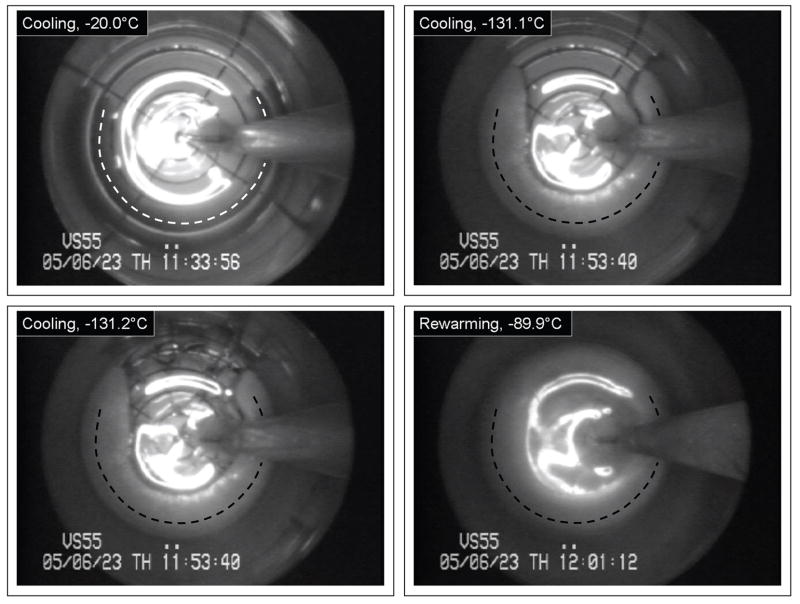
The outcome of cryopreservation via vitrification is a clear and transparent domain of cryoprotectant containing the blood vessel specimens. For illustration purposes of the application of cryomacroscopy in the current study, Fig. 2 displays results from an unsuccessful cryopreservation protocol. Above the heterogeneous nucleation point of the cryoprotectant, it appears as a transparent fluid (top left of Fig. 2). In this particular experiment, crystallization formed around (and probably inside) the specimen, while the remaining part of the domain vitrified. The crystallized region started to grow in a cylindrical shape, having a curved centerline roughly coinciding with the specimen’s centerline. The rate of growth of the crystallized region decreased with the decreasing temperature. The top right image in Fig. 2 is taken at a temperature of −131.1°C, which is 11.3°C below the glass transition temperature of −119.8°C for VS55. Due to thermal expansion mismatch between the crystallized and the vitrified regions, and possibly between the glass vial and the vitrified material,20,21 fractures form at the center top of the bottom left image. Note that these fractures occurred very rapidly, propagating in a time frame of milliseconds. Finally, devitrification is demonstrated in the bottom left image of Fig. 2. Devitrification is the formation of crystals during rewarming, whereas recrystallization is the crystal growth around already existing nuclei. Because a crystallization front was demonstrated in this particular experiment (its effect can only be seen in the video recording), while recrystallization is expected to be observed as a volumetric effect, the current process is identified as devitrification rather than recrystallization. Figure 3 displays representative images of a vitrified blood vessel segment (top) and rings (bottom), where macroscopic events of crystallization or fracture formation were not observed. The thermal protocol developed for the current study consistently led to the results displayed in Fig. 3.
Figure 2.
Representative cryomacroscopy images of a blood vessel segment (its centerline is represented by a dash line) immersed in VS55, subject to H1=6.3°C/min, H2=4.4°C/min, H3=18.2°C/min, and H4=137.1°C/min, and minimum temperature of −137.2°C; top-left: the specimen surrounded by CPA at the beginning of cooling (−20°C); top-right: the specimen is surrounded by crystallized material, while the remaining of the domain is vitrified (−131.1°C); bottom-left: fracture formation in the glassy region of the VS55, at the top-center portion of the image; bottom-right: complete devitrification during rewarming (−89.9°C).
Figure 3.
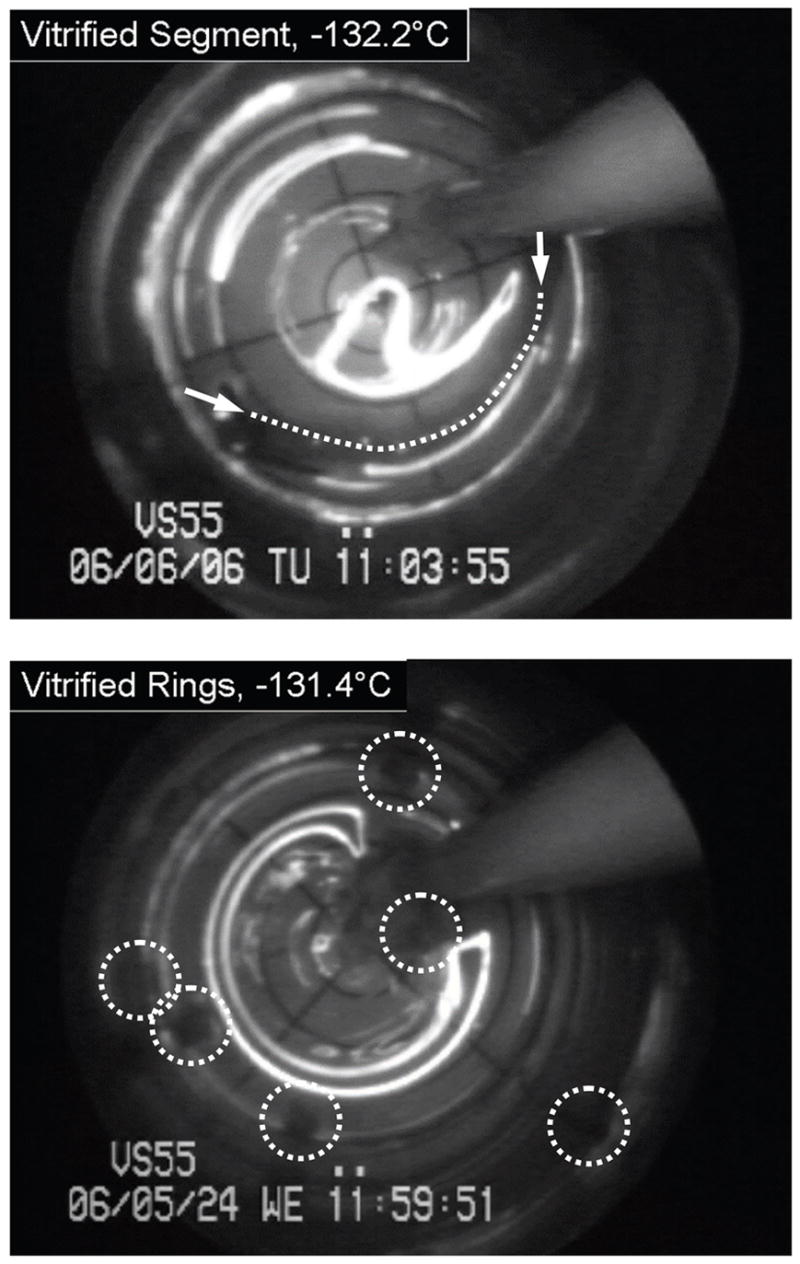
Representative cryomacroscopy images of vitrification of a blood vessel segment. at a minimum temperature of −132.2°C (top, the arrows point to the ends of the specimen and the dot line represents its centerline), and rings at a temperature of −131.4°C (bottom, selected rings are circled). In either image, the visibility of the polar grid underneath the CPA indicates that the VS55 vitrified, in contrast to the bottom-right image of Fig. 2.
Experiments described in the current study focus on functional recovery of carotid artery samples post-vitrification, subject to the minimum cooling rate established previously.22 Additional experiments were performed at a reference (much higher) cooling rate, which yet does not lead to fracturing of the sample, as verified by means of imaging (all experiments were performed while the testing vial was connected to the cryomacroscope). More specifically, the current study was designed to compare the functional recovery of carotid artery samples prepared either as 3-mm-thick rings or 2.5-cm-long segments, on either one of the two thermal protocols described above and illustrated in Fig. 4. The 2.5-cm segments were selected as a model system that represents a significant increase in size when compared with the 3-mm rings, which have previously been used as a standard model system for developing a successful technique of tissue vitrification.1 Moreover, steps were taken to reproduce predetermined vitrification protocols on the cryomacroscope, as specified in Table 1, where the reference data was not available for the cryomacroscope setup. These steps represent empirical selection of appropriate thermal sleeves and cooling and rewarming media, as discussed previously.22
Figure 4.
Typical thermal protocols during the vitrification process on the cryomacroscope setup. Details on the experimental set-up used to achieve these thermal protocols are summarized in Table 1.
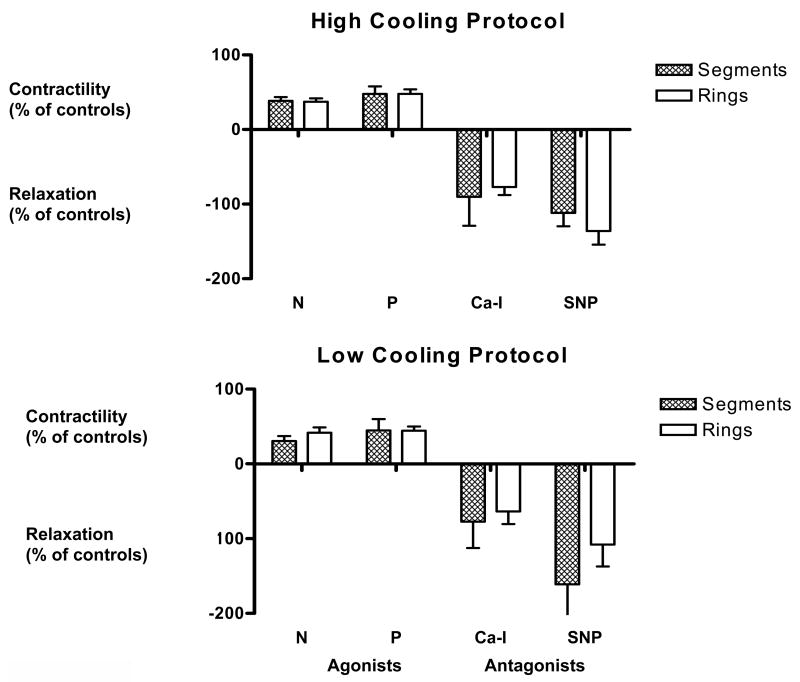
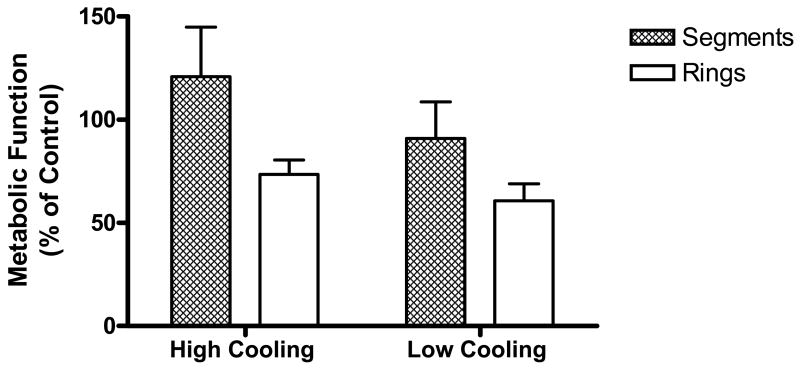
Figure 5 shows that for both cases of low and high cooling rates, there was no significant difference in the functional recovery between rings and segments. Contractile function in response to the agonists norepinephrine and phenylepinephrine was maintained at the same level (~50%), for the segments as for the rings, compared with noncryopreserved control samples. Moreover, relaxation in response to the antagonists calcium ionophore and sodium nitroprusside was maintained at between 75% and 100% of
Figure 5.
Functional recovery of artery segments and small artery rings after either the high or low cooling rate protocol (see Table 1). Function was assessed as either contractile or relaxation responses to pharmacological agonists (norepinephrine [N], phenylepinephrine [P]) or antagonists (calcium ionophore [Ca-I], sodium nitroprusside [SNP]), respectively, and normalized to the responses of control, untreated, tissue samples; controls were obtained from the same artery in each experiment. Data is expressed as the mean ± SEM.
The cooling and heating protocols used in the current experiments on tissue models were devised in a pervious cryomacroscopy study in the absence of tissue samples. The previous study focused on the minimum cooling rates necessary to attain vitrification and the minimum heating rates to avoid devitrification and/or recrystallization for the baseline VS55 medium.21,22 These predetermined conditions were emulated in the current study, which now incorporated biological tissue for evaluation of the effects of a lower cooling rate on the functional capabilities of the carotid vessel segments, coupled with observations of physical events in the samples by means of cryomacroscopy. Experimental data verifies that the level of contractile and relaxation function was maintained when samples were either cooled at a lower rate or the tissue sample size was increased from 3-mm rings to 2.5-cm segments. Furthermore, metabolic activity, as measured by the alamar Blue indicator (Fig. 6), was reduced on average with the decreasing cooling rate but was unaffected by increasing the size of the blood vessel segments. On the contrary, the 2.5-cm segments showed a higher mean value of functional recovery, although this was not statistically significant compared with the ring samples. We have no explanation for this observation and, because this was not statistically significant, it is unlikely that this is a real effect.
Figure 6.
Viability of artery rings and segments after vitrification, using either the high cooling rate (top), or low cooling rate protocol (bottom). Viability was determined using the alamarBlue metabolic indicator. Data was normalized with respect to fresh control tissue samples, obtained from the same artery as the vitrified samples. Data is expressed as the mean ± SEM.
Cryomacroscope images recorded during these experiments have been analyzed, but a detailed description of the observations is beyond the scope of this communication and will be reported separately. Nevertheless, we can report that no evidence of macroscopic crystallization or fractures was observed during cooling at high or low rates of any of the samples containing rings or segments. The cryomacroscope again proved to be a useful tool to verify the absence of ice crystallization and/or fractures that would compromise the function of the artery rings and segments demonstrated in this study.
In conclusion, we have verified that the rate of cooling and rewarming can be reduced from our baseline vitrification technique such that the function of larger tissue samples (segments) is not significantly different from that of smaller blood vessel rings. This represents a step toward improving recent developments in the vitrification of tissues to larger samples than the small model systems typically used in the development of these techniques.1,4,24 On going experimental efforts focus on increasing the surrounding cryoprotectant volume, as well as the tissue size, in an attempt to achieve successful vitrification of clinically determined dimensions, without danger of compromising structure and function due to thermal stress. We anticipate that this will be achieved by further systematic studies of the optimal thermal protocol for the specific formulation of the vitrification medium. Results of only one cryoprotectant are presented in this study, while other cryoprotectant formulations are being explored. Integration of the data presented here into mathematical modeling of vitrification and fracture formation is work in progress by the current research team. This study represents another step toward our long term goal of achieving vitreous cryopreservation of large tissues, while avoiding the destructive effect of thermal stress.
Acknowledgments
This study was supported by NIH/NHLBI, grant number R01HL06994401A1, 02, 03, 04.
References
- 1.Song YC, Khirabadi BS, Lightfoot FG, Brockbank KGM, Taylor MJ. Vitreous cryopreservation maintains the function of vascular grafts. Nature Biotechnol. 2000;18:296–299. doi: 10.1038/73737. [DOI] [PubMed] [Google Scholar]
- 2.Song YC, Lightfoot FG, Chen Z, Taylor MJ, Brockbank KGM. Vitreous Preservation of Rabbit Articular Cartilage. Cell Preserv Technol. 2004;2:67–74. [Google Scholar]
- 3.Song YC, An YH, Kang QK, Li C, Boggs JM, Chen Z, Taylor MJ, Brockbank KGM. Vitreous preservation of articular cartilage grafts. J Invest Surg. 2004;17:1–6. doi: 10.1080/08941930490422438. [DOI] [PubMed] [Google Scholar]
- 4.Taylor MJ, Song YC, Brockbank KGM. Vitrification in tissue preservation: new developments. In: Benson E, Fuller BJ, Lane N, editors. Life in the Frozen State. London: CRC Press; 2004. pp. 603–641. [Google Scholar]
- 5.Rabin Y, Steif PS. Solid mechanics aspects of cryobiology. In: Baust JG, Baust JM, editors. Advances in Bio-preservation. Boca Raton: CRC Press; 2006. pp. 353–375. [Google Scholar]
- 6.Karlsson JOM, Cravalho EG, Toner M. Intracellular ice formation: causes and consequences. Cryo-Letters. 1993;14:323–334. [Google Scholar]
- 7.Brockbank KG, Taylor MJ. Tissue preservation. In: Baust JG, Baust JM, editors. Advances in Biopreservation. Boca Raton: CRC Press; 2006. pp. 157–196. [Google Scholar]
- 8.Adam MJ, Hu F, Lange P, Wolfinbarger L. The effect of liquid nitrogen submersion on cryopreserved human heart valves. Cryobiology. 1990;27:605–614. doi: 10.1016/s0011-2240(05)80028-8. [DOI] [PubMed] [Google Scholar]
- 9.Wolfinbarger L, Jr, Adam M, Lange P, HU JF. Microfractures in cryopreserved heart valves: valve submersion in liquid nitrogen revisited. Applic Cryogenic Technol. 1991;10:227–233. [Google Scholar]
- 10.Kroener C, Luyet B. Discontinuous change in expansion coefficient at the glass transition temperature in aqueous solutions of glycerol. Biodynamica. 1966;10:41–45. [PubMed] [Google Scholar]
- 11.Kroener C, Luyet B. Formation of cracks during the vitrification of glycerol solutions and disappearance of the cracks during rewarming. Biodynamica. 1966;10:47–52. [PubMed] [Google Scholar]
- 12.Rubinsky B, Lee C, Bastacky J, Onik G. The process of freezing in the liver and the mechanisms of damage. Proceedings, CRYO 87-24th Annual Meeting; 1987. [DOI] [PubMed] [Google Scholar]
- 13.Rabin Y, Taylor MJ, Wolmark N. Thermal expansion measurements of frozen biological tissues at cryogenic temperatures. J Biomechan Eng. 1998;120:259–266. doi: 10.1115/1.2798310. [DOI] [PubMed] [Google Scholar]
- 14.Rabin Y, Bell E. Thermal expansion measurements of cryoprotective agents. Part II: measurements of DP6 and VS55, and comparison with DMSO. Cryobiology. 2003;46:264–270. doi: 10.1016/s0011-2240(03)00041-5. [DOI] [PubMed] [Google Scholar]
- 15.Plitz J, Rabin Y, Walsh JR. The effect of thermal expansion of ingredients on the cocktails VS55 and DP6. Cell Preserv Technol. 2004;2:215–226. [Google Scholar]
- 16.Rabin Y, Plitz J. Thermal expansion of blood vessels and muscle specimens permeated with DMSO, DP6, and VS55 at cryogenic temperatures. Ann Biomed Eng. 2005;33:1213–1228. doi: 10.1007/s10439-005-5364-0. [DOI] [PubMed] [Google Scholar]
- 17.Rios JL, Rabin Y. Thermal expansion of blood vessels in low cryogenic temperatures, Part II: Vitrification with VS55, DP6, and 7.05 M DMSO. Cryobiology. 2006;52:284–294. doi: 10.1016/j.cryobiol.2005.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rabin Y, Steif PS. Thermal stresses in a freezing sphere and its application to cryobiology. ASME J Appl Mechan. 1998;65:328–333. [Google Scholar]
- 19.Rabin Y, Steif PS. Thermal stress modeling in cryosurgery. Int J Solids Struct. 2000;37:2363–2375. [Google Scholar]
- 20.Rabin Y, Steif PS, Hess KC, Jimenez-Rios JL, Palastro MC. Fracture formation in vitrified thin films of cryoprotectants. Cryobiology. 2006;53:75–95. doi: 10.1016/j.cryobiol.2006.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Steif PS, Palastro M, Wan CR, Baicu S, Taylor MJ, Rabin Y. Cryomacroscopy of vitrification, Part II: Experimental observations and analysis of fracture formation in vitrified VS55 and DP6. Cell Preserv Technol. 2005;3:184–200. doi: 10.1089/cpt.2005.3.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rabin Y, Taylor MJ, Walsh JR, Baicu S, Steif PS. Cryomacroscopy of vitrification, Part I: A prototype and experimental observations on the cocktails VS55 and DP6. Cell Preserv Technol. 2005;3:169–183. doi: 10.1089/cpt.2005.3.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Taylor MJ, Song YC, Chen ZZ, Lee FS, Brockbank KG. Interactive determinants for optimized stabilization of autologous vascular grafts during surgery. Cell Preserv Technol. 2004;2:198–208. [Google Scholar]
- 24.Taylor MJ, Song YC, Khirabadi BS, Lightfoot FG, Brockbank KGM. Vitrification fulfills its promise as an approach to reducing freeze-induced injury in a multicellular tissue. Advan Heat Mass Transfer Biotechnol. 1999;44:93–102. [Google Scholar]
- 25.Song YC, Hagen PO, Lightfoot FG, Taylor MJ, Smith AC, Brockbank KGM. In vivo evaluation of the effects of a new ice-free cryopreservation process on autologous vascular grafts. J Invest Surg. 2000;13:279–288. doi: 10.1080/08941930050206300. [DOI] [PubMed] [Google Scholar]