Abstract
Both the thalamocortical and limbic systems generate a variety of brain state-dependent rhythms but the relationship between the oscillatory families is not well understood. Transfer of information across structures can be controlled by the offset oscillations. We suggest that slow oscillation of the neocortex, which was discovered by Mircea Steriade, temporally coordinates the self-organized oscillations in the neocortex, entorhinal cortex, subiculum and hippocampus. Transient coupling between rhythms can guide bidirectional information transfer among these structures and might serve to consolidate memory traces.
Keywords: Gamma oscillations, ripples, membrane potential, memory, current-source density, entorhinal cortex, subiculum
INTRODUCTION
Understanding how information is processed in the brain is a fundamental problem in neuroscience. We hypothesize that information is represented in the brain as a non-random, spatio–temporal pattern of firing in groups of neurons, also referred to as cell assemblies. In the simplest case, transfer of information involves two structures, or systems, which we refer to as ‘source’ (sender) and ‘target’ (receiver). To understand how information is transferred from source to target, one needs to know how the pattern of information is represented in the source, how this pattern is transferred to the target and, finally, how it is re-represented and stored in the target structure. The information-transfer process in the brain is usually considered unidirectional and passive: the source sends the information to an ever-ready recipient. Here, we suggest a different concept, which we call reciprocal information transfer. This reciprocal process implies that a target structure takes an active role in coordinating the time window within which it is most receptive by initiating the readout of the information from the source structure. Mechanisms of reciprocal information transfer and subsequent storage in the brain must match the following requirements: activation of ‘source’ and ‘recipient’ neuronal assemblies must be correlated temporally (‘source’ before ‘receiver’); and there must be sufficiently strong postsynaptic depolarization of the ‘receiver’ neurons. Oscillations provide the most efficient mechanism to match these requirements because they are apt to create transient periods of synchrony (Buzsáki and Draguhn, 2004; Buzsáki, 2006). Most neural oscillators have features of relaxation (nonharmonic) oscillators and exhibit distinct phases of input (when they can be easily perturbed, reset, advanced or delayed) and output (when they are refractory). In phase-coupled relaxation oscillator systems synchronization is fast, precise and persistent (Somers and Kopell, 1993). Mainly because of these features, coupled network oscillators comprise a natural substrate for information transfer. Synchronous activation of neurons on the fast time scale in local networks facilitates the formation of cell assemblies (Harris et al., 2003; Singer, 1993) and synaptic plasticity (Bliss and Lømo, 1973; Levy and Steward, 1983; Markram et al., 1997), whereas the timing of local events across structures can be coordinated by slower time scale oscillations. In the discussion below, we illustrate these principles by the interaction of thalamocortical and hippocampal oscillators.
Oscillations in the thalamocortical and hippocampal networks
Both thalamocortical and hippocampal systems have large numbers of oscillators. This brief review of selected rhythms is confined to a few of these patterns.
SLOW (<1 HZ) RHYTHMS
Mircea Steriade and his collaborators described a novel, cortical, oscillatory pattern in a series of papers in 1993 (Steriade et al., 1993e; Steriade et al., 1993a; Steriade et al., 1993f; Steriade et al., 1993b), which formed the cornerstone of Steriade's research to the end of his life. They referred to the newly discovered rhythm as ‘slow’ oscillation because of its frequency between 0.5 and 1.5 Hz. Slow oscillation was soon shown also in the human EEG during sleep (Achermann and Borbely, 1997; Amzica and Steriade, 1997; Molle et al., 2002), indicating its crucial importance for neocortical function. An important aspect of slow oscillation is its ability to temporally organize other cortical patterns, such as sleep spindles, gamma oscillations and K complexes (Achermann and Borbely, 1997; Amzica and Steriade, 1997; Molle et al., 2002; Steriade and Amzica, 1998a; Mukovski et al., 2006) and hippocampal sharp wave ripples (Sirota et al., 2003).
Slow oscillations are reflected in fluctuations in the membrane potential of neocortical neurons at the single-cell level. The membrane potential of virtually all neocortical neurons undergoes relatively abrupt transitions from a hyperpolarized DOWN state to a substantially more depolarized UP state (∼10–20mV difference) (Metherate and Ashe, 1993; Steriade et al., 1993e; Steriade et al., 1993f; Steriade et al., 1993b; Cowan and Wilson, 1994; Amzica and Steriade, 1995; Destexhe et al., 1999; Volgushev et al., 2006). The DOWN state is associated with a positive polarity local field potential (LFP) in infragranular layers. Rhythmical shifts between DOWN and UP states have also been described in striatal (Wilson et al., 1990; Stern et al., 1997) and thalamic (Steriade et al., 1993b; Timofeev and Steriade, 1996; Hughes et al., 2002) neurons. Similar dynamics have been described in vitro in the presence of near normal Ca2+/high K+/low Mg2+, either spontaneously or in response to electrical stimulation of the cortex (Shu et al., 2003; Steriade et al., 1993b; Sanchez-Vives and McCormick, 2000), and during application of either mGluR agonists or muscarinic agonists (Beierlein et al., 2000). It has been suggested that both intrinsic cellular and network mechanisms are crucial for the slow oscillations (Wilson et al., 1990; Stern et al., 1997; Shu et al., 2003; Sanchez-Vives and McCormick, 2000) but the necessary and sufficient conditions that determine its frequency (the duration of UP and DOWN states) are still debated. The UP state is accompanied by the synchronous recruitment of numerous cells in the network and changes in input resistance (Steriade et al., 2001; Shu et al., 2003; Waters and Helmchen, 2006). Extensive recurrent collaterals and balanced excitation and inhibition have been hypothesized to be the major requirements for the persistence of the UP state (Shu et al., 2003; Steriade et al., 1993e). Several factors can contribute to the transition from the UP to the DOWN state, including a gradual decrease of extracellular Ca2+ and a corresponding decrease in synaptic transmission (Massimini and Amzica, 2001), inactivation of Ih channels (Luthi and McCormick, 1998), and metabolic processes in the neurons (Cunningham et al., 2006).
Ca2+ imaging in vitro reveals somewhat similar, although irregular, dynamics in cortical networks, which also fluctuate between brief UP states of synchronous activation of spatially organized ensembles of neurons (attractors), and prolonged DOWN states. This dynamic, which has similar characteristics to the slow oscillation discussed above, depends on synaptic activity and intrinsic currents, but does not have a clear periodicity and is limited to small ensembles of cells (Mao et al., 2001; Cossart et al., 2003). Fluctuation of finite network patterns, similar to the spontaneous events, is also evoked by sensory stimulation in deeply anesthetized animals (Lampl et al., 1999; Tsodyks et al., 1999; Arieli et al., 1996; Anderson et al., 2000; Leopold et al., 2003; Petersen et al., 2003). Thus, the population dynamics of cortical networks might display a random walk between synchronous UP states in subpopulations of neurons, which seem to reflect behaviorally activated states and silence (Kenet et al., 2003). This mechanism might serve to persistently and sequentially activate different cell assemblies (attractors) that might be organized further into finer spatio–temporal dynamics by faster rhythms.
DELTA WAVES
The term ‘slow wave sleep’ is derived from the presence of ubiquitous large amplitude patterns (delta waves) in the cortical LFP and scalp EEG during non-REM sleep, immobility and deep anesthesia induced by various drugs (Gloor et al., 1977; Buzsáki et al., 1988; Steriade et al., 1993d). Steriade's discovery of slow oscillation allowed a redefinition of delta waves and delta oscillations. Neocortical delta waves represent transient (200–500 msec) cessation of synaptic and spiking activity of both principal cells and interneurons in all cortical layers, followed by episodes (0.3–1 sec) of sustained activity (Steriade and Buzsáki, 1990). The widespread cortical silence is associated with a positive wave in the infragranular layers and corresponding negative wave in supragranular recordings. Thus, the criteria of delta waves are identical with the DOWN state of the slow oscillation. From this perspective, therefore, the slow oscillations and delta waves are not separate patterns but the delta wave is DOWN part of the slow oscillation.
Although slow oscillations (and their delta wave component) arise from cortical networks, in the intact brain the thalamus also plays an important role. Oscillations in the delta frequency band (1–4 Hz) have been described in isolated thalamic slices, and the pattern depends on the interaction between IT and Ih currents, activated at hyperpolarized membrane potentials (McCormick and Pape, 1990; Soltesz et al., 1991; Steriade et al., 1991a; von Krosigk et al., 1993). The rhythmic thalamic output can advance the phase of the intrinsic neocortical slow oscillation, resulting in a rhythmic series of delta waves (DOWN states) at delta frequency during stage 4 of slow wave sleep (Kandel and Buzsáki, 1997; Amzica and Steriade, 1998; Steriade and Amzica, 1998b).
K-COMPLEXES AND SLEEP SPINDLES
Irregular, relatively sharp potentials occur sporadically in the scalp EEG during non-REM sleep (Loomis et al., 1938; Roth et al., 1956) and under anesthesia (Amzica and Steriade, 1997; Steriade and Amzica, 1998b). This pattern, termed K-complex (Loomis et al., 1938), can arise either spontaneously or be evoked by stimuli of all modalities, including slight positional changes in bed (Halasz et al., 1985; Halasz et al., 2004). Again, in light of the mechanisms of slow oscillations the origin of the K-complex can be redefined. Amzica and Steriade (Amzica and Steriade, 1997; Steriade and Amzica, 1998b) observed that the DOWN–UP transition of the slow oscillation cycle often takes the form of a K complex.
In view of these observations, the K complex is not a separate entity but corresponds to events when the DOWN–UP transition in many cortical neurons is particularly fast and synchronous, as reflected by the LFP. Because sensory stimuli can trigger UP states (Petersen et al., 2003), this might explain why at least some K complexes are associated with sensory inputs in sleeping subjects (Halasz et al., 2004).
Originally, the K complex was defined as a sharp potential followed by spindle activity (Loomis et al., 1938). Although K complexes and sleep spindles can occur in isolation, slow oscillations can organize these patterns in defined temporal sequences. The magnitude of DOWN–UP synchrony and associated discharges of neocortical neurons vary substantially. The more synchronous the DOWN–UP transition, the more its LFP correlate resembles a K complex. Synchronous discharges of neocortical neurons, in turn, can trigger a thalamocortical spindle. However, in the absence of a strong neocortical volley underlying the K complex, spindles can be still generated because the necessary and sufficient substrate for spindles resides in the thalamus (Mulle et al., 1985; Buzsáki, 1991; von Krosigk et al., 1993). Nevertheless, when a cortical volley arrives to the thalamus at the time a spontaneous spindle is about to occur (i.e. in the recipient phase of the rhythm that underlies spindle event repetitions), it can advance its occurrence.
Many reviews have discussed the mechanisms and functional significance of sleep spindles (McCormick and Bal, 1997; Destexhe and Sejnowski, 2001; Steriade et al., 2001). Briefly, the key EEG pattern of superficial sleep is a waxing–waning oscillatory pattern in the 12–18 Hz frequency range, known as the sleep spindle (Bremer, 1935; Steriade et al., 1993d). The frequency of sleep spindles is set by the cellular properties of reticular nucleus (RE) and the interplay between inhibitory and excitatory volleys of GABAergic neurons in the RE and glutamate-releasing thalamo–cortical cells (Buzsáki, 1991) as shown in vivo (Steriade et al., 1985; Steriade et al., 1987) and in vitro (von Krosigk et al., 1993). Importantly, the intrinsic properties of RE neurons, the interaction between IT and IKCa (Avanzini et al., 1989; Bal and McCormick, 1993), conspire to produce spindle frequency bursts when isolated from thalamic relay cells and the neocortex (Steriade et al., 1987; Bal and McCormick, 1993). If the reticular thalamic nucleus is either damaged or disconnected from the neocortex, sleep spindles are no longer observed either in the thalamus or in the neocortex (Steriade et al., 1985; Steriade et al., 1987; Buzsáki et al., 1988). Rhythmic bursts produced by RE neurons result in large IPSPs in thalamo–cortical cells, sufficient to generate rebound low-threshold Ca2+ bursts, which, in turn, produce long-lasting EPSPs in thalamic reticular cells and trigger the next oscillatory cycle (Deschenes et al., 1984; Buzsáki, 1991; Bal and McCormick, 1993; Huguenard and Prince, 1994; von Krosigk et al., 1993).
Although the thalamic circuit is sufficient to generate spindles (Morison and Bassett, 1945; Deschenes et al., 1984; Contreras et al., 1996; Timofeev and Steriade, 1996), in the intact brain the cortico–thalamic feedback has an important coordinating role. The thalamus is organized locally and does not have the means to produce global synchrony (McCormick and Bal, 1997). The emerging thalamic spindles are transferred to the neocortex via topographic thalamo–cortical projections, and the spatially contiguous or distinct thalamic oscillations are synchronized by cortico–thalamic feedback (Kim et al., 1995; Contreras et al., 1996). Thalamo–cortical spindles represent the first organized pattern in the neocortex in newborn rats and pre-term human babies (Khazipov et al., 2004; Hanganu et al., 2006; Milh et al., 2006), although these early spindles are confined spatially. After the establishment of long-range cortico–cortical connections, spindles typically occur virtually synchronously across wide areas of the thalamus and cortex (Verzeano and Negishi, 1960; Contreras et al., 1996; Contreras and Steriade, 1996; Bal et al., 2000). Therefore, we conclude that the cortical synchronizer of sleep spindles during natural slow wave sleep is the slow (<1 Hz) oscillation whose global synchrony depends on long-range cortical connections (Steriade et al., 1993b; Timofeev et al., 2000).
HIPPOCAMPAL THETA RHYTHMS
The most prominent oscillation in the mammalian brain is the hippocampal theta rhythm (6–12 Hz) (Green and Arduni, 1954) that is present mainly during locomotion and other ‘voluntary’ movements (Vanderwolf, 1969) and REM sleep (Jouvet, 1969). A crucial structure involved in theta oscillations is the medial septum–diagonal band of Broca (MSDB), because its lesion or disconnection from the hippocampus abolishes theta (Green and Arduni, 1954; Petsche et al., 1962). GABAergic and cholinergic neurons of the MSDB contribute to the theta rhythm by feed-forward disinhibition of CA1 pyramidal cells via the interneurons, and cholinergic activation of an intrahippocampal CA3 theta oscillator (Buzsáki, 2002). As is the case with thalamo-cortical spindles in which corticothalamic feedback serves as a synchronizer, hippocampal feedback to the medial septum is crucial for producing widespread synchrony (King et al., 1998). However, the hippocampo–septal feedback path is established by long-range GABAergic interneurons (Sik et al., 1994; Dragoi et al., 1999; Gulyas et al., 2003). The septal and intrahippocampal pathways produce a current source in the CA1 pyramidal layer and a sink in the stratum radiatum of CA1. Indeed, these current generators are abolished by atropine (acetylcholine antagonist), but remain intact after lesions of the entorhinal cortex (Buzsáki et al., 1983; Vanderwolf and Leung, 1983; Ylinen et al., 1995b). In addition to the MSDB, the hippocampus receives rhythmic subcortical modulatory inputs from several sources (Kocsis and Vertes, 1994; Vertes and Kocsis, 1997).
A second, extrahippocampal, theta oscillator is located in the entorhinal cortex. Perforant path input to distal dendrites of the CA1 and CA3 pyramidal cells and dentate granule cells is believed to produce current dipoles that are responsible for the large amplitude of theta at around the hippocampal fissure (Buzsáki et al., 1983). This latter oscillator is atropine-resistant, but is abolished by urethane anesthesia and lesions of the entorhinal cortex and originates mainly in layers 2/3 of the entorhinal cortex (Vanderwolf and Leung, 1983; Ylinen et al., 1995b; Kamondi et al., 1998). In support of this hypothesis, many layer 2/3 cells of the entorhinal cortex are phase-locked to the hippocampal theta (Alonso and Garcia-Austt, 1987b; Chrobak and Buzsáki, 1998b; Alonso and Llinás, 1989), and the theta waveshape reverses around layer 2 of the entorhinal cortex (Alonso and Garcia-Austt, 1987a; Mitchell and Ranck, 1980). The entorhinal and intrahippocampal CA3 theta oscillators vary their frequency and phase relatively independently (Kocsis et al., 1999). Pyramidal neurons do not have a fixed phase relationship with the ongoing theta waves but display systematic phase shifts with behavior. The timing of spikes within the theta cycle (i.e. their phase) is determined by the dynamic interaction between intra- and extra-hippocampal networks so that the phase of spikes correlates with the spatial position of the animal (O'Keefe and Recce, 1993; Skaggs et al., 1996; Harris et al., 2002; Mehta et al., 2002; Dragoi and Buzsáki, 2006).
The precise timing of principal cell spikes is assisted by a consortium of interneurons. Each class of interneuron has its own preferred phase of discharge (Klausberger et al., 2003; Klausberger et al., 2004). In addition to the hippocampus and entorhinal cortex, theta-modulated neurons have been identified in all limbic structures, including the perirhinal cortex (Muir and Bilkey, 1998), cingulate cortex (Leung and Borst, 1987; Colom et al., 1988), prefrontal cortex (Siapas et al., 2005), amygdala (Pare and Gaudreau, 1996; Collins et al., 1999; Pare and Collins, 2000), anterior thalamus (Vertes et al., 2001), mammillary bodies and the supramammillary nucleus (Kocsis and Vertes, 1994), and the subiculum (Bullock et al., 1990; Anderson and O'Mara, 2003). Some of these networks also generate their own theta fields. However, to what extent hippocampal theta affects the neocortex and whether neocortical structures can generate their own local theta resonance and oscillation is an open question (Kahana et al., 2001).
GAMMA OSCILLATIONS
Several types of cortical (Llinás et al., 1991; Nuñez et al., 1992) and thalamic neurons (Steriade et al., 1991b; Steriade et al., 1993c) display either fast subthreshold resonance or act as bone fide high frequency pacemakers (Gray and McCormick, 1996). Mutually coupled networks of interneurons are sufficient to produce gamma oscillations under certain circumstances (30–90 Hz) in cortical circuits (Jefferys et al., 1996; Steriade et al., 1996; Wang and Buzsáki, 1996; Traub et al., 1999; Bartos et al., 2002). Gamma oscillations are a characteristic feature of network activity in ‘desynchronized’ cortical areas involved in processing of incoming sensory information in the awake state or during REM sleep (Singer and Gray, 1995; Maloney et al., 1997). Gamma oscillations have also been observed in vivo in the hippocampus and the entorhinal cortex of rats (Stumpf, 1965; Buzsáki et al., 1983; Chrobak and Buzsáki, 1998a; Bragin et al., 1995a), the amygdala, the entorhinal and perirhinal cortices and the neocortex of cats (Boeijinga and Lopes da Silva, 1988; Gray et al., 1989; Engel et al., 1991; Collins et al., 2001), the neocortex of the monkeys (Kreiter and Singer, 1996) and in the human neocortex (Llinás and Ribary, 1993; Uchida et al., 2001). Gamma oscillations are also induced by various pharmacological manipulations in vitro (Whittington et al., 1996; Buhl et al., 1998; Fisahn et al., 1998). In the hippocampus, at least two distinct gamma generators have been identified: one depends on the perforant path input from the entorhinal cortex, whereas the other emerges in the CA3 region (Bragin et al., 1995a; Csicsvari et al., 2003). Cortical neurons are phase-locked to the local gamma oscillation (Gray et al., 1989; Murthy and Fetz, 1992; Steriade et al., 1996; Csicsvari et al., 2003), which allows them to synchronize within a narrow temporal window. This phase-locking is brought about by volleys of inhibitory currents produced by local interneurons (Buzsáki et al., 1983; Csicsvari et al., 2003; Bragin et al., 1995a; Hasenstaub et al., 2005).
In the hippocampus and the entorhinal cortex, the gamma power is modulated by theta oscillations (Bragin et al., 1995a; Chrobak and Buzsáki, 1998a). Several reviews summarize the mechanisms and functional significance of gamma oscillations (Singer and Gray, 1995; Traub et al., 1998; Engel et al., 2001).
FAST CORTICAL OSCILLATIONS
During slow wave sleep, immobility and consummatory behaviors, the hippocampus produces synchronous bursts termed sharp waves (SPWs) (Buzsáki et al., 1983; Buzsáki, 1986). SPWs are large amplitude field potentials that occur irregularly in the CA1 stratum radiatum as a result of a strong depolarization by the CA3 Schaffer collaterals, caused by the synchronous bursting of CA3 pyramidal cells. SPWs are associated with fast-field oscillations (∼140–200 Hz), or ‘ripples’, that are confined to the CA1 pyramidal cell layer (O'Keefe and Nadel, 1978; Buzsáki et al., 1992). The importance of SPWs derives from the observation that during its time window, 50 000–100 000 neurons discharge synchronously in the CA3–CA1–subicular complex–entorhinal complex (Chrobak and Buzsáki, 1996; Csicsvari et al., 1999; Csicsvari et al., 2000). Neuronal participation in the population discharge is not random; instead, each SPW event represents an exhaustive ‘search’ in the autoassociative CA3 recurrent network (Ylinen et al., 1995a; Csicsvari et al., 2000). Importantly, the ‘content’ of the SPW events is determined by previous experience of the animal (Buzsáki, 1989; Skaggs and McNaughton, 1996; Wilson and McNaughton, 1994; Kudrimoti et al., 1999; Nadasdy et al., 1999; Lee and Wilson, 2002). Because of its behavior-relevant content and because of the 3–5-fold gain of network excitability during the SPW (Csicsvari et al., 1999), this endogeneous hippocampal-output pattern, which is active during ‘off-line’ states of the hippocampus, might have a crucial role in transferring transient memories from the hippocampus to the neocortex for permanent storage (Buzsáki, 1998; Buzsáki, 1989; Buzsáki and Chrobak, 1995; Chrobak and Buzsáki, 1998b; Siapas and Wilson, 1998; Lorincz and Buzsáki, 2000).
The mechanisms of SPWs and ripple generation are not fully understood. Lesions of the entorhinal cortex or medial septum do not abolish SPWs (Buzsáki et al., 1983), in fact, the incidence of SPWs increases after severing the entorhinal inputs. This might be caused by the removal of tonic and phasic feed-forward activation of local inhibitory neurons by the mossy fibers of granule cells (Bragin et al., 1995b; Penttonen et al., 1997; Acsady et al., 1998). The physiological role and mechanisms of the fast oscillatory ripple that accompanies SPW events (Ylinen et al., 1995a; Csicsvari et al., 2000) are not well understood. Nevertheless, its importance is illustrated by the observation that the temporal structure of ripples changes in disease, and the emergence of superfast ripples are pathognostic in the diagnosis of temporal lobe epilepsy in humans (Bragin et al., 1999; Staba et al., 2004). High-frequency ripples are also present in the neocortex where they occur either spontaneously or in response to sensory stimulation (Kandel and Buzsáki, 1997; Grenier et al., 2001; Jones and Barth, 2002).
Slow oscillations, delta waves, K complexes, sleep spindles, theta waves, gamma oscillations and fast, transient (ripple) oscillations are present in all mammalian species and are associated with similar behaviors. Network oscillators of each kind have remarkably similar temporal frequencies and duration, although the presence of hippocampal theta oscillations in higher mammals is debated (Kahana et al., 2001), despite several orders of magnitude difference in brain size (Steriade, 2001; Buzsáki, 2006).
Information transfer by oscillatory coupling
Network oscillations and the neuronal synchrony they create offer a plausible mechanism for the storage, readout and transfer of information between different structures. Oscillations impose a spatio–temporal structure on neural ensemble activity within and across different brain areas. In general, synchronous activation of neurons by local, fast oscillations can facilitate activation of cell assemblies and synaptic plasticity, whereas the timing of local events can be coordinated by the slower oscillations. The key factors in this process are the limited conduction velocities of axons and spike transmission delays, (i.e. the time required from the arrival of a presynaptic volley to the discharge of the postsynaptic cell). Recruitment of neurons is usually limited to a single cycle because the spread of activity is either terminated or constrained by the build-up of inhibition. Therefore, during one cycle of fast oscillations only a small volume of neurons is recruited. By contrast, slow oscillations allow for the recruitment of neurons in a large neuronal space and, therefore, temporal organization of events across structures. Because slower oscillations affect larger neuronal populations, they often modulate the power of faster oscillatory events. Simply by changing the phase offset, the phase modulation mechanism can reverse instantaneously the direction of information transfer (Bragin et al., 1995a; Steriade et al., 1996; Chrobak and Buzsáki, 1998b; Steriade and Amzica, 1998a; Molle et al., 2002; Sirota et al., 2003; Buzsáki and Draguhn, 2004; Siapas et al., 2005; Buzsáki, 2006). In the remainder of the review, we illustrate how the slow oscillations described by Steriade interact with various other rhythms across brain structures.
Slow oscillations unify neocortical and paleocortical networks
Because the size of participating neuronal pool correlates with the duration of the oscillatory cycle, various brain rhythms can set differential functional boundaries between brain structures. In the waking, exploring animal, the prominent hippocampal theta oscillations functionally define the limbic system, including all those structures whose neurons are routinely phase-modulated by hippocampal theta rhythm (Buzsáki, 2002). As discussed above, hippocampal and paleocortical networks are engaged in coherent theta oscillations. This might not be surprising given the bidirectional connectivity between paleocortical and hippocampal (archicortical) structures (Witter, 1993; Suzuki and Amaral, 2004). How do these systems interact with each other in the absence of theta oscillations?
Changes in brain state are associated with different oscillatory regimes in all brain areas, so the state changes result in different dynamics of the activity across the brain (Fig. 1). As discussed earlier, deep, slow-wave sleep is characterized by widespread, synchronized, oscillatory patterns that are defined primarily by spatially coherent delta waves (Achermann and Borbely, 1997) during which spiking activity of nearly all principal cells and interneurons in the neocortex is suspended (Steriade and Buzsáki, 1990; Battaglia et al., 2004). Under anesthesia, similar but longer silent periods alternate regularly with active, spiking epochs (Steriade et al., 1993e; Steriade et al., 1993f; Steriade et al., 1993b; Amzica and Steriade, 1995; Destexhe et al., 1999). Steriade and collaborators have emphasized that the silent and active periods of slow oscillation involve brain areas several millimeters from each other (Steriade et al., 1993f; Amzica and Steriade, 1995; Achermann and Borbely, 1997; Battaglia et al., 2004) but the spatial extent of slow oscillations has not been determined. In fact, the front of neocortical DOWN–UP transition can form a traveling wave (Massimini et al., 2004) and less synchronous DOWN states can be relatively local (Fig. 2).
Fig. 1. Characterization of the slow oscillation state.
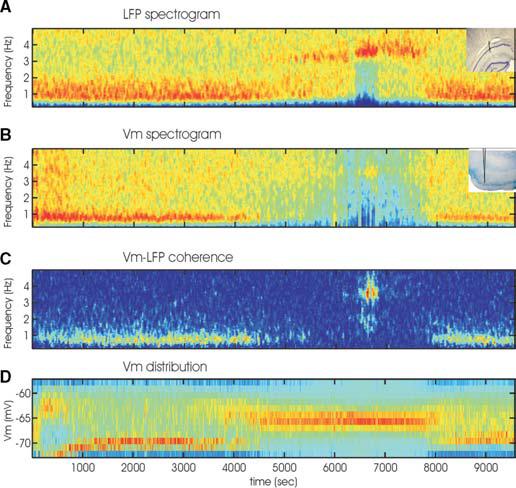
(A) Power spectrogram of LFP in CA1 pyramidal layer (inset shows electrode track) during >2.5 hours recording. Note a clear, isolated band of spontaneously occurring theta epoch at 3–4 Hz. (B) Power spectrogram of membrane potential fluctuation in a layer 3 entorhinal cortical neuron (inset). (C) Coherence between LFP and the intracellular potential. Note the high coherence values (yellow to red) at ∼0.7 Hz from 0 to 4000 sec changing gradually to coherence at theta frequency. (D) Distribution of membrane potential values as a function of time. Note the bimodal fluctuation of membrane potential during slow oscillation and the persistent depolarization during UP state, which is characterized by hippocampal theta activity. Only epochs with strong power of slow oscillations were used in subsequent analyses.
Fig. 2. Global and local neocortical DOWN states (delta waves) during SWS.
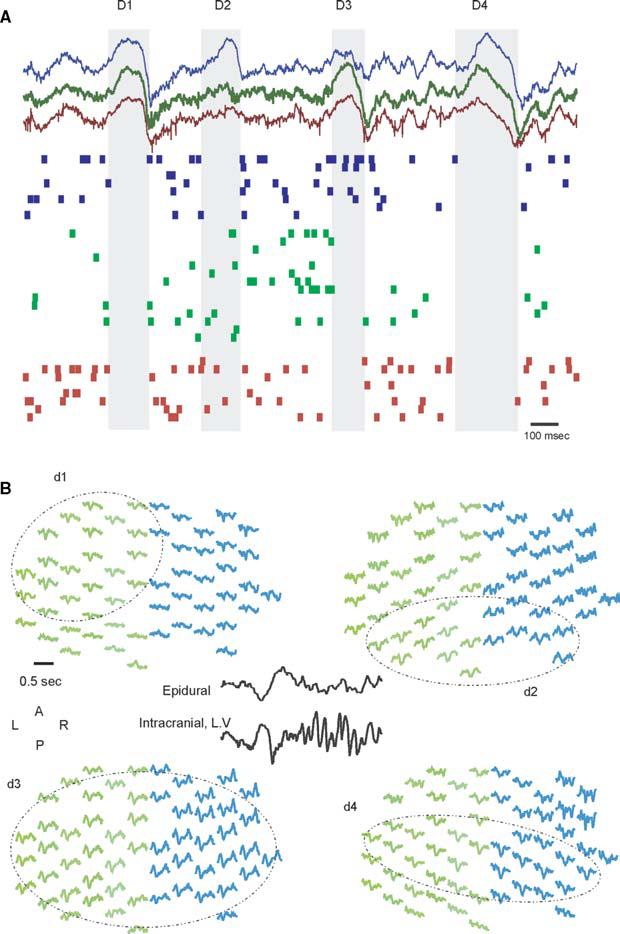
(A) Example of simultaneously recorded LFP and unit activity at three intracortical locations (∼1 mm spaced). Note that DOWN states (shaded area) can be synchronous and global (D1, D4) and local (D2, D3). (B) Example of bilateral, 64-channel, epidural EEG recordings in the rat during SWS. Negativity in the EEG is associated with deep delta wave (inset). Typically, delta waves are globally synchronized (d3), but can be localized to one side (d1), posterior (d2) or central (d4) regions.
Our investigations in rats confirm and extend Steriade's observations in the cat. Using simultaneous recordings of local field potentials and extracellular-unit activity in one cortical area and intracellular activity in another cortical area, we found that the extent of cortical silence is coordinated through virtually the entire neocortex and paleocortex (Isomura et al., 2006). DOWN states are recognized visually by the hyperpolarized membrane potential in intracellular recordings and by positive going local field potentials in deep layers associated with a reduction of fast activity and either the absence or extreme paucity of extracellularly recorded spikes. As Fig. 3 illustrates, UP–DOWN fluctuations of the membrane potential and associated rhythmic alternation of population discharge and silence occur coherently across the neocortex, entorhinal cortex and subiculum. The strong coherence between the intracellular and extracellular signals and the significant correlation between the power fluctuations of LFP and the intracellular signal are confined to the slow oscillation band. The mechanisms of such widespread synchrony are yet to be elucidated. The thalamus is unlikely to have a crucial role because it does not have the necessary anatomical networks to generate widespread synchrony (Contreras et al., 1996). A further ‘paradox’ is that the delay between activity in the deep and superficial layers at the same cortical site is often longer than the delay between the activity in deep layers at distant neocortical and paleocortical locations (Kandel and Buzsáki, 1997; Krupa et al., 2004). This is a paradox because distant intercortical communication arises from the axon collaterals of superficial neurons whereas axon collaterals of deep layer neurons are largely local with major subcortical projections (Szentagothai, 1978). One potential explanation is that slow oscillations emerge in the strongly local and recurrent circuits of layer 5 (Shu et al., 2003; Luczak et al., 2007). If oscillations in various neocortical and paleocortical modules emerge with a relatively similar frequency, then weak coupling through even sparse connections and occasional resetting of phase might be sufficient to maintain coherent local oscillations.
Fig. 3. Synchronization of the neocortex and paleocortex by the slow oscillation.
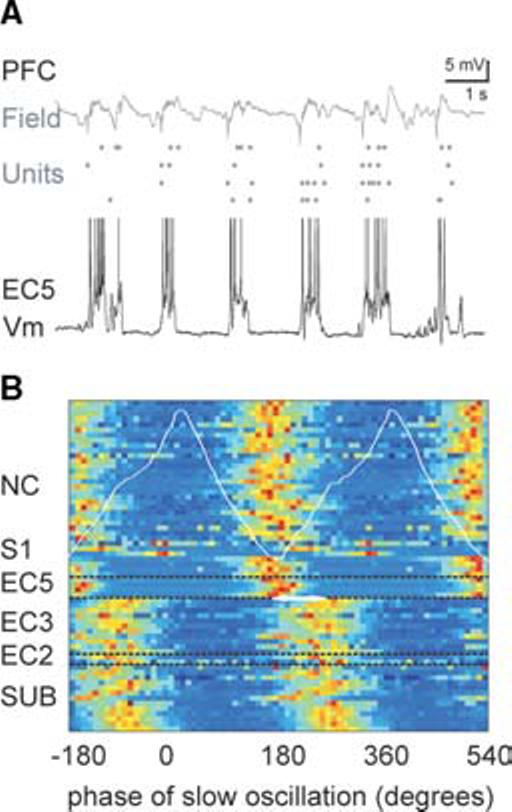
(A) Field potential recordings in superficial (s) and deep (d) layers of the prefrontal cortex (PFC) (dots represtent unit discharges) and intracellular activity in a layer 5 entorhinal cell (EC5). (b) Phase-locking of DOWN–UP transitions in different neurons to the neocortical slow oscillation. Each row represents a normalized slow oscillation phase histogram (color-coded for magnitude) of DOWN–UP transitions of membrane potential in neocortical (NC), EC5 and superficial (EC2 and EC3) cells, and subiculum (SUB). The reference LFP electrode was in CA1 pyramidal layer in each experiment. White trace, average LFP. Note the similar preferred phase of DOWN–UP transitions in neocortical and deep entorhinal neurons, and consistent phase shift (∼60°) in superficial cells and subiculum.
Hippocampal network patterns are affected by slow oscillations
The excitatory front of the UP states can spread to the hippocampus via the entorhinal cortex. This is evidenced by sinks in the dentate molecular layer and str. lacunosum-moleculare of the CA1 region, the main targets of the entorhinal cortex, that follow DOWN–UP transition in either the neocortex (Sirota et al., 2003; Wolansky et al., 2006) or the entorhinal cortex (Fig. 4a) (Isomura et al., 2006). Moreover, UP-state-associated sleep spindles are associated with the spindle-frequency currents in the CA1 str. lacunosum-moleculare and the dentate molecular layer (Sirota et al., 2003), the targets of the entorhinal inputs (Amaral and Witter, 1989). As expected, granule cells and other neurons of the dentate area fire coherently with the input from the entorhinal neurons during the slow oscillation (Fig. 4b). The engagement of the dentate circuit is demonstrated further by the power increase of the gamma oscillation during the UP phase of the slow oscillation (Fig. 5). By contrast, the phasic inputs in the distal dendrites of CA1 pyramidal cells are not reflected faithfully by the population output of this region. Although a large fraction of CA1 pyramidal neurons tends to discharge coherently with the cortical UP state, several individual neurons show no apparent phase relationship and others discharge preferentially during the cortical DOWN state.
Fig. 4. Neo-/paleocortical input-dependent activity of hippocampus during slow wave sleep.
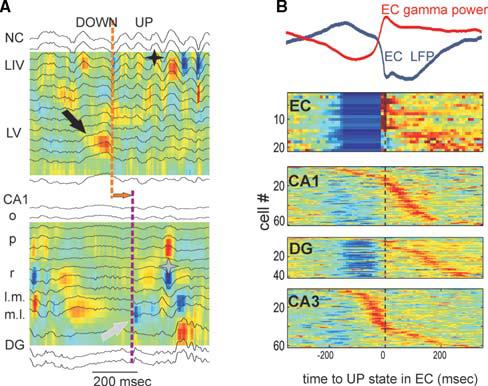
(A) Current-source density (CSD) and superimposed LFP traces of simultaneously recorded events in the neocortex (top) and the hippocampus (bottom). Delta wave (red source in layer 5, LV, marked by black arrow) is followed by a sleep spindle (black star). DOWN–UP state transition is indicated by orange dashed line. In the hippocampus, the entorhinal cortex-mediated DOWN–UP transition is reflected by a phase-reversal of LFP and a sink (blue) in the molecular layer (white arrow). Horizontal arrow, neocorticodentate time lag (∼100 msec). Gray star, sharp wave in CA1 striatum radiatum. (B) Activity in the entorhinal cortex and hippocampus relative to the onset of the UP state in the entorhinal cortex (time zero in all plots). From top to bottom: color-coded stacked cross-correlograms of EC neurons, average LFP (layer 5; blue) and gamma power (red) in EC, color-coded, stacked, cross-correlograms of DG, CA1 and CA3 neurons (recorded in separate sleep sessions). Each line is a single neuron.
Fig. 5. Slow oscillation phase- and layer-dependence of hippocampal gamma oscillation.
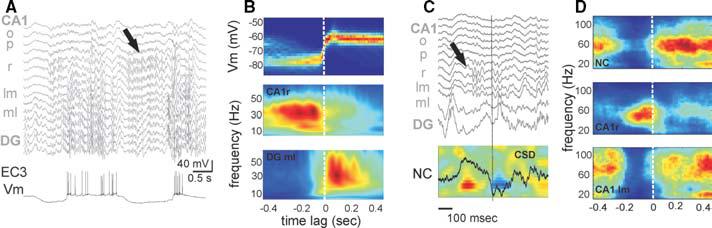
(A) Multisite local-field recording in the hippocampus and intracellular trace from an entorhinal layer 3 neuron (EC3) during anesthesia. (B) Distribution of the membrane (top) and average spectrogram of the local field potential in str. radiatum (middle) and molecular layer (bottom) triggered by DOWN–UP transitions (n = 2298 epochs). Note increased gamma power in str. radiatum during the DOWN state and in the molecular layer during the UP state. (C,D) Data from natural sleep. (c) Multisite LFP recording in the hippocampus (traces, top) and CSD map (bottom) from the neocortex (NC; bottom panel). LFP trace in NC is from layer 5. Note train of gamma oscillation in str. radiatum (black arrow) during the DOWN state of the neocortex (source, red). (d) Average spectrograms of LFP in NC, CA1 str. radiatum (CA1r) and lacunosummolecular e (CA1lm) triggered by the onset of activity following the delta wave (n = 98 events). Note similar patterns to (a and b).
The dentate circuit appears to act as a ‘barrier’ because it largely shields the effects of entorhinal inputs on neurons of the intrahippocampal regions. Intracellular and extracellular unit recordings reveal that substantial numbers of CA3 pyramidal cells fire preferentially when both entorhinal and dentate gyrus neurons are silent (DOWN state). At the network level this firing activity is expressed in the form of self-organized gamma oscillations as evidenced by gamma power increase in the CA1 str. radiatum, the major target of the CA3 output (Fig. 5). As a result, populations of CA1 neurons can be active together during the UP state, driven either directly by the layer 3 entorhinal input or by the dentate–CA3–CA1 trisynaptic pathway, whereas in the DOWN state the self-organized activity in the CA3 region is their main driving force. In contrast to neocortical and paleocortical areas, the membrane potential of most hippocampal cells has a unimodal, though skewed, membrane potential distribution. However, there is an apparent phase-specific modulation of the membrane potential of hippocampal cells by the neocortical slow oscillation, which is in register with unit and field observations (Isomura et al., 2006). The differential recruitment of different hippocampal regions by the slow oscillation is distinct from the neocortex. Quiescence and gamma frequency power in the neocortex is modulated by slow oscillations in all layers (Steriade et al., 1993e; Hasenstaub et al., 2005; Mukovski et al., 2007). If the dentate, CA3 and CA1 regions are conceived of as layers, our findings show that regions of the hippocampus can work either together as a feed-forward multi-layer system or separately, because of the gating property of the dentate and the ability of the CA3 recurrent system to produce self-organized patterns.
Hippocampal output is biased by neocortical inputs
In addition to gamma oscillations, the CA3–CA1 region also gives rise to the most synchronous hippocampal pattern, the SPW-ripple complex (Buzsáki, 1996; Chrobak and Buzsáki, 1996), but at a significantly reduced rate in anesthetized compared to drug-free rats (Ylinen et al., 1995a). The synchronous discharge of CA1 neurons and downstream subicular and entorhinal neurons provides the most effective output to the neocortex (Chrobak and Buzsáki, 1994). In addition to self-organized gamma oscillations, SPW ripples also emerge in the DOWN state. Nevertheless, the majority of hippocampal ripples in both anesthetized and sleeping animals occur during the UP state, particularly after the DOWN–UP transition (Fig. 6) (Isomura et al., 2006; Moelle et al., 2006). As discussed earlier, the DOWN–UP transition is a crucial state because it can trigger K complexes and sleep spindles (Amzica and Steriade, 1997; Massimini et al., 2004; Molle et al., 2002). The temporal association of hippocampal ripples and sleep spindles in the neocortex on the scale of ∼1 sec has led investigators to hypothesize that the hippocampal SPW output is responsible for the DOWN–UP shift and the occurrence of sleep spindles in neocortical networks (Siapas and Wilson, 1998; Battaglia et al., 2004).
Fig. 6. Relationship between slow oscillation and hippocampal sharp wave/ripple complex.
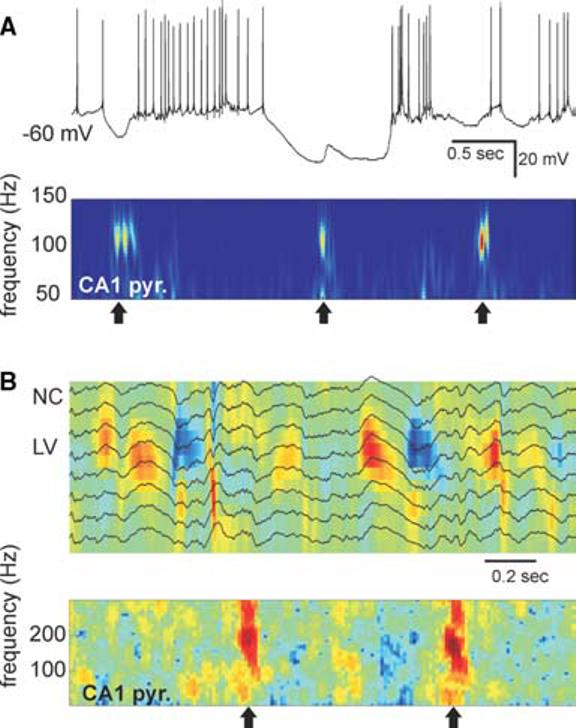
(A) Example of activity in a layer 3 entorhinal neuron and associated spectrogram of LFP in CA1 pyramidal layer under anesthesia (arrows, ripples). Note transient depolarizations during both DOWN and UP states after ripples. (B) Example of neocortical delta waves (white arrows, LFP traces and CSD map) and associated spectrogram of LFP in CA1 pyramidal layer during SWS.
However, even if such functional link exists, it is extremely week. Intracellular recordings in layer 5 entorhinal cortical neurons reveal that SPW ripples can depolarize and discharge these neurons in both UP and DOWN states (Chrobak and Buzsáki, 1996; Isomura et al., 2006). However, this depolarization is transient and the membrane potential returns quickly to its previous level (Fig. 6a), which argues in favor of circuit as opposed to intrinsic cellular mechanisms of slow oscillations. More refined analysis of the SPW/ripple-sleep spindle relationship reveals that the temporal sequence of neuronal organization is in the reverse direction; namely, the neocortical input can temporally bias the occurrence of CA3 spiking and consequent CA1 ripples (Sirota et al., 2003; Isomura et al., 2006). The synchronous cortical unit discharges associated with the thalamocortical spindles occasionally led to an increased firing of hippocampal neurons within 30–50 msec, and this increased activity was often associated with a SPW/ripple complex.
Functional implications of the periodic ‘rebooting’ of neocortical, paleocortical and hippocampal activity by slow oscillations
Our findings indicate that the slow rhythm described by Steriade resets local activity in vast neocortical areas, and that the paleocortical entorhinal and subicular structures and the dentate gyrus of the hippocampus are integral parts of the transient quiescence of slow oscillations (Isomura et al., 2006). Although the CA3 and CA1 networks can sustain organized patterns in the DOWN state, the hippocampal output, as represented by SPW/ripples, is also biased by the slow oscillation. The temporal bias on hippocampal SPWs and thalamocortical spindles brought about by the cortical slow oscillations probably have important functional consequences for the transfer of information among these structures. Although the crucial details of this mechanism are not known, the following framework might guide further experimentation.
After each DOWN state, the neocortex self-organizes its global activity from locally generated patterns (Steriade and Amzica, 1998a; Luczak et al., 2007). The behavioral relevance of these organized oscillatory patterns is illustrated by the finding that the amplitude and interregional coherence of slow oscillation increase following a declarative memory task (Molle et al., 2004). Furthermore, brain stimulation at the frequency of slow oscillation during non-REM sleep improves post-sleep memory performance (Marshall et al., 2004; Marshall et al., 2006). Recent observations in both anesthetized and resting, nonanesthetized animals reveals that activity of neocortical neurons has a non-random spatiotemporal pattern (Prut et al., 1998; Hoffman and McNaughton, 2002; Ikegaya et al., 2004; MacLean et al., 2005; Mokeichev et al., 2007), and these events are triggered by synchrony onset (UP state) (Luczak et al., 2007). Similarly, at the level of the hippocampus recruitment of neurons during SPW/ripples has a non-random spatiotemporal structure (Csicsvari et al., 2000). Often, the spatiotemporal pattern of firing of neurons during sleep correlates with that in the previous waking state (Wilson and McNaughton, 1994; Skaggs and McNaughton, 1996; Nadasdy et al., 1998; Buzsáki, 1989; Hirase et al., 2001; Lee and Wilson, 2002; Ribeiro et al., 2004; Ji and Wilson, 2007). Furthermore, activation of such spatiotemporal patterns is more probable during a sleep session following than preceding a learning experience in familiar (Lee and Wilson, 2002; Ribeiro et al., 2004; Ji and Wilson, 2007) or novel (Nadasdy et al., 1998; Kudrimoti et al., 1999) environments, which may indicate plastic changes in the synaptic matrix. Alternatively, differences in firing statistics of neurons between two sleep episodes might be explained by changes in the structure of sleep rhythms induced by the learning experience (Gais et al., 2002; Cantero et al., 2002; Molle et al., 2004).
It has been hypothesized that transfer of information between the neocortex and hippocampus takes place in different states and different times. During learning, the neocortical information that enters the hippocampus by way of the entorhinal cortex alters the synaptic weights in hippocampal circuits. During subsequent sleep the self-organized patterns that underlie SPWs will utilize the strongest synaptic connections, including those that are recently modified. These new patterns, in turn, can transfer the learned hippocampal information to the neocortex by repeatedly depolarizing and modifying cortical synapses (Buzsáki, 1989).
A caveat in the hypothetical, two-stage process of information transfer and memory is the absence of a mechanism to coordinate the time of the hippocampal output with the ongoing activity in the neocortex and guide the hippocampal output to the neocortical circuits that gave rise to input to the hippocampus during the experience. The temporal coordination of neocortical sleep spindles, gamma bursts and hippocampal SPWs by the slow oscillations offers such a mechanism. DOWN–UP transition, which, perhaps, involves thalamocortical spindles, triggers organized firing patterns of cortical neurons which, in turn, lead to activation of specific subpopulations of hippocampal neurons. These activated hippocampal neurons give rise to SPW-related synchronous outputs to readdress the neocortex. Because SPW is a punctuate event whereas the sleep spindle is temporally protracted, the hippocampal output can be directed to the still active neocortical assemblies. The temporal coordination of these events facilitates conditions in which unique neocortical inputs to the hippocampus and, in turn, hippocampal outputs to the neocortex might be modified selectively (King et al., 1999; Sirota et al., 2003; Steriade and Timofeev, 2003). In this reciprocal information-transfer process, the neocortex serves as the target (‘receiver’) of the information from the ‘source’ (sender) hippocampus.
Neocortical slow oscillations might coordinate both the time and content of the information transfer from the hippocampus. Providing experimental support for this framework requires demonstration that specific assemblies in the neocortex–entorhinal cortex–hippocampus axis during sleep are brought about by waking experiences (Huber et al., 2004). Nevertheless, the discovery of slow oscillations in the neocortex by Mircea Steriade offers clues to an important missing link in this process.
REFERENCES
- Achermann P, Borbely AA. Low-frequency (<1 Hz) oscillations in the human sleep electroencephalogram. Neuroscience. 1997;81:213–222. doi: 10.1016/s0306-4522(97)00186-3. [DOI] [PubMed] [Google Scholar]
- Acsady L, Kamondi A, Sik A, Freund T, Buzsáki G. GABAergic cells are the major postsynaptic targets of mossy fibers in the rat hippocampus. Journal of Neuroscience. 1998;18:3386–3403. doi: 10.1523/JNEUROSCI.18-09-03386.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso A, Garcia-Austt E. Neuronal sources of theta rhythm in the entorhinal cortex of the rat. I. Laminar distribution of theta field potentials. Experimental Brain Research. 1987a;67:493–501. doi: 10.1007/BF00247282. [DOI] [PubMed] [Google Scholar]
- Alonso A, Garcia-Austt E. Neuronal sources of theta rhythm in the entorhinal cortex of the rat. II. Phase relations between unit discharges and theta field potentials. Experimental Brain Research. 1987b;67:502–509. doi: 10.1007/BF00247283. [DOI] [PubMed] [Google Scholar]
- Alonso A, Llinás RR. Subthreshold Na+-dependent theta-like rhythmicity in stellate cells of entorhinal cortex layer II. Nature. 1989;342:175–177. doi: 10.1038/342175a0. [DOI] [PubMed] [Google Scholar]
- Amaral DG, Witter MP. The three-dimensional organization of the hippocampal formation: a review of anatomical data. Neuroscience. 1989;31:571–591. doi: 10.1016/0306-4522(89)90424-7. [DOI] [PubMed] [Google Scholar]
- Amzica F, Steriade M. The K-complex: its slow (<1-Hz) rhythmicity and relation to delta waves. Neurology. 1997;49:952–959. doi: 10.1212/wnl.49.4.952. [DOI] [PubMed] [Google Scholar]
- Amzica F, Steriade M. Electrophysiological correlates of sleep delta waves. Electroencephalography and clinical neurophysiology. 1998;107:69–83. doi: 10.1016/s0013-4694(98)00051-0. [DOI] [PubMed] [Google Scholar]
- Amzica F, Steriade M. Disconnection of intracortical synaptic linkages disrupts synchronization of a slow oscillation. Journal of Neuroscience. 1995;15:4658–4677. doi: 10.1523/JNEUROSCI.15-06-04658.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson J, Lampl I, Reichova I, Carandini M, Ferster D. Stimulus dependence of two-state fluctuations of membrane potential in cat visual cortex. Nature Neuroscience. 2000;3:617–621. doi: 10.1038/75797. [DOI] [PubMed] [Google Scholar]
- Anderson MI, O'Mara SM. Analysis of recordings of single-unit firing and population activity in the dorsal subiculum of unrestrained, freely moving rats. Journal of Neurophysiology. 2003;90:655–665. doi: 10.1152/jn.00723.2002. [DOI] [PubMed] [Google Scholar]
- Arieli A, Sterkin A, Grinvald A, Aertsen A. Dynamics of ongoing activity: explanation of the large variability in evoked cortical responses. Science. 1996;273:1868–1871. doi: 10.1126/science.273.5283.1868. [DOI] [PubMed] [Google Scholar]
- Avanzini G, de Curtis M, Panzica F, Spreafico R. Intrinsic properties of nucleus reticularis thalami neurones of the rat studied in vitro. Journal of Physiology. 1989;416:111–122. doi: 10.1113/jphysiol.1989.sp017752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bal T, Debay D, Destexhe A. Cortical feedback controls the frequency and synchrony of oscillations in the visual thalamus. Journal of Neuroscience. 2000;20:7478–7488. doi: 10.1523/JNEUROSCI.20-19-07478.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bal T, McCormick DA. Mechanisms of oscillatory activity in guinea-pig nucleus reticularis thalami in vitro: a mammalian pacemaker. Journal of Physiology. 1993;468:669–691. doi: 10.1113/jphysiol.1993.sp019794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartos M, Vida I, Frotscher M, Meyer A, Monyer H, Geiger JRP, Jonas P. Fast synaptic inhibition promotes synchronized gamma oscillations in hippocampal interneuron networks. Proceedings of the National Academy of Sciences of the USA. 2002;99:13222–13227. doi: 10.1073/pnas.192233099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battaglia FP, Sutherland GR, McNaughton BL. Hippocampal sharp wave bursts coincide with neocortical “up-state” transitions. Learning and Memory. 2004;11:697–704. doi: 10.1101/lm.73504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beierlein M, Gibson JR, Connors BW. A network of electrically coupled interneurons drives synchronized inhibition in neocortex. Nature Neuroscience. 2000;3:904–910. doi: 10.1038/78809. [DOI] [PubMed] [Google Scholar]
- Bliss TV, Lomo T. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. Journal of Physiology. 1973;232:331–356. doi: 10.1113/jphysiol.1973.sp010273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeijinga PH, Lopes da Silva FH. Differential distribution of beta and theta EEG activity in the entorhinal cortex of the cat. Brain Research. 1988;448:272–286. doi: 10.1016/0006-8993(88)91264-4. [DOI] [PubMed] [Google Scholar]
- Bragin A, Engel J, Jr., Wilson CL, Fried I, Buzsáki G. High-frequency oscillations in human brain. Hippocampus. 1999;9:137–142. doi: 10.1002/(SICI)1098-1063(1999)9:2<137::AID-HIPO5>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Bragin A, Jando G, Nadasdy Z, Hetke J, Wise K, Buzsáki G. Gamma (40–100 Hz) oscillation in the hippocampus of the behaving rat. Journal of Neuroscience. 1995a;15:47–60. doi: 10.1523/JNEUROSCI.15-01-00047.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bragin A, Jando G, Nadasdy Z, van Landeghem M, Buzsáki G. Dentate EEG spikes and associated interneuronal population bursts in the hippocampal hilar region of the rat. Journal of Neurophysiology. 1995b;73:1691–1705. doi: 10.1152/jn.1995.73.4.1691. [DOI] [PubMed] [Google Scholar]
- Bremer F. Cerveau “isole” et physiologie du sommeil. Comptes rendus des séances de la Société de biologie et de ses filiales. 1935;118:1235–1241. [Google Scholar]
- Buhl EH, Tamas G, Fisahn A. Cholinergic activation and tonic excitation induce persistent gamma oscillations in mouse somatosensory cortex in vitro. Journal of Physiology. 1998;513:117–126. doi: 10.1111/j.1469-7793.1998.117by.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullock TH, Buzsáki G, McClune MC. Coherence of compound field potentials reveals discontinuities in the CA1-subiculum of the hippocampus in freely moving rats. Neuroscience. 1990;38:609–619. doi: 10.1016/0306-4522(90)90055-9. [DOI] [PubMed] [Google Scholar]
- Buzsáki G. Hippocampal sharp waves: their origin and significance. Brain Research. 1986;398:242–252. doi: 10.1016/0006-8993(86)91483-6. [DOI] [PubMed] [Google Scholar]
- Buzsáki G. The thalamic clock: emergent network properties. Neuroscience. 1991;41:351–364. doi: 10.1016/0306-4522(91)90332-i. [DOI] [PubMed] [Google Scholar]
- Buzsáki G. Rhythms of the brain. Oxford University Press; 2006. [Google Scholar]
- Buzsáki G. Theta oscillations in the hippocampus. Neuron. 2002;33:325–340. doi: 10.1016/s0896-6273(02)00586-x. [DOI] [PubMed] [Google Scholar]
- Buzsáki G. Two-stage model of memory trace formation: a role for “noisy” brain states. Neuroscience. 1989;31:551–570. doi: 10.1016/0306-4522(89)90423-5. [DOI] [PubMed] [Google Scholar]
- Buzsáki G. The hippocampo-neocortical dialogue. Cerebral Cortex. 1996;6:81–92. doi: 10.1093/cercor/6.2.81. [DOI] [PubMed] [Google Scholar]
- Buzsáki G. Memory consolidation during sleep: a neurophysiological perspective. Journal of Sleep Research. 1998;7(Suppl 1):17–23. doi: 10.1046/j.1365-2869.7.s1.3.x. [DOI] [PubMed] [Google Scholar]
- Buzsáki G, Bickford RG, Ponomareff G, Thal LJ, Mandel R, Gage FH. Nucleus basalis and thalamic control of neocortical activity in the freely moving rat. Journal of Neuroscience. 1988;8:4007–4026. doi: 10.1523/JNEUROSCI.08-11-04007.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsáki G, Chrobak JJ. Temporal structure in spatially organized neuronal ensembles: a role for interneuronal networks. Current Opinion in Neurobiology. 1995;5:504–510. doi: 10.1016/0959-4388(95)80012-3. [DOI] [PubMed] [Google Scholar]
- Buzsáki G, Draguhn A. Neuronal oscillations in cortical networks. Science. 2004;304:1095–9203. doi: 10.1126/science.1099745. [DOI] [PubMed] [Google Scholar]
- Buzsáki G, Horvath Z, Urioste R, Hetke J, Wise K. High-frequency network oscillation in the hippocampus. Science. 1992;256:1025–1027. doi: 10.1126/science.1589772. [DOI] [PubMed] [Google Scholar]
- Buzsáki G, Leung LW, Vanderwolf CH. Cellular bases of hippocampal EEG in the behaving rat. Brain Research. 1983;287:139–171. doi: 10.1016/0165-0173(83)90037-1. [DOI] [PubMed] [Google Scholar]
- Cantero JL, Atienza M, Salas RM, Dominguez-Marin E. Effects of prolonged waking-auditory stimulation on electroencephalogram synchronization and cortical coherence during subsequent slow-wave sleep. Journal of Neuroscience. 2002;22:4702–4708. doi: 10.1523/JNEUROSCI.22-11-04702.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrobak JJ, Buzsáki G. Selective activation of deep layer (V-VI) retrohippocampal cortical neurons during hippocampal sharp waves in the behaving rat. Journal of Neuroscience. 1994;14:6160–6170. doi: 10.1523/JNEUROSCI.14-10-06160.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrobak JJ, Buzsáki G. Gamma oscillations in the entorhinal cortex of the freely behaving rat. Journal of Neuroscience. 1998a;18:388–398. doi: 10.1523/JNEUROSCI.18-01-00388.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrobak JJ, Buzsáki G. Operational dynamics in the hippocampalentorhinal axis. Neuroscience and Biobehavioral Reviews. 1998b;22:303–310. doi: 10.1016/s0149-7634(97)00016-x. [DOI] [PubMed] [Google Scholar]
- Chrobak JJ, Buzsáki G. High-frequency oscillations in the output networks of the hippocampal-entorhinal axis of the freely behaving rat. Journal of Neuroscience. 1996;16:3056–3066. doi: 10.1523/JNEUROSCI.16-09-03056.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins DR, Lang EJ, Pare D. Spontaneous activity of the perirhinal cortex in behaving cats. Neuroscience. 1999;89:1025–1039. doi: 10.1016/s0306-4522(98)00396-0. [DOI] [PubMed] [Google Scholar]
- Collins DR, Pelletier JG, Pare D. Slow and fast (gamma) neuronal oscillations in the perirhinal cortex and lateral amygdala. Journal of Neurophysiology. 2001;85:1661–1672. doi: 10.1152/jn.2001.85.4.1661. [DOI] [PubMed] [Google Scholar]
- Colom LV, Christie BR, Bland BH. Cingulate cell discharge patterns related to hippocampal EEG and their modulation by muscarinic and nicotinic agents. Brain Research. 1988;460:329–338. doi: 10.1016/0006-8993(88)90377-0. [DOI] [PubMed] [Google Scholar]
- Contreras D, Destexhe A, Sejnowski TJ, Steriade M. Control of spatiotemporal coherence of a thalamic oscillation by corticothalamic feedback. Science. 1996;274:771–774. doi: 10.1126/science.274.5288.771. [DOI] [PubMed] [Google Scholar]
- Contreras D, Steriade M. Spindle oscillation in cats: the role of corticothalamic feedback in a thalamically generated rhythm. Journal of Physiology. 1996;490:159–179. doi: 10.1113/jphysiol.1996.sp021133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cossart R, Aronov D, Yuste R. Attractor dynamics of network UP states in the neocortex. Nature. 2003;423:283–288. doi: 10.1038/nature01614. [DOI] [PubMed] [Google Scholar]
- Cowan RL, Wilson CJ. Spontaneous firing patterns and axonal projections of single corticostriatal neurons in the rat medial agranular cortex. Journal of Neurophysiology. 1994;71:17–32. doi: 10.1152/jn.1994.71.1.17. [DOI] [PubMed] [Google Scholar]
- Csicsvari J, Hirase H, Czurko A, Mamiya A, Buzsáki G. Fast network oscillations in the hippocampal CA1 region of the behaving rat. Journal of Neuroscience. 1999;19:RC20. doi: 10.1523/JNEUROSCI.19-16-j0001.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csicsvari J, Hirase H, Mamiya A, Buzsáki G. Ensemble patterns of hippocampal CA3-CA1 neurons during sharp wave-associated population events. Neuron. 2000;28:585–594. doi: 10.1016/s0896-6273(00)00135-5. [DOI] [PubMed] [Google Scholar]
- Csicsvari J, Jamieson B, Wise KD, Buzsáki G. Mechanisms of gamma oscillations in the hippocampus of the behaving rat. Neuron. 2003;37:311–322. doi: 10.1016/s0896-6273(02)01169-8. [DOI] [PubMed] [Google Scholar]
- Cunningham MO, Pervouchine DD, Racca C, Kopell NJ, Davies CH, Jones RS, et al. Neuronal metabolism governs cortical network response state. Proceedings of the National Academy of Sciences of the USA. 2006;103:5597–5601. doi: 10.1073/pnas.0600604103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deschenes M, Paradis M, Roy JP, Steriade M. Electrophysiology of neurons of lateral thalamic nuclei in cat: resting properties and burst discharges. Journal of Neurophysiology. 1984;51:1196–1219. doi: 10.1152/jn.1984.51.6.1196. [DOI] [PubMed] [Google Scholar]
- Destexhe A, Contreras D, Steriade M. Spatiotemporal analysis of local field potentials and unit discharges in cat cerebral cortex during natural wake and sleep states. Journal of Neuroscience. 1999;19:4595–4608. doi: 10.1523/JNEUROSCI.19-11-04595.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Destexhe A, Sejnowski T. Thalamocortical Assemblies - How Ion Channels, Single Neurons and large-Scale Networks Organize Sleep Oscillations. Oxford University Press; 2001. [Google Scholar]
- Dragoi G, Buzsáki G. Temporal encoding of place sequences by hippocampal cell assemblies. Neuron. 2006;50:145–157. doi: 10.1016/j.neuron.2006.02.023. [DOI] [PubMed] [Google Scholar]
- Dragoi G, Carpi D, Recce M, Csicsvari J, Buzsáki G. Interactions between hippocampus and medial septum during sharp waves and theta oscillation in the behaving rat. Journal of Neuroscience. 1999;19:6191–6199. doi: 10.1523/JNEUROSCI.19-14-06191.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel AK, Fries P, Singer W. Dynamic predictions: oscillations and synchrony in top-down processing. Nature Reviews Neuroscience. 2001;2:704–716. doi: 10.1038/35094565. [DOI] [PubMed] [Google Scholar]
- Engel AK, Konig P, Kreiter AK, Singer W. Interhemispheric synchronization of oscillatory neuronal responses in cat visual cortex. Science. 1991;252:1177–1179. doi: 10.1126/science.252.5009.1177. [DOI] [PubMed] [Google Scholar]
- Fisahn A, Pike FG, Buhl EH, Paulsen O. Cholinergic induction of network oscillations at 40 Hz in the hippocampus in vitro. Nature. 1998;394:186–189. doi: 10.1038/28179. [DOI] [PubMed] [Google Scholar]
- Gais S, Molle M, Helms K, Born J. Learning-dependent increases in sleep spindle density. Journal of Neuroscience. 2002;22:6830–6834. doi: 10.1523/JNEUROSCI.22-15-06830.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gloor P, Ball G, Schaul N. Brain lesions that produce delta waves in the EEG. Neurology. 1977;27:326–333. doi: 10.1212/wnl.27.4.326. [DOI] [PubMed] [Google Scholar]
- Gray CM, Konig P, Engel AK, Singer W. Oscillatory responses in cat visual cortex exhibit inter-columnar synchronization which reflects global stimulus properties. Nature. 1989;338:334–347. doi: 10.1038/338334a0. [DOI] [PubMed] [Google Scholar]
- Gray CM, McCormick DA. Chattering cells: superficial pyramidal neurons contributing to the generation of synchronous oscillations in the visual cortex. Science. 1996;274:109–113. doi: 10.1126/science.274.5284.109. [DOI] [PubMed] [Google Scholar]
- Green JD, Arduni AA. Hippocampal electrical activity in arousal. Journal of Neurophysiology. 1954;17:533–557. doi: 10.1152/jn.1954.17.6.533. [DOI] [PubMed] [Google Scholar]
- Grenier F, Timofeev I, Steriade M. Focal synchronization of ripples (80–200 hz) in neocortex and their neuronal correlates. Journal of Neurophysiology. 2001;86:1884–1898. doi: 10.1152/jn.2001.86.4.1884. [DOI] [PubMed] [Google Scholar]
- Gulyas AI, Hajos N, Katona I, Freund TF. Interneurons are the local targets of hippocampal inhibitory cells which project to the medial septum. European Journal of Neuroscience. 2003;17:1861–1872. doi: 10.1046/j.1460-9568.2003.02630.x. [DOI] [PubMed] [Google Scholar]
- Halasz P, Pal I, Rajna P. K-complex formation of the EEG in sleep. A survey and new examinations. Acta Physiologica Hungarica. 1985;65:3–35. [PubMed] [Google Scholar]
- Halasz P, Terzano M, Parrino L, Bodizs R. The nature of arousal in sleep. Journal of Sleep Research. 2004;13:1–23. doi: 10.1111/j.1365-2869.2004.00388.x. [DOI] [PubMed] [Google Scholar]
- Hanganu IL, Ben-Ari Y, Khazipov R. Retinal waves trigger spindle bursts in the neonatal rat visual cortex. Journal of Neuroscience. 2006;26:6728–6736. doi: 10.1523/JNEUROSCI.0752-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris KD, Csicsvari J, Hirase H, Dragoi G, Buzsáki G. Organization of cell assemblies in the hippocampus. Nature. 2003;424:552–556. doi: 10.1038/nature01834. [DOI] [PubMed] [Google Scholar]
- Harris KD, Henze DA, Hirase H, Leinekugel X, Dragoi G, Czurko A, et al. Spike train dynamics predicts theta-related phase precession in hippocampal pyramidal cells. Nature. 2002;417:738–741. doi: 10.1038/nature00808. [DOI] [PubMed] [Google Scholar]
- Hasenstaub A, Shu Y, Haider B, Kraushaar U, Duque A, McCormick DA. Inhibitory postsynaptic potentials carry synchronized frequency information in active cortical networks. Neuron. 2005;47:423–435. doi: 10.1016/j.neuron.2005.06.016. [DOI] [PubMed] [Google Scholar]
- Hirase H, Leinekugel X, Czurko A, Csicsvari J, Buzsáki G. Firing rates of hippocampal neurons are preserved during subsequent sleep episodes and modified by novel awake experience. Proceedings of the National Academy of Sciences of the USA. 2001;98:9386–9390. doi: 10.1073/pnas.161274398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman KL, McNaughton BL. Coordinated reactivation of distributed memory traces in primate neocortex. Science. 2002;297:2070–2073. doi: 10.1126/science.1073538. [DOI] [PubMed] [Google Scholar]
- Huber R, Ghilardi MF, Massimini M, Tononi G. Local sleep and learning. Nature. 2004;430:78–81. doi: 10.1038/nature02663. [DOI] [PubMed] [Google Scholar]
- Hughes SW, Cope DW, Blethyn KL, Crunelli V. Cellular mechanisms of the slow (<1 Hz) oscillation in thalamocortical neurons in vitro. Neuron. 2002;33:947–958. doi: 10.1016/s0896-6273(02)00623-2. [DOI] [PubMed] [Google Scholar]
- Huguenard JR, Prince DA. Intrathalamic rhythmicity studied in vitro: nominal T-current modulation causes robust antioscillatory effects. Journal of Neuroscience. 1994;14:5485–5502. doi: 10.1523/JNEUROSCI.14-09-05485.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikegaya Y, Aaron G, Cossart R, Aronov D, Lampl I, Ferster D, et al. Synfire chains and cortical songs: temporal modules of cortical activity. Science. 2004;304:559–564. doi: 10.1126/science.1093173. [DOI] [PubMed] [Google Scholar]
- Isomura Y, Sirota A, Ozen S, Mongomery S, Mizuseki K, Henze D, et al. Integration and segregation of activity in entorhinalhippocampal subregions by neocortical slow oscillations. Neuron. 2006;52:871–882. doi: 10.1016/j.neuron.2006.10.023. [DOI] [PubMed] [Google Scholar]
- Jefferys JG, Traub RD, Whittington MA. Neuronal networks for induced ‘40 Hz’ rhythms. Trends in Neurosciences. 1996;19:202–208. doi: 10.1016/s0166-2236(96)10023-0. [DOI] [PubMed] [Google Scholar]
- Ji D, Wilson MA. Coordinated memory replay in the visual cortex and hippocampus during sleep. Nature Neuroscience. 2007;10:100–107. doi: 10.1038/nn1825. [DOI] [PubMed] [Google Scholar]
- Jones MS, Barth DS. Effects of bicuculline methiodide on fast (>200 Hz) electrical oscillations in rat somatosensory cortex. Journal of Neurophysiology. 2002;88:1016–1025. doi: 10.1152/jn.2002.88.2.1016. [DOI] [PubMed] [Google Scholar]
- Jouvet M. Biogenic amines and the states of sleep. Science. 1969;163:32–41. doi: 10.1126/science.163.3862.32. [DOI] [PubMed] [Google Scholar]
- Kahana MJ, Seelig D, Madsen JR. Theta returns. Current Opinions in Neurobiology. 2001;11:739–744. doi: 10.1016/s0959-4388(01)00278-1. [DOI] [PubMed] [Google Scholar]
- Kamondi A, Acsady L, Wang XJ, Buzsáki G. Theta oscillations in somata and dendrites of hippocampal pyramidal cells in vivo: activity-dependent phaseprecession of action potentials. Hippocampus. 1998;8:244–261. doi: 10.1002/(SICI)1098-1063(1998)8:3<244::AID-HIPO7>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Kandel A, Buzsáki G. Cellular-synaptic generation of sleep spindles, spikeand- wave discharges, and evoked thalamocortical responses in the neocortex of the rat. Journal of Neuroscience. 1997;17:6783–6797. doi: 10.1523/JNEUROSCI.17-17-06783.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenet T, Bibitchkov D, Tsodyks M, Grinvald A, Arieli A. Spontaneously emerging cortical representations of visual attributes. Nature. 2003;425:954–956. doi: 10.1038/nature02078. [DOI] [PubMed] [Google Scholar]
- Khazipov R, Sirota A, Leinekugel X, Holmes GL, Ben-Ari Y, Buzsáki G. Early motor activity drives spindle bursts in the developing somatosensory cortex. Nature. 2004;432:758–761. doi: 10.1038/nature03132. [DOI] [PubMed] [Google Scholar]
- Kim U, Bal T, McCormick DA. Spindle waves are propagating synchronized oscillations in the ferret LGNd in vitro. Journal of Neurophysiology. 1995;74:1301–1323. doi: 10.1152/jn.1995.74.3.1301. [DOI] [PubMed] [Google Scholar]
- King C, Henze DA, Leinekugel X, Buzsáki G. Hebbian modification of a hippocampal population pattern in the rat. Journal of Physiology. 1999;521:159–167. doi: 10.1111/j.1469-7793.1999.00159.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King C, Recce M, O'Keefe J. The rhythmicity of cells of the medial septum/diagonal band of Broca in the awake freely moving rat: relationships with behaviour and hippocampal theta. European Journal of Neuroscience. 1998;10:464–477. doi: 10.1046/j.1460-9568.1998.00026.x. [DOI] [PubMed] [Google Scholar]
- Klausberger T, Magill PJ, Marton LF, Roberts JD, Cobden PM, Buzsáki G, et al. Brain-state- and cell-type-specific firing of hippocampal interneurons in vivo. Nature. 2003;421:844–848. doi: 10.1038/nature01374. [DOI] [PubMed] [Google Scholar]
- Klausberger T, Marton LF, Baude A, Roberts JD, Magill PJ, Somogyi P. Spike timing of dendrite-targeting bistratified cells during hippocampal network oscillations in vivo. Nature Neuroscience. 2004;7:41–47. doi: 10.1038/nn1159. [DOI] [PubMed] [Google Scholar]
- Kocsis B, Bragin A, Buzsáki G. Interdependence of multiple theta generators in the hippocampus: a partial coherence analysis. Journal of Neuroscience. 1999;19:6200–6212. doi: 10.1523/JNEUROSCI.19-14-06200.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocsis B, Vertes RP. Characterization of neurons of the supramammillary nucleus and mammillary body that discharge rhythmically with the hippocampal theta rhythm in the rat. Journal of Neuroscience. 1994;14:7040–7052. doi: 10.1523/JNEUROSCI.14-11-07040.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreiter AK, Singer W. Stimulus-dependent synchronization of neuronal responses in the visual cortex of the awake macaque monkey. Journal of Neuroscience. 1996;16:2381–2396. doi: 10.1523/JNEUROSCI.16-07-02381.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krupa DJ, Wiest MC, Shuler MG, Laubach M, Nicolelis MA. Layerspecific somatosensory cortical activation during active tactile discrimination. Science. 2004;304:1989–1992. doi: 10.1126/science.1093318. [DOI] [PubMed] [Google Scholar]
- Kudrimoti HS, Barnes CA, McNaughton BL. Reactivation of hippocampal cell assemblies: effects of behavioral state, experience, and EEG dynamics. Journal of Neuroscience. 1999;19:4090–4101. doi: 10.1523/JNEUROSCI.19-10-04090.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampl I, Reichova I, Ferster D. Synchronous membrane potential fluctuations in neurons of the cat visual cortex. Neuron. 1999;22:361–374. doi: 10.1016/s0896-6273(00)81096-x. [DOI] [PubMed] [Google Scholar]
- Lee AK, Wilson MA. Memory of sequential experience in the hippocampus during slow wave sleep. Neuron. 2002;36:1183–1194. doi: 10.1016/s0896-6273(02)01096-6. [DOI] [PubMed] [Google Scholar]
- Leopold DA, Murayama Y, Logothetis NK. Very slow activity fluctuations in monkey visual cortex: implications for functional brain imaging. Cerebral Cortex. 2003;13:422–433. doi: 10.1093/cercor/13.4.422. [DOI] [PubMed] [Google Scholar]
- Leung LS, Borst JG. Electrical activity of the cingulate cortex. I. Generating mechanisms and relations to behavior. Brain Research. 1987;407:68–80. doi: 10.1016/0006-8993(87)91220-0. [DOI] [PubMed] [Google Scholar]
- Levy WB, Steward O. Temporal contiguity requirements for long-term associative potentiation/depression in the hippocampus. Neuroscience. 1983;8:791–797. doi: 10.1016/0306-4522(83)90010-6. [DOI] [PubMed] [Google Scholar]
- Llinás R, Ribary U. Coherent 40-Hz oscillation characterizes dream state in humans. Proceedings of the National Academy of Sciences of the USA. 1993;90:2078–2081. doi: 10.1073/pnas.90.5.2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás RR, Grace AA, Yarom Y. In vitro neurons in mammalian cortical layer 4 exhibit intrinsic oscillatory activity in the 10- to 50-Hz frequency range. Proceedings of the National Academy of Sciences of the USA. 1991;88:897–901. doi: 10.1073/pnas.88.3.897. erratum appears in Proceedings of the National Academy of Sciences of the USA 88, 3510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loomis A, Harvey N, Hobart G. Distribution of disturbance patterns in human electroencephalogram, with special reference to sleep. Journal of Neurophysiology. 1938;1:413–430. [Google Scholar]
- Lorincz A, Buzsáki G. Two-phase computational model training long-term memories in the entorhinal-hippocampal region. Annals of the New York Academy of Sciences. 2000;911:83–111. doi: 10.1111/j.1749-6632.2000.tb06721.x. [DOI] [PubMed] [Google Scholar]
- Luczak A, Barthó P, Marguet SL, Buzsáki G, Harris KD. Neocortical spontaneous activity in vivo: cellular heterogeneity and sequential structure. Proceedings of the National Academy of Sciences of the USA. 2007;104:347–352. doi: 10.1073/pnas.0605643104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luthi A, McCormick DA. Periodicity of thalamic synchronized oscillations: the role of Ca2+-mediated upregulation of Ih. Neuron. 1998;20:553–563. doi: 10.1016/s0896-6273(00)80994-0. [DOI] [PubMed] [Google Scholar]
- MacLean JN, Watson BO, Aaron GB, Yuste R. Internal dynamics determine the cortical response to thalamic stimulation. Neuron. 2005;48:811–823. doi: 10.1016/j.neuron.2005.09.035. [DOI] [PubMed] [Google Scholar]
- Maloney KJ, Cape EG, Gotman J, Jones BE. High-frequency gamma electroencephalogram activity in association with sleep-wake states and spontaneous behaviors in the rat. Neuroscience. 1997;76:541–555. doi: 10.1016/s0306-4522(96)00298-9. [DOI] [PubMed] [Google Scholar]
- Mao BQ, Hamzei-Sichani F, Aronov D, Froemke RC, Yuste R. Dynamics of spontaneous activity in neocortical slices. Neuron. 2001;32:883–898. doi: 10.1016/s0896-6273(01)00518-9. [DOI] [PubMed] [Google Scholar]
- Markram H, Lubke J, Frotscher M, Sakmann B. Regulation of synaptic efficacy by coincidence of postsynaptic APs and EPSPs. Science. 1997;275:213–215. doi: 10.1126/science.275.5297.213. [DOI] [PubMed] [Google Scholar]
- Marshall L, Molle M, Hallschmid M, Born J. Transcranial direct current stimulation during sleep improves declarative memory. Journal of Neuroscience. 2004;24:9985–9992. doi: 10.1523/JNEUROSCI.2725-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall L, Helgadottir H, Molle M, Born J. Boosting slow oscillations during sleep potentiates memory. Nature. 2006;444:610–613. doi: 10.1038/nature05278. [DOI] [PubMed] [Google Scholar]
- Massimini M, Amzica F. Extracellular calcium fluctuations and intracellular potentials in the cortex during the slow sleep oscillation. Journal of Neurophysiology. 2001;85:1346–1350. doi: 10.1152/jn.2001.85.3.1346. [DOI] [PubMed] [Google Scholar]
- Massimini M, Huber R, Ferrarelli F, Hill S, Tononi G. The sleep slow oscillation as a traveling wave. Journal of Neuroscience. 2004;24:6862–6870. doi: 10.1523/JNEUROSCI.1318-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick DA, Bal T. Sleep and arousal: thalamocortical mechanisms. Annual Review of Neuroscience. 1997;20:185–215. doi: 10.1146/annurev.neuro.20.1.185. [DOI] [PubMed] [Google Scholar]
- McCormick DA, Pape HC. Properties of a hyperpolarization-activated cation current and its role in rhythmic oscillation in thalamic relay neurones. Journal of Physiology. 1990;431:291–318. doi: 10.1113/jphysiol.1990.sp018331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta MR, Lee AK, Wilson MA. Role of experience and oscillations in transforming a rate code into a temporal code. Nature. 2002;417:741–746. doi: 10.1038/nature00807. [DOI] [PubMed] [Google Scholar]
- Metherate R, Ashe JH. Nucleus basalis stimulation facilitates thalamocortical synaptic transmission in the rat auditory cortex. Synapse. 1993;14:132–143. doi: 10.1002/syn.890140206. [DOI] [PubMed] [Google Scholar]
- Milh M, Kaminska A, Huon C, Lapillonne A, Ben-Ari Y, Khazipov R. Rapid cortical oscillations and early motor activity in premature human neonate. Cerebral Cortex. 2006 doi: 10.1093/cercor/bhl069. PMID: 16950867 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Mitchell SJ, Ranck JB., Jr. Generation of theta rhythm in medial entorhinal cortex of freely moving rats. Brain Research. 1980;189:49–66. doi: 10.1016/0006-8993(80)90006-2. [DOI] [PubMed] [Google Scholar]
- Moelle M, Yeshenko O, Marshall L, Sara SJ, Born J. Hippocampal sharp wave-ripples linked to slow oscillations in rat slow-wave sleep. Journal of Neurophysiology. 2006;96:62–70. doi: 10.1152/jn.00014.2006. [DOI] [PubMed] [Google Scholar]
- Mokeichev A, Okun M, Barak O, Katz Y, Ben-Shahar O, Lampl I. Stochastic emergence of repeating cortical motifs in spontaneous membrane potential fluctuations in vivo. Neuron. 2007;53:413–425. doi: 10.1016/j.neuron.2007.01.017. [DOI] [PubMed] [Google Scholar]
- Molle M, Marshall L, Gais S, Born J. Learning increases human electroencephalographic coherence during subsequent slow sleep oscillations. Proceedings of the National Academy of Sciences of the USA. 2004;101:13963–13968. doi: 10.1073/pnas.0402820101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molle M, Marshall L, Gais S, Born J. Grouping of spindle activity during slow oscillations in human non-rapid eye movement sleep. Journal of Neuroscience. 2002;22:10941–10947. doi: 10.1523/JNEUROSCI.22-24-10941.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morison RS, Bassett DL. Electrical activity of the thalamus and basal ganglia in decorrticated cats. Journal of Neurophysiology. 1945;8:309–314. [Google Scholar]
- Muir GM, Bilkey DK. Synchronous modulation of perirhinal cortex neuronal activity during cholinergically mediated (type II) hippocampal theta. Hippocampus. 1998;8:526–532. doi: 10.1002/(SICI)1098-1063(1998)8:5<526::AID-HIPO10>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Mukovski M, Chauvette S, Timofeev I, Volgushev M. Detection of active and silent states in neocortical neurons from the field potential signal during slow-wave sleep. Cerebral Cortex. 2007;17:400–414. doi: 10.1093/cercor/bhj157. [DOI] [PubMed] [Google Scholar]
- Mulle C, Steriade M, Deschenes M. Absence of spindle oscillations in the cat anterior thalamic nuclei. Brain Research. 1985;334:169–171. doi: 10.1016/0006-8993(85)90581-5. [DOI] [PubMed] [Google Scholar]
- Murthy VN, Fetz EE. Coherent 25- to 35-Hz oscillations in the sensorimotor cortex of awake behaving monkeys. Proceedings of the National Academy of Sciences of the USA. 1992;89:5670–5674. doi: 10.1073/pnas.89.12.5670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadasdy Z, Csicsvari J, Penttonen M, Hetke J, Wise K, Buzsáki G. Extracellular recording and analysis of neuronal activity: from single cells to ensembles. In: Eichenbaum HB, Davis JL, editors. Neuronal Ensembles: Strategies for Recording and Decoding. Wiley-Liss; 1998. pp. 17–55. [Google Scholar]
- Nadasdy Z, Hirase H, Czurko A, Csicsvari J, Buzsáki G. Replay and time compression of recurring spike sequences in the hippocampus. Journal of Neuroscience. 1999;19:9497–9507. doi: 10.1523/JNEUROSCI.19-21-09497.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuñez A, Amzica F, Steriade M. Voltage-dependent fast (20–40 Hz) oscillations in long-axoned neocortical neurons [letter] Neuroscience. 1992;51:7–10. doi: 10.1016/0306-4522(92)90464-d. [DOI] [PubMed] [Google Scholar]
- O'Keefe J, Nadel L. The Hippocampus as a Cognitive Map. Clarendon Press; 1978. [Google Scholar]
- O'Keefe J, Recce ML. Phase relationship between hippocampal place units and the EEG theta rhythm. Hippocampus. 1993;3:317–330. doi: 10.1002/hipo.450030307. [DOI] [PubMed] [Google Scholar]
- Pare D, Collins DR. Neuronal correlates of fear in the lateral amygdala: multiple extracellular recordings in conscious cats. Journal of Neuroscience. 2000;20:2701–2710. doi: 10.1523/JNEUROSCI.20-07-02701.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pare D, Gaudreau H. Projection cells and interneurons of the lateral and basolateral amygdala: distinct firing patterns and differential relation to theta and delta rhythms in conscious cats. Journal of Neuroscience. 1996;16:3334–3350. doi: 10.1523/JNEUROSCI.16-10-03334.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penttonen M, Kamondi A, Sik A, Acsady L, Buzsáki G. Feed-forward and feed-back activation of the dentate gyrus in vivo during dentate spikes and sharp wave bursts. Hippocampus. 1997;7:437–450. doi: 10.1002/(SICI)1098-1063(1997)7:4<437::AID-HIPO9>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Petersen CC, Hahn TT, Mehta M, Grinvald A, Sakmann B. Interaction of sensory responses with spontaneous depolarization in layer 2/3 barrel cortex. Proceedings of the National Academy of Sciences of the USA. 2003;100:13638–13643. doi: 10.1073/pnas.2235811100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petsche H, Stumpf C, Gogolak G. The significance of the rabbit's septum as a relay station between the midbrain and the hippocampus. I. The control of hippocampus arousal activity by the septum cells. Electroencephalography and clinical neurophysiology. 1962;14:202–211. doi: 10.1016/0013-4694(62)90030-5. [DOI] [PubMed] [Google Scholar]
- Prut Y, Vaadia E, Bergman H, Haalman I, Slovin H, Abeles M. Spatiotemporal structure of cortical activity: properties and behavioral relevance. Journal of Neurophysiology. 1998;79:2857–2874. doi: 10.1152/jn.1998.79.6.2857. [DOI] [PubMed] [Google Scholar]
- Ribeiro S, Gervasoni D, Soares ES, Zhou Y, Lin SC, Pantoja J, et al. Long-lasting novelty-induced neuronal reverberation during slowwave sleep in multiple forebrain areas. PLoS Biology. 2004;2:E24. doi: 10.1371/journal.pbio.0020024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth M, Shaw J, Green J. The form, voltage distribution and physiological significance of the K-complex. Electroencephalography and clinical neurophysiology. 1956;8:385–402. doi: 10.1016/0013-4694(56)90004-9. [DOI] [PubMed] [Google Scholar]
- Sanchez-Vives MV, McCormick DA. Cellular and network mechanisms of rhythmic recurrent activity in neocortex. Nature Neuroscience. 2000;3:1027–1034. doi: 10.1038/79848. [DOI] [PubMed] [Google Scholar]
- Shu Y, Hasenstaub A, McCormick DA. Turning on and off recurrent balanced cortical activity. Nature. 2003;423:288–293. doi: 10.1038/nature01616. [DOI] [PubMed] [Google Scholar]
- Siapas AG, Lubenov EV, Wilson MA. Prefrontal phase locking to hippocampal theta oscillations. Neuron. 2005;46:141–151. doi: 10.1016/j.neuron.2005.02.028. [DOI] [PubMed] [Google Scholar]
- Siapas AG, Wilson MA. Coordinated interactions between hippocampal ripples and cortical spindles during slow-wave sleep. Neuron. 1998;21:1123–1128. doi: 10.1016/s0896-6273(00)80629-7. [DOI] [PubMed] [Google Scholar]
- Sik A, Ylinen A, Penttonen M, Buzsáki G. Inhibitory CA1-CA3-hilar region feedback in the hippocampus. Science. 1994;265:1722–1724. doi: 10.1126/science.8085161. [DOI] [PubMed] [Google Scholar]
- Singer W. Synchronization of cortical activity and its putative role in information processing and learning. Annual Review of Physiology. 1993;55:349–374. doi: 10.1146/annurev.ph.55.030193.002025. [DOI] [PubMed] [Google Scholar]
- Singer W, Gray CM. Visual feature integration and the temporal correlation hypothesis. Annual Review of Neuroscience. 1995;18:555–586. doi: 10.1146/annurev.ne.18.030195.003011. [DOI] [PubMed] [Google Scholar]
- Sirota A, Csicsvari J, Buhl D, Buzsáki G. Communication between neocortex and hippocampus during sleep in rodents. Proceedings of the National Academy of Sciences of the USA. 2003;100:2065–2069. doi: 10.1073/pnas.0437938100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skaggs WE, McNaughton BL. Replay of neuronal firing sequences in rat hippocampus during sleep following spatial experience. Science. 1996;271:1870–1873. doi: 10.1126/science.271.5257.1870. [DOI] [PubMed] [Google Scholar]
- Skaggs WE, McNaughton BL, Wilson MA, Barnes CA. Theta phase precession in hippocampal neuronal populations and the compression of temporal sequences. Hippocampus. 1996;6:149–172. doi: 10.1002/(SICI)1098-1063(1996)6:2<149::AID-HIPO6>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Soltesz I, Lightowler S, Leresche N, Jassik-Gerschenfeld D, Pollard CE, Crunelli V. Two inward currents and the transformation of low-frequency oscillations of rat and cat thalamocortical cells. Journal of Physiology. 1991;441:175–197. doi: 10.1113/jphysiol.1991.sp018745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somers D, Kopell N. Rapid synchronization through fast threshold modulation. Biological Cybernetics. 1993;68:393–407. doi: 10.1007/BF00198772. [DOI] [PubMed] [Google Scholar]
- Staba RJ, Wilson CL, Bragin A, Jhung D, Fried I, Engel J., Jr. High frequency oscillations recorded in human medial temporal lobe during sleep. Annals of Neurology. 2004;56:108–115. doi: 10.1002/ana.20164. [DOI] [PubMed] [Google Scholar]
- Steriade M. Impact of network activities on neuronal properties in corticothalamic systems. Journal of Neurophysiology. 2001;86:1–39. doi: 10.1152/jn.2001.86.1.1. [DOI] [PubMed] [Google Scholar]
- Steriade M, Amzica F. Slow sleep oscillation, rhythmic K-complexes, and their paroxysmal developments. Journal of Sleep Research. 1998b;7(Suppl 1):30–35. doi: 10.1046/j.1365-2869.7.s1.4.x. [DOI] [PubMed] [Google Scholar]
- Steriade M, Amzica F. Coalescence of sleep rhythms and their chronology in corticothalamic networks. Sleep Research Online. 1998a;1:1–10. [PubMed] [Google Scholar]
- Steriade M, Amzica F, Contreras D. Synchronization of fast (30–40 Hz) spontaneous cortical rhythms during brain activation. Journal of Neuroscience. 1996;16:392–417. doi: 10.1523/JNEUROSCI.16-01-00392.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M, Amzica F, Nuñez A. Cholinergic and noradrenergic modulation of the slow (approximately 0.3 Hz) oscillation in neocortical cells. Journal of Neurophysiology. 1993a;70:1385–1400. doi: 10.1152/jn.1993.70.4.1385. [DOI] [PubMed] [Google Scholar]
- Steriade M, Buzsáki G. Parallel activation of thalamic and cortical neurons by brainstem and basal forebrain cholinergic systems. In: Steriade M, Biesold D, editors. Brain Cholinergic System. Oxford University Press; 1990. pp. 3–64. [Google Scholar]
- Steriade M, Contreras D, Curro DR, Nuñez A. The slow (<1 Hz) oscillation in reticular thalamic and thalamocortical neurons: scenario of sleep rhythm generation in interacting thalamic and neocortical networks. Journal of Neuroscience. 1993b;13:3284–3299. doi: 10.1523/JNEUROSCI.13-08-03284.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M, Curro Dossi R, Contreras D. Electrophysiological properties of intralaminar thalamocortical cells discharging rhythmic (approximately 40 HZ) spikebursts at approximately 1000 HZ during waking and rapid eye movement sleep. Neuroscience. 1993c;56:1–9. doi: 10.1016/0306-4522(93)90556-u. [DOI] [PubMed] [Google Scholar]
- Steriade M, Deschenes M, Domich L, Mulle C. Abolition of spindle oscillations in thalamic neurons disconnected from nucleus reticularis thalami. Journal of Neurophysiology. 1985;54:1473–1497. doi: 10.1152/jn.1985.54.6.1473. [DOI] [PubMed] [Google Scholar]
- Steriade M, Domich L, Oakson G, Deschenes M. The deafferented reticular thalamic nucleus generates spindle rhythmicity. Journal of Neurophysiology. 1987;57:260–273. doi: 10.1152/jn.1987.57.1.260. [DOI] [PubMed] [Google Scholar]
- Steriade M, Dossi RC, Nuñez A. Network modulation of a slow intrinsic oscillation of cat thalamocortical neurons implicated in sleep delta waves: cortically induced synchronization and brainstem cholinergic suppression. Journal of Neuroscience. 1991a;11:3200–3217. doi: 10.1523/JNEUROSCI.11-10-03200.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M, Dossi RC, Pare D, Oakson G. Fast oscillations (20–40 Hz) in thalamocortical systems and their potentiation by mesopontine cholinergic nuclei in the cat. Proceedings of the National Academy of Sciences of the USA. 1991b;88:4396–4400. doi: 10.1073/pnas.88.10.4396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M, McCormick DA, Sejnowski TJ. Thalamocortical oscillations in the sleeping and aroused brain. Science. 1993d;262:679–685. doi: 10.1126/science.8235588. [DOI] [PubMed] [Google Scholar]
- Steriade M, Nuñez A, Amzica F. A novel slow (<1 Hz) oscillation of neocortical neurons in vivo: depolarizing and hyperpolarizing components. Journal of Neuroscience. 1993e;13:3252–3265. doi: 10.1523/JNEUROSCI.13-08-03252.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M, Nuñez A, Amzica F. Intracellular analysis of relations between the slow (<1 Hz) neocortical oscillation and other sleep rhythms of the electroencephalogram. Journal of Neuroscience. 1993f;13:3266–3283. doi: 10.1523/JNEUROSCI.13-08-03266.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M, Timofeev I. Neuronal plasticity in thalamocortical networks during sleep and waking oscillations. Neuron. 2003;37:563–576. doi: 10.1016/s0896-6273(03)00065-5. [DOI] [PubMed] [Google Scholar]
- Steriade M, Timofeev I, Grenier F. Natural waking and sleep states: a view from inside neocortical neurons. Journal of Neurophysiology. 2001;85:1969–1985. doi: 10.1152/jn.2001.85.5.1969. [DOI] [PubMed] [Google Scholar]
- Stern EA, Kincaid AE, Wilson CJ. Spontaneous subthreshold membrane potential fluctuations and action potential variability of rat corticostriatal and striatal neurons in vivo. Journal of Neurophysiology. 1997;77:1697–1715. doi: 10.1152/jn.1997.77.4.1697. [DOI] [PubMed] [Google Scholar]
- Stumpf C. Drug action on the electrical activity of the hippocampus. International Review of Neurobiology. 1965;8:77–138. doi: 10.1016/s0074-7742(08)60756-4. [DOI] [PubMed] [Google Scholar]
- Suzuki WA, Amaral DG. Functional neuroanatomy of the medial temporal lobe memory system. Cortex. 2004;40:220–222. doi: 10.1016/s0010-9452(08)70958-4. [DOI] [PubMed] [Google Scholar]
- Szentagothai J. The Ferrier Lecture, 1977. The neuron network of the cerebral cortex: a functional interpretation. (Series B).Proceedings of the Royal Society of London. 1978;201:219–248. doi: 10.1098/rspb.1978.0043. [DOI] [PubMed] [Google Scholar]
- Timofeev I, Grenier F, Bazhenov M, Sejnowski TJ, Steriade M. Origin of slow cortical oscillations in deafferented cortical slabs. Cerebral Cortex. 2000;10:1185–1199. doi: 10.1093/cercor/10.12.1185. [DOI] [PubMed] [Google Scholar]
- Timofeev I, Steriade M. Low-frequency rhythms in the thalamus of intactcortex and decorticated cats. Journal of Neurophysiology. 1996;76:4152–4168. doi: 10.1152/jn.1996.76.6.4152. [DOI] [PubMed] [Google Scholar]
- Traub RD, Jefferys JG, Whittington MA. Fast Oscillations in Cortical Circuits. MIT Press; 1999. [Google Scholar]
- Traub RD, Spruston N, Soltesz I, Konnerth A, Whittington MA, Jefferys GR. Gamma-frequency oscillations: a neuronal population phenomenon, regulated by synaptic and intrinsic cellular processes, and inducing synaptic plasticity. Progress in Neurobiology. 1998;55:563–575. doi: 10.1016/s0301-0082(98)00020-3. [DOI] [PubMed] [Google Scholar]
- Tsodyks M, Kenet T, Grinvald A, Arieli A. Linking spontaneous activity of single cortical neurons and the underlying functional architecture. Science. 1999;286:1943–1946. doi: 10.1126/science.286.5446.1943. [DOI] [PubMed] [Google Scholar]
- Uchida S, Maehara T, Hirai N, Okubo Y, Shimizu H. Cortical oscillations in human medial temporal lobe during wakefulness and all-night sleep. Brain Research. 2001;891:7–19. doi: 10.1016/s0006-8993(00)03154-1. [DOI] [PubMed] [Google Scholar]
- Vanderwolf CH. Hippocampal electrical activity and voluntary movement in the rat. Electroencephalography and Clinical Neurophysiology. 1969;26:407–418. doi: 10.1016/0013-4694(69)90092-3. [DOI] [PubMed] [Google Scholar]
- Vanderwolf CH, Leung LS. Hippocampal rhythmical slow activity: a brief history and effects of entorhinal lesions and phencycidine. In: Seifer W, editor. The Neurobiology of the Hippocampus. Academic Press; 1983. pp. 275–302. [Google Scholar]
- Vertes RP, Albo Z, Viana DP. Theta-rhythmically firing neurons in the anterior thalamus: implications for mnemonic functions of Papez's circuit. Neuroscience. 2001;104:619–625. doi: 10.1016/s0306-4522(01)00131-2. [DOI] [PubMed] [Google Scholar]
- Vertes RP, Kocsis B. Brainstem-diencephalo-septohippocampal systems controlling the theta rhythm of the hippocampus. Neuroscience. 1997;81:893–926. doi: 10.1016/s0306-4522(97)00239-x. [DOI] [PubMed] [Google Scholar]
- Verzeano M, Negishi K. Neuronal activity in cortical and thalamic networks. The Journal of General Physiology. 1960;43(Suppl):177–195. doi: 10.1085/jgp.43.6.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volgushev M, Chauvette S, Mukovski M, Timofeev I. Precise long-range synchronization of activity and silence in neocortical neurons during slow-wave oscillations. Journal of Neuroscience. 2006;26:5665–5672. doi: 10.1523/JNEUROSCI.0279-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Krosigk M, Bal T, McCormick DA. Cellular mechanisms of a synchronized oscillation in the thalamus. Science. 1993;261:361–364. doi: 10.1126/science.8392750. [DOI] [PubMed] [Google Scholar]
- Wang XJ, Buzsáki G. Gamma oscillation by synaptic inhibition in a hippocampal interneuronal network model. Journal of Neuroscience. 1996;16:6402–6413. doi: 10.1523/JNEUROSCI.16-20-06402.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters J, Helmchen F. Background synaptic activity is sparse in neocortex. Journal of Neuroscience. 2006;26:8267–8277. doi: 10.1523/JNEUROSCI.2152-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittington MA, Jefferys JG, Traub RD. Effects of intravenous anaesthetic agents on fast inhibitory oscillations in the rat hippocampus in vitro. British Journal of Pharmacology. 1996;118:1977–1986. doi: 10.1111/j.1476-5381.1996.tb15633.x. erratum appears in British Journal of Pharmacology 119, 1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson CJ, Chang HT, Kitai ST. Firing patterns and synaptic potentials of identified giant aspiny interneurons in the rat neostriatum. Journal of Neuroscience. 1990;10:508–519. doi: 10.1523/JNEUROSCI.10-02-00508.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson MA, McNaughton BL. Reactivation of hippocampal ensemble memories during sleep. Science. 1994;265:676–679. doi: 10.1126/science.8036517. [DOI] [PubMed] [Google Scholar]
- Witter MP. Organization of the entorhinal-hippocampal system: a review of current anatomical data. Hippocampus. 1993;3:33–44. [PubMed] [Google Scholar]
- Wolansky T, Clement EA, Peters SR, Palczak MA, Dickson CT. Hippocampal slow oscillation: a novel EEG state and its coordination with ongoing neocortical activity. Journal of Neuroscience. 2006;26:6213–6229. doi: 10.1523/JNEUROSCI.5594-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ylinen A, Bragin A, Nadasdy Z, Jando G, Szabo I, Sik A, et al. Sharp wave-associated high-frequency oscillation (200 Hz) in the intact hippocampus: network and intracellular mechanisms. Journal of Neuroscience. 1995a;15:30–46. doi: 10.1523/JNEUROSCI.15-01-00030.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ylinen A, Soltesz I, Bragin A, Penttonen M, Sik A, Buzsáki G. Intracellular correlates of hippocampal theta rhythm in identified pyramidal cells, granule cells, and basket cells. Hippocampus. 1995b;5:78–90. doi: 10.1002/hipo.450050110. [DOI] [PubMed] [Google Scholar]


