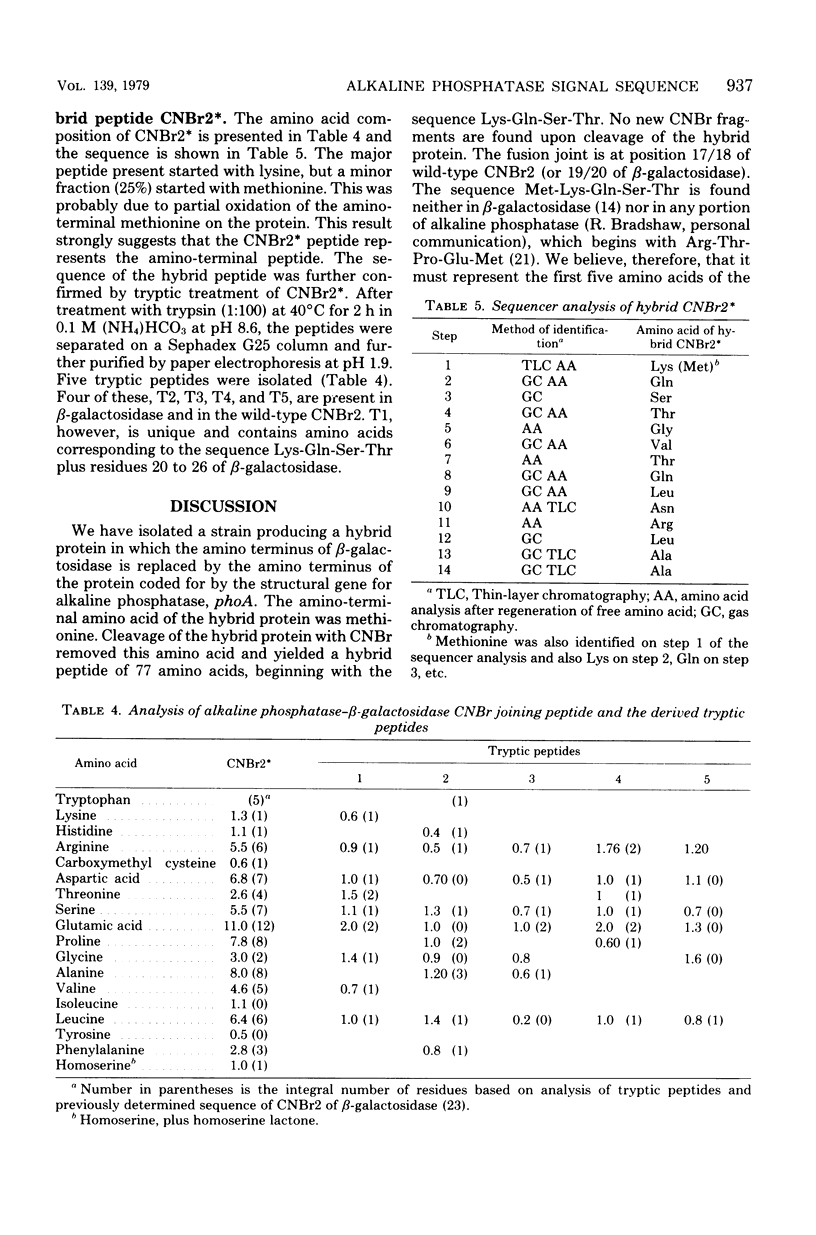
Abstract
We have isolated strains of Escherichia coli in which an amino-terminal portion of the cytoplasmic enzyme beta-galactosidase is replaced by an amino-terminal portion of the periplasmic enzyme alkaline phosphatase. The synthesis of these hybrid proteins is regulated by inorganic phosphate and they are located in the cytoplasm. One of these proteins was purified, and 14 amino acids of the amino-terminal sequence were determined. The first five amino acids, Met-Lys-Gln-Ser-Thr, appear to represent a portion of the signal sequence of the precursor of alkaline phosphatase, and the remaining sequence corresponds to that of beta-galactosidase, beginning at amino acid residue 20. The approach described here could be used for the analysis of signal sequences of exported proteins and for partial amino acid sequence determination of certain of certain other proteins.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ambler R. P., Scott G. K. Partial amino acid sequence of penicillinase coded by Escherichia coli plasmid R6K. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3732–3736. doi: 10.1073/pnas.75.8.3732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambler R. P., Scott G. K. Partial amino acid sequence of penicillinase coded by Escherichia coli plasmid R6K. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3732–3736. doi: 10.1073/pnas.75.8.3732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann B. J., Low K. B., Taylor A. L. Recalibrated linkage map of Escherichia coli K-12. Bacteriol Rev. 1976 Mar;40(1):116–167. doi: 10.1128/br.40.1.116-167.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassford P., Beckwith J. Escherichia coli mutants accumulating the precursor of a secreted protein in the cytoplasm. Nature. 1979 Feb 15;277(5697):538–541. doi: 10.1038/277538a0. [DOI] [PubMed] [Google Scholar]
- Blobel G., Dobberstein B. Transfer of proteins across membranes. I. Presence of proteolytically processed and unprocessed nascent immunoglobulin light chains on membrane-bound ribosomes of murine myeloma. J Cell Biol. 1975 Dec;67(3):835–851. doi: 10.1083/jcb.67.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brake A. J., Celada F., Fowler A. V., Zabin I. An immunochemical aid to sequence determination of proteins. Anal Biochem. 1977 May 15;80(1):108–115. doi: 10.1016/0003-2697(77)90630-3. [DOI] [PubMed] [Google Scholar]
- Brake A. J., Fowler A. V., Zabin I., Kania J., Müller-Hill B. beta-Galactosidase chimeras: primary structure of a lac repressor-beta-galactosidase protein. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4824–4827. doi: 10.1073/pnas.75.10.4824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadaban M. J. Transposition and fusion of the lac genes to selected promoters in Escherichia coli using bacteriophage lambda and Mu. J Mol Biol. 1976 Jul 5;104(3):541–555. doi: 10.1016/0022-2836(76)90119-4. [DOI] [PubMed] [Google Scholar]
- Fowler A. V. Amino acid sequence of beta-galactosidase. VII. Isolation of the 24 cyanogen bromide peptides. J Biol Chem. 1978 Aug 10;253(15):5499–5504. [PubMed] [Google Scholar]
- Fowler A. V. High-level production of -galactosidase by Escherichia coli merodiploids. J Bacteriol. 1972 Nov;112(2):856–860. doi: 10.1128/jb.112.2.856-860.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler A. V., Zabin I. Amino acid sequence of beta-galactosidase. XI. Peptide ordering procedures and the complete sequence. J Biol Chem. 1978 Aug 10;253(15):5521–5525. [PubMed] [Google Scholar]
- Goldberg A. L., St John A. C. Intracellular protein degradation in mammalian and bacterial cells: Part 2. Annu Rev Biochem. 1976;45:747–803. doi: 10.1146/annurev.bi.45.070176.003531. [DOI] [PubMed] [Google Scholar]
- HORIUCHI T., TOMIZAWA J. I., NOVICK A. Isolation and properties of bacteria capable of high rates of beta-galactosidase synthesis. Biochim Biophys Acta. 1962 Jan 22;55:152–163. doi: 10.1016/0006-3002(62)90941-1. [DOI] [PubMed] [Google Scholar]
- Hardy S. J., Randall L. L. Position of the extra amino acid sequence in the precursor arabinose-binding protein of Escherichia coli. J Bacteriol. 1978 Jul;135(1):291–293. doi: 10.1128/jb.135.1.291-293.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inouye H., Beckwith J. Synthesis and processing of an Escherichia coli alkaline phosphatase precursor in vitro. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1440–1444. doi: 10.1073/pnas.74.4.1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inouye S., Wang S., Sekizawa J., Halegoua S., Inouye M. Amino acid sequence for the peptide extension on the prolipoprotein of the Escherichia coli outer membrane. Proc Natl Acad Sci U S A. 1977 Mar;74(3):1004–1008. doi: 10.1073/pnas.74.3.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley P. M., Neumann P. A., Shriefer K., Cancedda F., Schlesinger M. J., Bradshaw R. A. Amino acid sequence of Escherichia coli alkaline phosphatase. Amino- and carboxyl-terminal sequences and variations between two isozymes. Biochemistry. 1973 Aug 28;12(18):3499–3503. doi: 10.1021/bi00742a023. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Langley K. E., Fowler A. V., Zabin I. Amino acid sequence of beta-galactosidase. IV. Sequence of an alpha-complementing cyanogen bromide peptide, residues 3 to 92. J Biol Chem. 1975 Apr 10;250(7):2587–2592. [PubMed] [Google Scholar]
- Lin S., Zabin I. Beta-galactosidase. Rates of synthesis and degradation of incomplete chains. J Biol Chem. 1972 Apr 10;247(7):2205–2211. [PubMed] [Google Scholar]
- PLOCKE D. J., LEVINTHAL C., VALLEE B. L. Alkaline phosphatase of Escherichia coli: a zinc metalloenzyme. Biochemistry. 1962 May 25;1:373–378. doi: 10.1021/bi00909a001. [DOI] [PubMed] [Google Scholar]
- Pagès J. M., Piovant M., Varenne S., Lazdunski C. Mechanistic aspects of the transfer of nascent periplasmic proteins across the cytoplasmic membrane in Escherichia coli. Eur J Biochem. 1978 May 16;86(2):589–602. doi: 10.1111/j.1432-1033.1978.tb12343.x. [DOI] [PubMed] [Google Scholar]
- Pratt C., Gallant J. A dominant constitutive phoR mutation in Escherichia coli. Genetics. 1972 Oct;72(2):217–226. doi: 10.1093/genetics/72.2.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall L. L., Hardy S. J. Synthesis of exported proteins by membrane-bound polysomes from Escherichia coli. Eur J Biochem. 1977 May 2;75(1):43–53. doi: 10.1111/j.1432-1033.1977.tb11502.x. [DOI] [PubMed] [Google Scholar]
- Silberstein S., Inouye M. Studies on the role of bacteriophage T7 lysozyme during phage infection. J Mol Biol. 1975 Jul 25;96(1):1–11. doi: 10.1016/0022-2836(75)90178-3. [DOI] [PubMed] [Google Scholar]
- Silhavy T. J., Shuman H. A., Beckwith J., Schwartz M. Use of gene fusions to study outer membrane protein localization in Escherichia coli. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5411–5415. doi: 10.1073/pnas.74.12.5411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith W. P., Tai P. C., Thompson R. C., Davis B. D. Extracellular labeling of nascent polypeptides traversing the membrane of Escherichia coli. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2830–2834. doi: 10.1073/pnas.74.7.2830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimoto K., Sugisaki H., Okamoto T., Takanami M. Studies on bacteriophage fd DNA. IV. The sequence of messenger RNA for the major coat protein gene. J Mol Biol. 1977 Apr 25;111(4):487–507. doi: 10.1016/s0022-2836(77)80065-x. [DOI] [PubMed] [Google Scholar]
- TORRIANI A. Influence of inorganic phosphate in the formation of phosphatases by Escherichia coli. Biochim Biophys Acta. 1960 Mar 11;38:460–469. doi: 10.1016/0006-3002(60)91281-6. [DOI] [PubMed] [Google Scholar]
- Yamada S., Itano H. Phenanthrenequinone as an analytical reagent for arginine and other monosubstituted guanidines. Biochim Biophys Acta. 1966 Dec 28;130(2):538–540. doi: 10.1016/0304-4165(66)90256-x. [DOI] [PubMed] [Google Scholar]
- Zabin I., Fowler A. V., Beckwith J. R. Position of the mutation in beta-galactosidase ochre mutant U118. J Bacteriol. 1978 Jan;133(1):437–438. doi: 10.1128/jb.133.1.437-438.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]



