Abstract
A mutant of Mycobacterium tuberculosis (Δ19) lacking the 19kDa lipoprotein grows well in culture in vitro but was shown here to be essentially incapable of any significant replication in mice, even in mice lacking the gamma interferon gene. Despite this, mice inoculated with Δ19 were equally protected against an aerosol delivered challenge with M. tuberculosis compared to the conventional BCG vaccine. Cellular responses, including the generation of activated CD4 and CD8 cells secreting gamma interferon, were produced in similar numbers, and lung cells, particularly dendritic macrophages, exhibited high levels of Class-II MHC expression. These data show that despite being highly attenuated, the Δ19 mutant strongly retained vaccinogenic properties.
Keywords: 19kDa-lipoprotein, tuberculosis, mouse model, vaccination
The expression of TH1 immunity to tuberculosis infection has recently been shown to be initially driven by the interaction between lipid containing mycobacterial products and Toll-like receptors [pattern recognition molecules] on the surface of the engulfing macrophage [1–6]. A primary example is the 19kDa lipoprotein (Rv3763; LpqH) of M. tuberculosis, which interacts with the Toll-like receptor 2 (TLR2) inducing the production of IL-12 p40 when added to macrophages [3], thus initiating the subsequent production of IFNγ by sensitized T cells. However, the signaling effects of the interaction between the 19kDa lipoprotein and the host cell are complex, and prolonged exposure of cells has negative effects [7–10]. This results in inhibition of the macrophage expression of a subset of IFNγ-induced genes, including CIITA that regulates the expression of MHC class II (MHC-II), interfering with subsequent antigen presentation [9,11].
The development of a mutant of M. tuberculosis lacking the 19kDa lipoprotein [Δ19] has allowed investigation of these mechanisms further [12]. Cells infected with this mutant in vitro did not show any reduction in surface expression of MHC Class-II, but IL-1β secretion was lower [12]. In the present report, we extended these studies to in vivo experiments. These showed that expression of the 19kDa lipoprotein is essential to replication of the organism in the lungs of mice, both normal and immunocompromised, and that without it the bacilli persisted as a low grade chronic infection. Interestingly, despite this, mice inoculated with Δ19 had similar resistance to aerosol challenge infection to that engendered by BCG. Fewer CD4 and CD8, including IFNγ secreting cells, were needed to control the lung infections compared to controls. Finally, analysis of lung macrophage populations indicated far higher expression of Class-II MHC in Δ19 vaccinated mice compared to control mice.
1. Methods and materials
1.1 Animals
Specific pathogen-free female, 6–8-week-old, C57BL/6 (resistant) and CBA/J (susceptible) mice from Jackson laboratories, Bar Harbor, ME. They were kept under barrier conditions in an ABL-III laboratory and fed sterile water and chow.
1.2. Aerosol infection with M. tuberculosis
M. tuberculosis strain H37Rv, H37Rv-Δ19(19kDa−/−), H37Rv-Δ19 complemented, and the BCG Pasteur vaccine strain were grown in Proskauer-Beck liquid medium containing 0.05% Tween-80 to mid-log phase and then frozen in aliquots at −70°C until needed. For low-dose aerosol infections, bacterial stocks were diluted in 5 ml of sterile distilled water to 2×106 colony-forming units/ml and placed in a nebulizer attached to an airborne infection system (Glass-Col, Terre Haute, IN). Mice were exposed to 40 min of aerosol where approximately 100 bacteria were deposited in the lungs of each animal. Bacterial load was determined by plating right lobe homogenates onto nutrient 7H11 agar plates supplemented with OADC. Colonies were counted after 21 days of incubation at 37°C. For the vaccination studies, 106 CFU of H37Rv-Δ19 or BCG Pasteur were injected subcutaneously. Four weeks afterwards mice were challenged by low-dose aerosol exposure with H37Rv as above.
1.3. Lung cell digestion
Mice were euthanized by CO2 asphyxiation, and the thoracic cavity was opened. The lung was cleared of blood by perfusion through the pulmonary artery with 10 ml of ice cold phosphate-buffered saline (PBS) containing 50 U/ml of heparin (Sigma, St Louis, MO). Lungs were aseptically removed, teased apart and treated with a solution of deoxyribonuclease IV (DNAse) (Sigma Chemical, 30 μg/ml) and collagenase XI (Sigma Chemical, 0.7 mg/ml) for 45 min at 37°C. To obtain a single-cell suspension, the organs were gently passed through cell strainers (Becton Dickinson, Labware, Lincoln Park, NJ). The remaining erythrocytes were lysed with Gey’s solution (0.15 M NH4Cl, 10 mM HCO3) and the cells were washed with Dulbecco’s modified Eagle’s minimal essential medium. Total cell numbers were determined by using a Neubauer chamber.
1.4. Flow Cytometry for surface markers and intracellular cytokines
For flow cytometry analysis, single-cell suspensions of lung from each mice were re-suspended in PBS (Sigma-Aldrich) containing 0.1% of Sodium Azide (PBS+Na/Az). Cells were incubated in the dark for 25 min at 4°C with specific antibody (directly conjugated to fluorescein isothiocyanate (FITC), phycoerythrin (PE), peridin-Cholorophyll-protein (PerCP) or allophycocyanin (APC), at 3μg/ml), followed by two washes in PBS containing 4% sodium azide. Cell surface markers were analyzed with the following specific antibodies: FITC anti-CD44 (clone 7D4), FITC anti-MHC-II (clone AF6-120.1 and 2G9), PE anti-CD25(clone 3C7), PE anti-GR.1 (cloneRB6-8C5), PerCP anti-CD4 (clone RM4-5), PerCP anti-CD8 (clone 53-6.7), PerCP anti-CD11b (clone M1/70), APC antiCD11c (clone HL3) and APC anti-CD62L (clone MEL-14) all antibodies from BD Pharmingen. Measurement of intracellular IFN-γ was conducted by pre-incubating lung cells with monensin (3 μM) (Golgi stop, BD Pharmingen), anti-CD3 and anti-CD28 (both at 0.2 μg/106 cells) for 4 h at 37°C, 5% CO2. For TNFα staining, lung cells were pre-incubated with monensin (3 μM), and 5 μg/ml of LPS (Sigma-Aldrich, San Louis, MO) for 6 hr at 37°C, 5% CO2. The cells were then surface stained, washed and then fixed and permeabilized with Perm Fix/Perm Wash (BD Pharmingen). Finally, the cells were stained for intracellular anti-IFNγ (clone XMG1.2) or anti-TNFα (clone MP6-XT22) or its respective isotype controls (BD Pharmingen) for a further 30 min. All the samples were analyzed on a Becton Dickinson FACScalibur and data were analyzed using CELLQUEST (Becton Dickinson Immunocytometry Systems) or Summit software (DakoCytomation). Cells were gated on lymphocytes or based on characteristic forward and side scatter profiles. Individual cell populations were identified according to their presence of specific fluorescent-labeled antibody. All the analyses were performed with an acquisition of 250000 events.
1.5. Statistics
Statistical significance was determined using two-way ANOVA with Bonferroni post tests using GraphPad Prism 4.00 for Windows, GraphPad Software (San Diego CA).
2. Results
2.1. The Δ19 mutant is highly attenuated
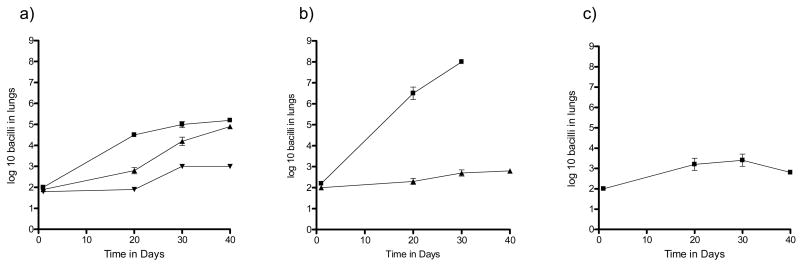
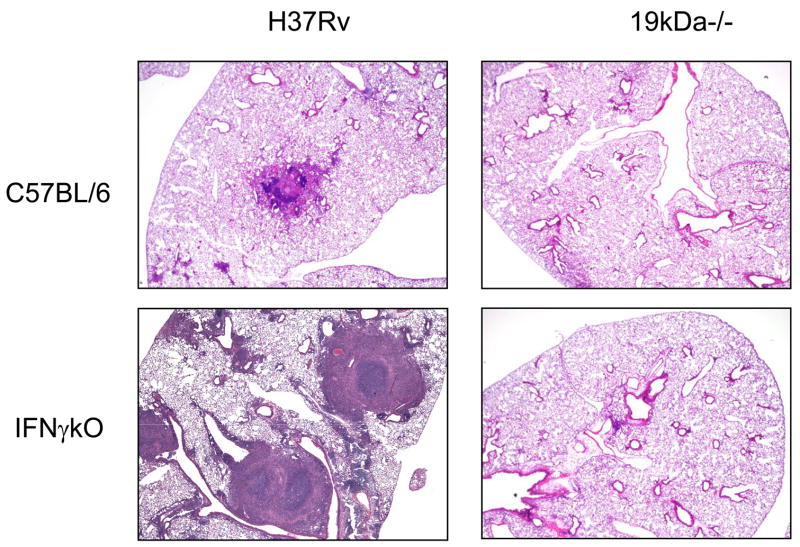
In a first experiment the Δ19 mutant was compared to wild type H37Rv and an H37Rv-Δ19 complemented strain for its ability to grow in normal C57BL/6 mice. As shown in Fig. 1a, the Δ19 mutant showed only minimal growth in the C57BL/6 mice whereas H37Rv grew approximately 3 logs, and while the H37Rv-Δ19 complemented strain grew more slowly it reached similar levels by day 40. There was no statistical difference between the growth of H37Rv and the complemented strain at day 40 (P=0.08). In mice lacking IFNγ [GKO mice] H37Rv grew progressively, killing them in 30–40 days, whereas the Δ19 showed only a slight increase over this time (Fig. 1b). In fact, in both experiments the Δ19 grew comparably to BCG given by aerosol (Fig. 1c). This was further illustrated by histological examination of the infected lungs (Fig. 2). In mice infected with H37Rv granulomatous inflammation was seen as expected, whereas in the GKO necrotizing lesions consolidated much of the lung tissues. In the mice infected with Δ19, only minimal, scarce, cellular infiltrations were seen, even in the GKO mice.
Figure 1.
a) Growth of M. tuberculosis H37Rv [squares], H37Rv Δ19 complemented [up triangles] and H37Rv Δ19 [down triangles] in C57BL/6 mice. b) Growth of M. tuberculosis H37Rv [squares] and H37Rv Δ19 [triangles] in IFNγ gene disrupted mice after low dose aerosol infection. c) Growth of BCG after low dose aerosol in C57BL/6 shown for comparative purposes. The bacterial load was calculated by plating lung homogenates onto 7H11 agar at 37°C and counting colonies 21 days later. The data are expressed as the mean ± the standard error from five mice per group. Each graph is representative of two independent experiments.
Figure 2.
Representative photomicrographs of hematoxylin and eosin stained, formalin fixed lungs from C57BL/6 or IFNγ gene knockout mice infected by aerosol one month earlier.
2.2. Mice vaccinated with Δ19 are as well protected as mice vaccinated with BCG
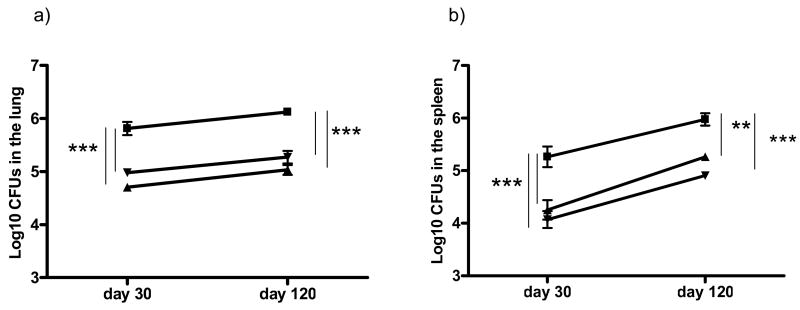
Given the very poor growth of the Δ19, as well as the negligible inflammatory response to it, it seemed unlikely that this organism was capable of inducing immunity in vivo. However, to verify this, mice were vaccinated with 106 CFU of Δ19, and compared to BCG vaccinated mice. As shown in Fig. 3, the lungs and spleen of the different groups were removed and plated 30 or 120 days later. Surprisingly, vaccination with Δ19 gave similar and sustained protection to that given by BCG.
Figure 3.
The bacterial load in the lungs [a] and spleens [b] of saline controls [squares], compared to BCG vaccinated [triangles] or Δ19 vaccinated [circles] mice 30 or 120 days after low dose aerosol challenge. The data are expressed as the mean ± the standard error of the mean from five mice per group. ** P<0.01 and *** P<0.001. The graph is representative of five mice per group and two independent experiments. Statistical significant differences between
Given the protection results, the histological data was as expected [data not shown], but with one major difference. Foamy macrophages are a characteristic of tuberculous granulomas and are interesting because they express dendritic cell markers [13]. These were seen in large numbers in the lungs of the BCG vaccinated mice, but not in the Δ19 vaccinated mice. Instead, in the latter, granulomas were highly lymphocytic, as shown by morphological and histological analysis.
2.3. The T cell response was similar in the Δ19 and BCG vaccinated mice
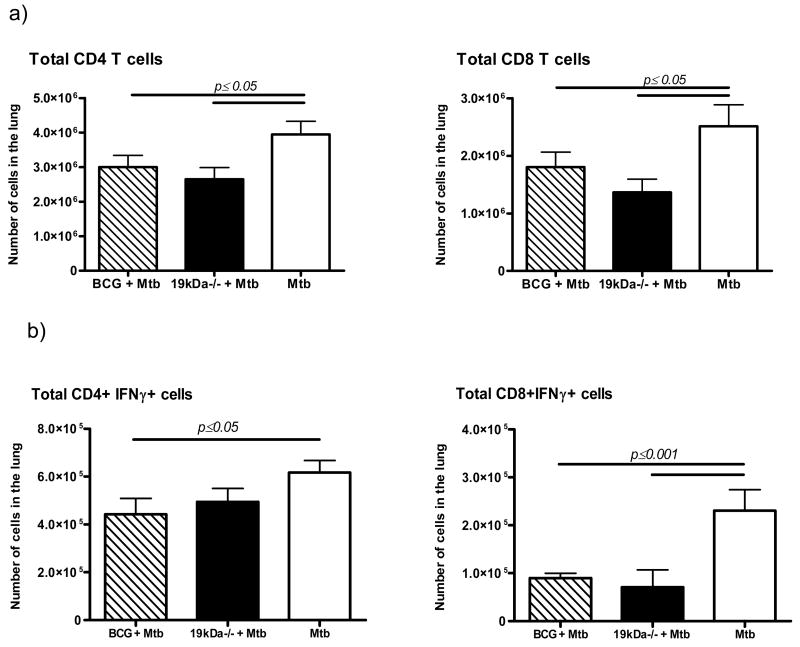
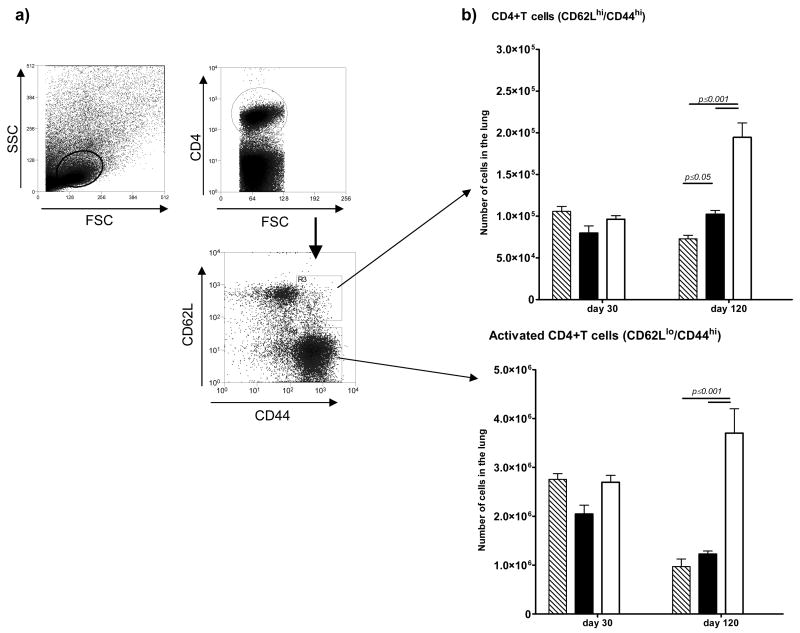
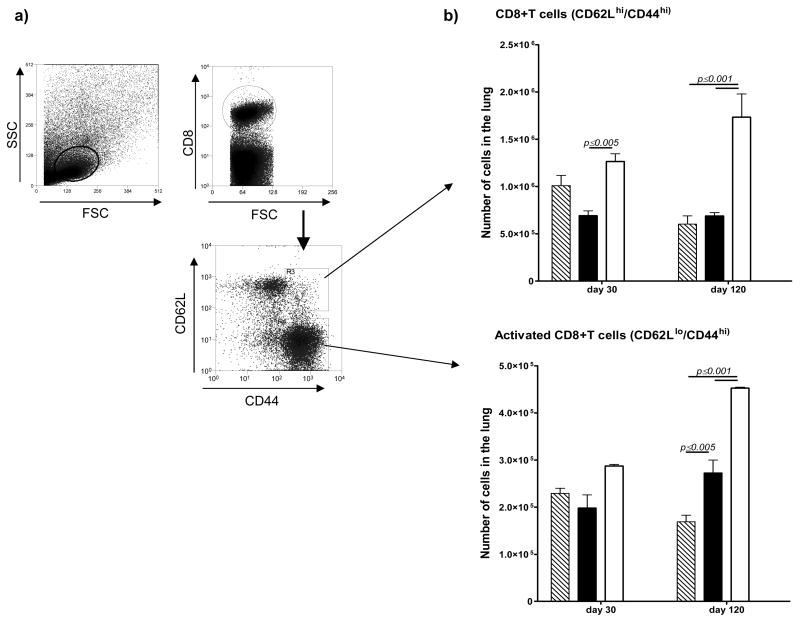
Immune animals need to focus less T cells into infected lesions to control the infection. As shown in Fig. 4 this was observed in both groups of vaccinated mice, and applied to both IFNγ-secreting CD4 and CD8 T cells. Further analysis [Fig. 5] separating activated [CD62Llo/CD44hi] and central memory [CD62Lhi/CD44hi] CD4 cells showed reductions in the BCG and Δ19 vaccinated populations by day 120, with significantly higher activated cells remaining in the unprotected controls. Similar observations were made regarding the CD8 T cell response [Fig. 6]. Surprisingly in this case, the activated CD8 T cells (CD62lo/CD44hi) remained increased in the Δ19 vaccinated group at day 120 after infection.
Figure 4.
Total CD4 and CD8 T cells numbers [a], and numbers of IFNγ secreting T cell [b] subsets entering the lungs four weeks after aerosol challenge. The data are expressed as the mean ± the standard error of the mean from five mice per group. Statistical significance between marker expression on lung T cells between the different groups was calculated using two-way ANOVA. Results are representative of two independent experiments.
Figure 5.
Numbers of CD4 T cell subsets harvested from the lungs 30 or 120 days after aerosol challenge infection. Mice vaccinated with BCG [hatched bars] or with Δ19 [filled] were compared to control mice [open]. After appropriate gating [a] CD4 cells in the lungs were analyzed for [b] CD62Lhi/CD44hi central memory cells or CD62Llo/CD44lo activated cells. Significance differences between marker expression was calculated using two-way ANOVA. Results are representative of two independent experiments.
Figure 6.
Numbers of CD8 T cell subsets harvested from the lungs 30 or 120 days after aerosol challenge infection. Mice vaccinated with BCG [hatched bars] or with Δ19 [filled] were compared to control mice [open]. After appropriate gating [a] CD8 cells in the lungs were analyzed for [b] CD62Lhi central memory cells or CD62Llo activated cells. Significance differences between marker expression was calculated using two-way ANOVA. Results are representative or two independent experiments.
2.4. MHC class II expression was higher on macrophages in the vaccinated groups
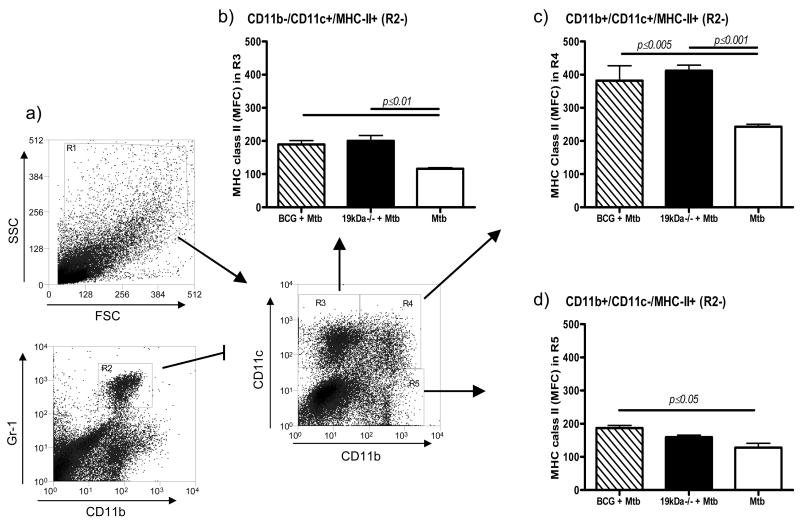
We used CD11b and CD11c expression on lung cells to identify macrophage subsets. To analyze the response of these macrophage subset, in vaccinated mice, we assessed their ability to express MHC Class-II molecules [Fig. 7]. The MHC-II fluorescence signal from alveolar macrophages CD11b−/CD11c+ (region 3) was modest, but increased in the vaccinated animals. It was also marginally higher in the CD11b+/CD11c− positive cells (region 5), which contains small macrophages and some monocytes. In contrast, CD11b+/CD11c+ positive cells (region 4), which are mainly dendritic cells, showed a large increase in MHC-II expression compared to infected controls.
Figure 7.
MHC Class-II expression on macrophages subsets in the lungs one month after aerosol challenge infection. Analysis was made based on CD11b and CD11c staining after Gr-1hi CD11bhi granulocytes were gated out. Data shows MFC levels of Class-II expression on CD11b−/CD11c+ cells (region 3) [alveolar macrophages], CD11b+/CD11c+ cells (region 4) [rich in mature dendritic macrophages], and CD11b+/CD11c− cells (region 5) [mostly small macrophages and monocytes]. Statistical significance between MFC was calculated using two-way ANOVA. Results are representative or two independent experiments.
3. Discussion
The results of this study show that loss of the ability to express the 19kDa-lipoprotein renders M. tuberculosis unable to replicate in vivo to any extent. Despite this it still remained immunogenic, generating IFNγ secreting CD4 and CD8 T cells, and protecting mice from low dose aerosol challenge infection to a similar degree to the BCG vaccine. Given that the Δ19 mutant grew perfectly well in culture media (data not shown), this seems to suggest that the 19kDa-lipoprotein plays an important role in the host cell phagosome, enabling pathogen cell division. This mutant thus falls into the category of live attenuated vaccines, several of which have been evaluated in animal models [14].
It was interesting to note that the inflammatory response in the lungs to the Δ19 was minimal, and in fact there is evidence that the 19kDa-lipoprotein is a major mediator of neutrophil activation, including down-regulation of CD62L (L-selectin) and up-regulation of CD35 (CR1) and CD11b/CD18 (CR3, Mac-1) [15]. In addition, exposure of neutrophils to MTB 19-kDa lipoprotein enhanced a subsequent oxidative burst [15]. Exposure of macrophages to this protein may also induce cell death, allowing escape and dissemination [16].
Results from the two vaccines tested in parallel were very similar, but one interesting observation was that BCG vaccinated mice had lung granulomas containing large numbers of vacuolated foamy macrophages after challenge infection, whereas the Δ19 vaccinated mice had granulomas that were much more lymphocytic. The foamy cells are a well known characteristic of tuberculosis granulomas, and are of interest because they express both DEC-205, a dendritic cell marker, as well anti-apoptotic TRAF markers [13]. Why these were not frequent in the granulomas of Δ19 vaccinated mice is unknown.
In this regard, dendritic macrophages were the population with the highest levels of MHC Class-II expression after challenge. This is in keeping with the hypothesis that both the Δ19 and BCG vaccines enabled the immune mice to more rapidly limit the growth of the challenge infection, thus reducing the levels of the 19kDa lipoprotein produced by the challenge organism and thus facilitating the ability of the host cells to present antigens using Class-II molecules without interference from this antigen.
An alternative approach to try to understand the role of the 19kDa lipoprotein has been to over-express it. It has been known for some time that over-expression in the saprophyte M. vaccae interferes with its vaccine properties [18]. When over-expressed in BCG, different results have been reported, with one laboratory showing enhanced IFNγ production but also increased TH2 cytokines, coupled with a failure of the rBCG to protect guinea pigs [19], although more recently it was found that over-expression or deletion of the lipoprotein in BCG did not affect its protective capacity [20].
These results have applications to new tuberculosis vaccine design [14]. In addition to creating considerable attenuation of M. tuberculosis, removal of the lipoprotein may also improve vaccinogenic properties in terms of reducing potential interference with antigen processing mechanisms in the host cell [8]. In addition, these results indicate that only minimal bacterial growth is needed to generate some degree of protective immunity in the lungs, given that the growth of the Δ19 mutant was fairly similar to that of BCG given by aerosol exposure shown here. This is obviously consistent with current experience with several other auxotrophic or live attenuated mutants of M. tuberculosis indicating that only limited exposure to these organisms seems sufficient to generate protective immunity, at least to the extent that this can be detected in these short term protection assays, making these attractive for future rational vaccine design.
Acknowledgments
This work was supported by grants AI-40488 and AI-44072 from the NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Beutler B. Toll-like receptors: how they work and what they do. Curr Opin Hematol. 2002;9(1):2–10. doi: 10.1097/00062752-200201000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Beutler B, Hoebe K, Du X, Ulevitch RJ. How we detect microbes and respond to them: the Toll-like receptors and their transducers. J Leukoc Biol. 2003;74(4):479–485. doi: 10.1189/jlb.0203082. [DOI] [PubMed] [Google Scholar]
- 3.Brightbill HD, Libraty DH, Krutzik SR, et al. Host defense mechanisms triggered by microbial lipoproteins through toll-like receptors. Science. 1999;285(5428):732–736. doi: 10.1126/science.285.5428.732. [DOI] [PubMed] [Google Scholar]
- 4.Brightbill HD, Modlin RL. Toll-like receptors: molecular mechanisms of the mammalian immune response. Immunology. 2000;101(1):1–10. doi: 10.1046/j.1365-2567.2000.00093.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Basu S, Fenton MJ. Toll-like receptors: function and roles in lung disease. Am J Physiol Lung Cell Mol Physiol. 2004;286(5):L887–892. doi: 10.1152/ajplung.00323.2003. [DOI] [PubMed] [Google Scholar]
- 6.Vogel SN, Fitzgerald KA, Fenton MJ. TLRs: differential adapter utilization by toll-like receptors mediates TLR-specific patterns of gene expression. Mol Interv. 2003;3(8):466–477. doi: 10.1124/mi.3.8.466. [DOI] [PubMed] [Google Scholar]
- 7.Pennini ME, Pai RK, Schultz DC, Boom WH, Harding CV. Mycobacterium tuberculosis 19-kDa lipoprotein inhibits IFN-gamma-induced chromatin remodeling of MHC2TA by TLR2 and MAPK signaling. J Immunol. 2006;176(7):4323–4330. doi: 10.4049/jimmunol.176.7.4323. [DOI] [PubMed] [Google Scholar]
- 8.Pai RK, Pennini ME, Tobian AA, Canaday DH, Boom WH, Harding CV. Prolonged toll-like receptor signaling by Mycobacterium tuberculosis and its 19-kilodalton lipoprotein inhibits gamma interferon-induced regulation of selected genes in macrophages. Infect Immun. 2004;72(11):6603–6614. doi: 10.1128/IAI.72.11.6603-6614.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gehring AJ, Rojas RE, Canaday DH, Lakey DL, Harding CV, Boom WH. The Mycobacterium tuberculosis 19-kilodalton lipoprotein inhibits gamma interferon-regulated HLA-DR and Fc gamma R1 on human macrophages through Toll-like receptor 2. Infect Immun. 2003;71(8):4487–4497. doi: 10.1128/IAI.71.8.4487-4497.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Noss EH, Harding CV, Boom WH. Mycobacterium tuberculosis inhibits MHC class II antigen processing in murine bone marrow macrophages. Cell Immunol. 2000;201(1):63–74. doi: 10.1006/cimm.2000.1633. [DOI] [PubMed] [Google Scholar]
- 11.Pai RK, Convery M, Hamilton TA, Boom WH, Harding CV. Inhibition of IFN-gamma-induced class II transactivator expression by a 19-kDa lipoprotein from Mycobacterium tuberculosis: a potential mechanism for immune evasion. J Immunol. 2003;171(1):175–184. doi: 10.4049/jimmunol.171.1.175. [DOI] [PubMed] [Google Scholar]
- 12.Stewart GR, Wilkinson KA, Newton SM, et al. Effect of deletion or overexpression of the 19-kilodalton lipoprotein Rv3763 on the innate response to Mycobacterium tuberculosis. Infect Immun. 2005;73(10):6831–6837. doi: 10.1128/IAI.73.10.6831-6837.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ordway D, Henao-Tamayo M, Orme IM, Gonzalez-Juarrero M. Foamy macrophages within lung granulomas of mice infected with Mycobacterium tuberculosis express molecules characteristic of dendritic cells and antiapoptotic markers of the TNF receptor-associated factor family. J Immunol. 2005;175(6):3873–3881. doi: 10.4049/jimmunol.175.6.3873. [DOI] [PubMed] [Google Scholar]
- 14.Orme IM. Preclinical testing of new vaccines for tuberculosis: a comprehensive review. Vaccine. 2006;24(1):2–19. doi: 10.1016/j.vaccine.2005.07.078. [DOI] [PubMed] [Google Scholar]
- 15.Neufert C, Pai RK, Noss EH, Berger M, Boom WH, Harding CV. Mycobacterium tuberculosis 19-kDa lipoprotein promotes neutrophil activation. J Immunol. 2001;167(3):1542–1549. doi: 10.4049/jimmunol.167.3.1542. [DOI] [PubMed] [Google Scholar]
- 16.Ciaramella A, Cavone A, Santucci MB, et al. Induction of apoptosis and release of interleukin-1 beta by cell wall-associated 19-kDa lipoprotein during the course of mycobacterial infection. J Infect Dis. 2004;190(6):1167–1176. doi: 10.1086/423850. [DOI] [PubMed] [Google Scholar]
- 17.Abou-Zeid C, Gares MP, Inwald J, et al. Induction of a type 1 immune response to a recombinant antigen from Mycobacterium tuberculosis expressed in Mycobacterium vaccae. Infect Immun. 1997;65(5):1856–1862. doi: 10.1128/iai.65.5.1856-1862.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rao V, Dhar N, Shakila H, et al. Increased expression of Mycobacterium tuberculosis 19 kDa lipoprotein obliterates the protective efficacy of BCG by polarizing host immune responses to the Th2 subtype. Scand J Immunol. 2005;61(5):410–417. doi: 10.1111/j.1365-3083.2005.01569.x. [DOI] [PubMed] [Google Scholar]
- 19.Yeremeev VV, Stewart GR, Neyrolles O, et al. Deletion of the 19kDa antigen does not alter the protective efficacy of BCG. Tuber Lung Dis. 2000;80(6):243–247. doi: 10.1054/tuld.2000.0252. [DOI] [PubMed] [Google Scholar]