Abstract
Endometrial cancer is the most common gynecological malignancy in the US, however, its underlying molecular mechanisms are poorly understood and few prognostic indicators have been identified. The Protein Kinase C (PKC) family have been shown to regulate pathways critical to malignant transformation, and in endometrial tumors, changes in PKC expression and activity have been linked to a more aggressive phenotype and poor prognosis. We have recently shown that PKCδ is a critical regulator of apoptosis and cell survival in endometrial cancer cells; however, PKCδ levels in endometrial tumors had not been determined. We used immunohistochemistry to examine PKCδ protein levels in normal endometrium and endometrioid carcinomas of increasing grade. Normal endometrium exhibited abundant nuclear and cytoplasmic staining of PKCδ, confined to glandular epithelium. In endometrial tumors, decreased PKCδ expression, both in intensity and fraction of epithelial cells stained, was observed with increasing tumor grade, with PKCδ being preferentially lost from the nucleus. Consistent with these observations, endometrial cancer cell lines derived from poorly differentiated tumors exhibited reduced PKCδ levels relative to well-differentiated lines. Treatment of endometrial cancer cells with etoposide resulted in a translocation of PKCδ from cytoplasm to nucleus concomitant with induction of apoptosis. Decreased PKCδ expression, particularly in the nucleus, may compromise the ability of cells to undergo apoptosis, perhaps conferring resistance to chemotherapy. Our results indicate that loss of PKCδ is an indicator of endometrial malignancy and increasing grade of cancer. Thus, PKCδ may function as a tumor suppressor in endometrial cancer.
Keywords: PKCδ, endometrial cancer, immunohistochemistry, expression, nucleus
1. Introduction
Endometrial cancer is the most common invasive gynecological malignancy in the US with an estimated 40,000 new cases and 7,500 deaths annually. It is the fourth most common cancer in women, and the seventh leading cause of death related to malignancy (1). Despite the evident health risks endometrial cancer poses to women, its underlying molecular mechanisms are poorly understood and, unlike breast cancer, few molecular markers have been identified as indicators of progression and prognosis (1).
The Protein Kinase C (PKC) family is comprised of 11 isoforms, which differ in expression pattern, function, and response to extracellular stimuli (2). PKCs have been shown to regulate pathways critical to malignant transformation, including cell proliferation, cellular invasion, and programmed cell death or apoptosis (3–6). Alterations in the expression profile or activities of members of the protein kinase C (PKC) family are postulated to contribute to endometrial neoplasia and transformation (7 –9).
Alterations in expression of PKCδ, are reported in several endocrine-related cancers, including breast, thyroid, pancreas and ovary, and have been linked to regulation of proliferation, survival and metastasis (10–13). We have recently shown that PKCδ is required for apoptosis in endometrial cancer cells and that relative levels of the active kinase can modulate sensitivity to chemotherapeutic agents (14). Based upon these observations, we hypothesized that PKCδ functions as a tumor suppressor and that endometrial cancers would exhibit a reduction in PKCδ levels, contributing to the evasion of programmed cell death characteristic of cancers (15). However, differential expression of PKCδ in endometrial tumors had not been evaluated.
The objective of this study was to analyze PKCδ expression in endometrial cancers. Immunohistochemical analysis was used to examine PKCδ protein levels in a series of normal and endometrial tumor tissues and to correlate changes with tumor grade and proliferative cells assessed by Ki-67 staining. Our results demonstrate a progressive reduction in PKCδ expression, particularly in the nucleus, correlating with increasing grade. Endometrial cancer cell lines derived from well-differentiated (grade 1) to poorly differentiated (grade 3) cancers exhibited a corresponding reduction in PKCδ levels. We have previously demonstrated a critical role for PKCδ in regulation of apoptosis in endometrial cancer cells (14) and nuclear import of PKCδ is necessary for apoptosis in other epithelial cancer cell lines (3, 4, 16). Therefore, based upon the loss of nuclear PKCδ in endometrial tumors observed in these studies, we used endometrial cancer cell lines to examine changes in PKCδ nuclear localization upon induction of apoptosis.
2. Materials and Methods
2.1. Tissue Collection
Thirty-two cases of uterine endometrial adenocarcinoma were obtained from the Department of Pathology at the University of Colorado Health Science Center under Colorado Multiple Institutional Review Board protocol number 00-1094. Samples were selected, from an archival de-identified data set, to provide a cross section of tumor grades. According to the International Federation of Gynecology and Obstetrics (FIGO) criteria, seventeen cases were classified as grade one, seven cases were classified as grade two, and eight cases were classified as grade three. Of these cases, four grade 1 tumors contained adjacent, normal atrophic endometrium. Additionally, the study included 11 cases of normal endometrium with four in the proliferative phase, three in the secretory phase, and four atrophic. The mean age of patients was 54.6 ± 13. Since additional clinical information was unobtainable, patient samples were stratified by age based on National Institute of Aging estimates of the onset of menopause in US women at a mean age of 51. A subset of nine grade one tumors was derived from patients under 50, typically arising in pre- or perimenopausal women in a background of secretory, or proliferative endometrium and unopposed estrogen exposure (1). The remaining tumors were obtained from patients over 50 years old, likely arising post menopausally, in a background of atrophic endometrium.
2.2. Immunohistochemistry
Endometrial tissue was formalin-fixed and paraffin-embedded. The tissue was sectioned into 5 μm thick slices and blocked for endogenous peroxidase activity using 3% (v/v) hydrogen peroxide. Antigen retrieval was performed in citrate buffer (20mM, pH 6.0) for 10 min. at 60°C. Sections were incubated with antibodies specific for PKCδ (0.17mg/ml, C-20, Santa-Cruz Biotechnology, Santa Cruz, CA) or Ki-67 (DakoCytomation, Carpinteria, CA) for one hour, and stained using an indirect avidin biotin immunoperoxidase method on a DAKO Autostainer™ as described (17). Negative controls were run using an equivalent concentration of subclass-matched IgG (BD Pharmingen, San Diego, CA) or polyclonal IgG (VECTOR Laboratories, Burlingame, CA). Specificity of staining was demonstrated by competition with peptide antigens to block staining (Santa Cruz Biotechnology) and verified by lack of staining in PKCδ null mice (18) in comparison to wild type mouse tissue (data not shown).
PKCδ stained sections were evaluated through a blinded review of the entire histological section by five independent observers and an average proportion of cells stained and intensity were calculated. Intensity was scored from no staining (0), faint (1+), moderate (2+) to strong (3+). Proliferative index was calculated as the proportion of cancer cells expressing Ki-67, based on an evaluation of 3 randomly selected fields of at least 400 cells.
2.3. Cell Culture
Relative levels of PKCδ were evaluated in endometrial cancer cell lines derived from and characteristic of well- to poorly- differentiated tumors (19). Ishikawa cells, low grade, well-differentiated; HEC-1-A, medium grade, moderate-differentiation; and HEC-50, high grade poorly differentiated. Ishikawa endometrial adenocarcinoma cells and HEC-50 endometrial adenocarcinoma cells were a gift from Dr. K. K. Leslie (University of New Mexico, Albuquerque). HEC-1-A endometrial adenocarcinoma cells were purchased from the American Tissue Culture Collection (Manassas, VA). Cells were grown in DMEM (Cellgro, Herndon, VA) supplemented with 12.5% horse serum (Invitrogen, Carlsbad, CA), 2.5% fetal bovine serum (Gibco, Carlsbad, CA), 10 U/ml penicillin (Fisher, Hampton, NH), 10 μg/ml streptomycin (Cellgro), and 200 μM L-glutamine (Fisher) and maintained at 37 º C in 5% CO2.
2.4. Western Blot Analysis
Endometrial cancer cells (Ishikawa, HEC-1-A, HEC-50) were washed twice with ice-cold phosphate buffered saline (PBS) and lysed in buffer (50mM Tris, 150mM NaCl, 0.5% Triton X-100; pH 7.4) supplemented with protease inhibitors (Roche Diagnostics, Mannheim, Germany). Cells lysates were clarified by centrifugation at 13,000 g for 10 min at 4ºC, and protein concentration determined by Bradford assay (Bio-Rad Laboratories, Hercules, CA). Aliquots (35μg) were resolved by SDS polyacrylamide-gel electrophoresis, transferred to PVDF membrane and processed as described previously (14). PKCδ levels were assessed with anti-PKCδ antibody (C-20, Santa Cruz Biotechnology) and equal loading was assessed using a monoclonal anti-β-actin antibody (A5316, Sigma). Band intensity was quantitated by densitometry using Quantity One software (v4.5.1) and a GelDoc™ Imaging System (Bio-Rad).
2.5. Immunofluorescence and confocal microscopy
HEC-1-A endometrial cancer cells were plated at 50% confluency on 6 well chamber slides (Nalgene Nunc International Corp., Naperville, IL). Twenty-four hours later, cells were treated ± 50μM etoposide (Calbiochem, San Diego, CA) for six hours. Cells were washed twice in ice-cold PBS, then fixed and permeabilized in 50% methanol/50% acetone (v/v) under slow agitation for 2 minutes. Cells were rinsed five times for five minutes each with PBS and incubated in PKCδ antibody (C-20, Santa Cruz Biotechnology) 1:1000 (v/v) in PBS for one hour, at room temperature. Cells were rinsed in PBS and incubated in the dark with goat anti-rabbit; Alexa Fluor 594 (Molecular Probes) diluted 1:150 in PBS for 45 minutes at room temperature. Slides were dried and mounted using Pro-long Gold Antifade Reagent with DAPI (Molecular Probes, Invitrogen). Immunofluorescence images were captured on a Nikon Diaphot fluorescent microscope equipped with a Cooke SensiCam CCD camera (Tonawand, NY) and digitally deconvolved using Slidebook software (Intelligent Imaging Innovations Inc., Denver CO).
2.6. Statistical Analysis
Results are expressed as means ± standard error of the mean (s.e.m). Data were analyzed by paired Student’s t-test. P values of less than 0.05 were considered significant.
3. Results
Staining of PKCδ was analyzed in glandular and stromal elements of normal endometrium and endometrioid carcinomas of increasing grade. In addition, relative cytoplasmic and nuclear expression of PKCδ was assessed and correlated with tumor grade.
3.1. PKCδ expression in normal endometrium
Seven cases of normal cycling endometrium and four atrophic endometrial samples were stained for PKCδ. Normal atrophic tissue adjacent to grade 1 tumors was also analyzed in four additional sections. In cycling endometrium, four cases were in the proliferative phase and three in the secretory phase of the menstrual cycle. PKCδ expression was detected in all normal endometrium. Staining was homogenous within each section and localized primarily to the epithelial glandular portions of the tissue. In the adjacent stroma, less than 5% of cells stained for PKCδ, which was attributed to infiltrating lymphocytes (Fig. 1A). In proliferative endometrium, PKCδ staining was observed in all cells, localized mainly in the cytoplasm with an average intensity of 2.1+. Relative to cycling endometrium, atrophic glands exhibited higher PKCδ staining in the cytoplasm, with an average intensity of 2.7+. Cytoplasmic expression in proliferative and secretory endometrium exhibited more variation between cases and was consistently less intense with an average intensity of 2.0+ for secretory endometrium and 2.1+ for proliferative endometrium (Fig. 1A, Table 1).
Fig. 1.
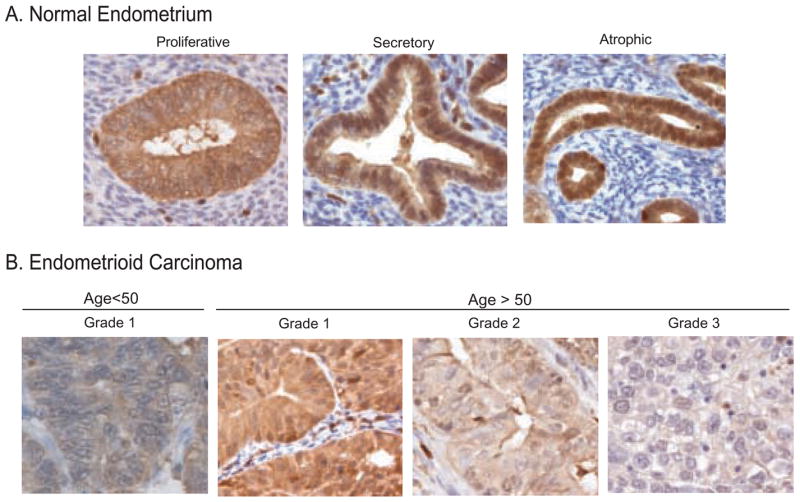
PKCδ expression is reduced in malignant epithelium relative to normal tissue: Representative images of (A) normal proliferative, secretory or atrophic endometrium and (B) endometrioid carcinoma of increasing grade. Samples were processed and stained for PKCδ, as described in Materials and Methods.
Table 1.
Summary of immunohistochemical analysis of PKCδ expression.
| Histological Diagnosis | Proliferative Index | Cytoplasmic Staining | Nuclear Staining | N | ||
|---|---|---|---|---|---|---|
| Intensity | % Cells | Intensity | % Cells | |||
| Normal Endometrium | ||||||
| Proliferative | 13 ± 2.5 | 2.1 ± 0.3 | 100 ± 0.5 | 1.6 ± 0.6 | 55 ± 13 | 4 |
| Secretory | 0.0 ± 0.0 | 2.0 ± 0.2 | 100 ± 0.0 | 1.8 ± 0.1 | 64 ± 9.2 | 3 |
| Atrophic | 4.3 ± 1.1 | 2.7 ± 0.2 | 100 ± 0.2 | 2.7 ± 0.1 | 99 ± 0.5 | 8 |
| Carcinoma (< age 50) | + 30 ± 4.8 | **0.97 ± 0.1 | 96 ± 2.7 | *0.41 ± 0.2 | **14 ± 2.9 | 9 |
| Carcinoma (> age 50) | ||||||
| Grade 1 | + 35 ± 10 | **1.4 ± 0.1 | 98 ± 1.4 | **0.66 ± 0.3 | **29 ± 9.4 | 8 |
| Grade 2 | + 41 ± 8.2 | **1.2 ± 0.2 | 87 ± 8.6 | **0.55 ± 0.3 | **15 ± 3.9 | 7 |
| Grade 3 | + 52 ± 7.6 | **0.96 ± 0.1 | 89 ± 6.3 | **0.44 ± 0.3 | **6.9 ± 3.3 | 8 |
Data were obtained and processed as described in Materials and Methods and are expressed as mean ± standard error of the mean. Values for tumors from patients < age 50 are statistically different from normal cycling endometrium, and tumors from patients > age 50 differ significantly from normal atrophic endometrium as indicated: +p<0.05, *p<0.002, **p<0.0002. Grade 3 tumors differed significantly from grade 1 tumors with respect to cytoplasmic intensity and percentage nuclei stained (p<0.05). N: number of cases.
Nuclei of all normal endometrial glands stained positive for PKCδ. Nuclear staining was most prominent in atrophic endometrium at an average of 2.7+, comparable to that of the cytoplasm (Figs. 1A & 2). Lower levels of nuclear PKCδ were detected in secretory and proliferative endometrium, with mean intensities of 1.8 and 1.6, respectively (Table 1). Atrophic endometrium also displayed the highest fraction (99%) of nuclei staining positive for PKCδ. In secretory endometrium, an average of 65% of glandular cells demonstrated nuclear PKCδ expression, and the average dropped to 55% in proliferative endometrium (Table 1). No staining was detected in matched non-immune immunoglobulin controls (not shown).
Fig. 2.
Loss of PKCδ expression in the nuclei of malignant epithelium: (A) Representative image of a low-grade tumor with adjacent normal atrophic endometrium (40X magnification). Enlargement of normal tissue (B) showing robust nuclear and cytoplasmic expression of PKCδ compared to malignant epithelium (C) which exhibits reduction of cytoplasmic staining and loss of nuclear PKCδ. Sections were stained as in Materials and Methods.
3.2. PKCδ expression in endometrial carcinomas
A series of thirty-two cases of endometrioid endometrial carcinomas were analyzed for changes in the expression of PKCδ relative to normal endometrium. PKCδ staining in malignant epithelium was largely confined to glandular tissue with minimal stromal staining, again attributable to infiltrating lymphocytes (Fig. 1B). Comparison of normal and tumor stroma revealed no difference in PKCδ levels, in either proportion of positive cells or staining intensity.
3.3. Cytoplasmic expression of PKCδ
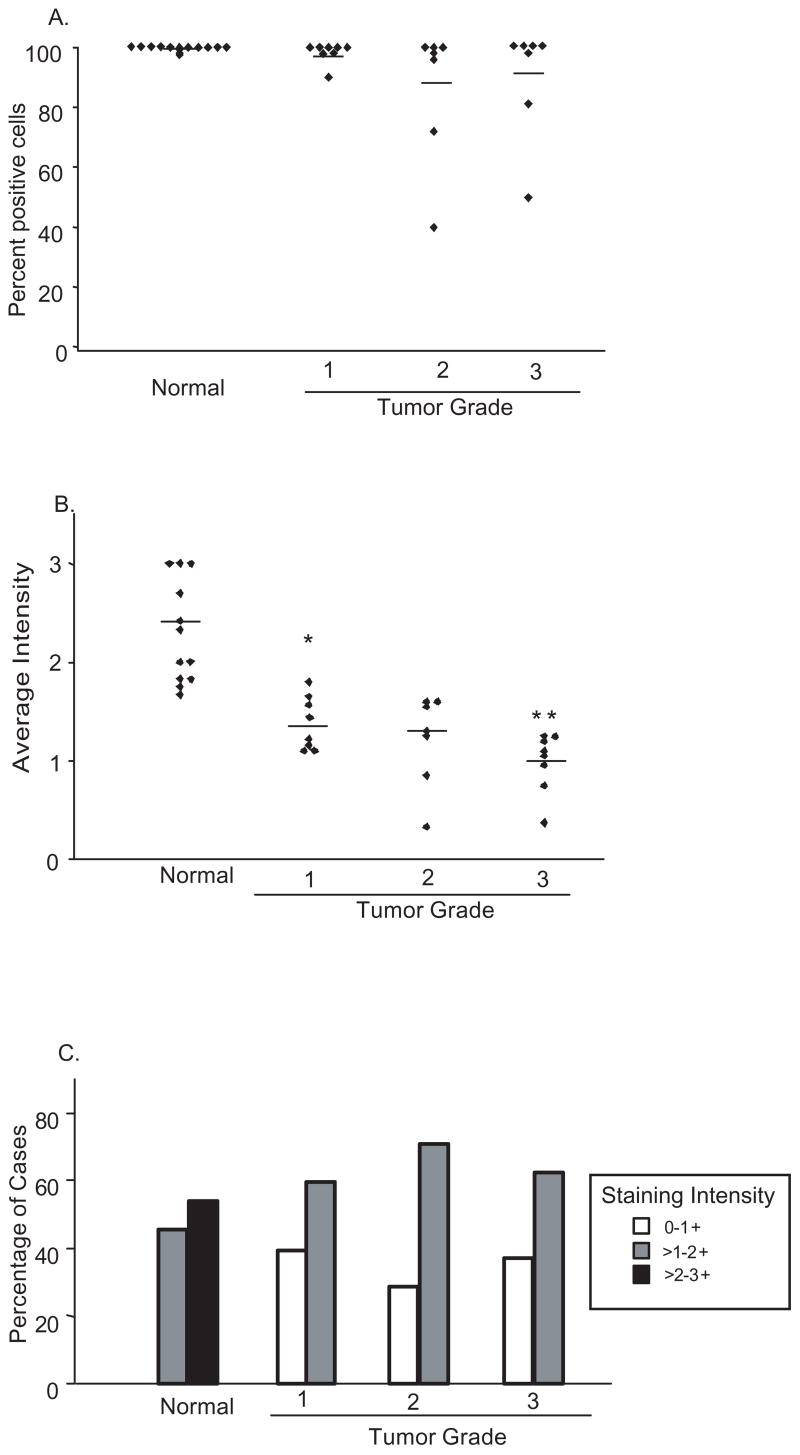
Changes in levels of PKCδ in the cytoplasm were assessed and analyzed for fraction of positive cells (Fig 3A), average intensity of cytoplasmic staining (Fig. 3B) and distribution of intensities by tertile according to grade (Fig. 3C).
Fig. 3.
PKCδ cytoplasmic expression is reduced in endometrial cancer: Tumor samples stained for PKCδ were analyzed and scored for (A) the fraction of cells per case staining and (B) average staining intensity in the cytoplasm. The mean percentage stained cells (A) and intensity for each sample population of normal or increasing tumor grade, is denoted by the horizontal line. (C) The fraction of cases falling into each tertile of staining intensity, according to tumor grade. * p <0.0001. ** p <0.05.
Some tumors of all grades showed a reduction in the fraction of positive cells relative to normal tissue, but this was not statistically significant and did not correlate with increasing grade (Fig. 3A). In contrast, tumors exhibited a significant loss in cytoplasmic intensity of PKCδ staining relative to normal endometrium. PKCδ levels in the cytoplasm progressively decreased with increasing tumor grade. Normal atrophic endometrium had an average intensity of 2.7+, grade one carcinomas, 1.4+, grade two, 1.2+ and in grade three, 1.0+ (Fig. 3B). Accordingly, approximately 57% of normal cases showed the highest tertile staining of 2–3+ with the remainder staining in the 1–2+ range, while 30–40% of tumors stained in the lowest tertile (0–1) and no tumors stained higher than 2+ (Fig. 3C). Grade one tumors from patients less than 50 years old (likely pre- or perimenopausal) exhibited a more pronounced, statistically significant (p<0.005) decrease in cytoplasmic intensity of PKCδ relative to patients (likely postmenopausal) over age 50 (Table 1).
Overall, endometrial carcinomas show a significant (p<0.0002) decrease in cytoplasmic PKCδ expression per cell upon transition from normal to malignant endometrium, and a trend of progressively reduced levels with increasing grade (Table 1).
3.4. Nuclear expression of PKCδ
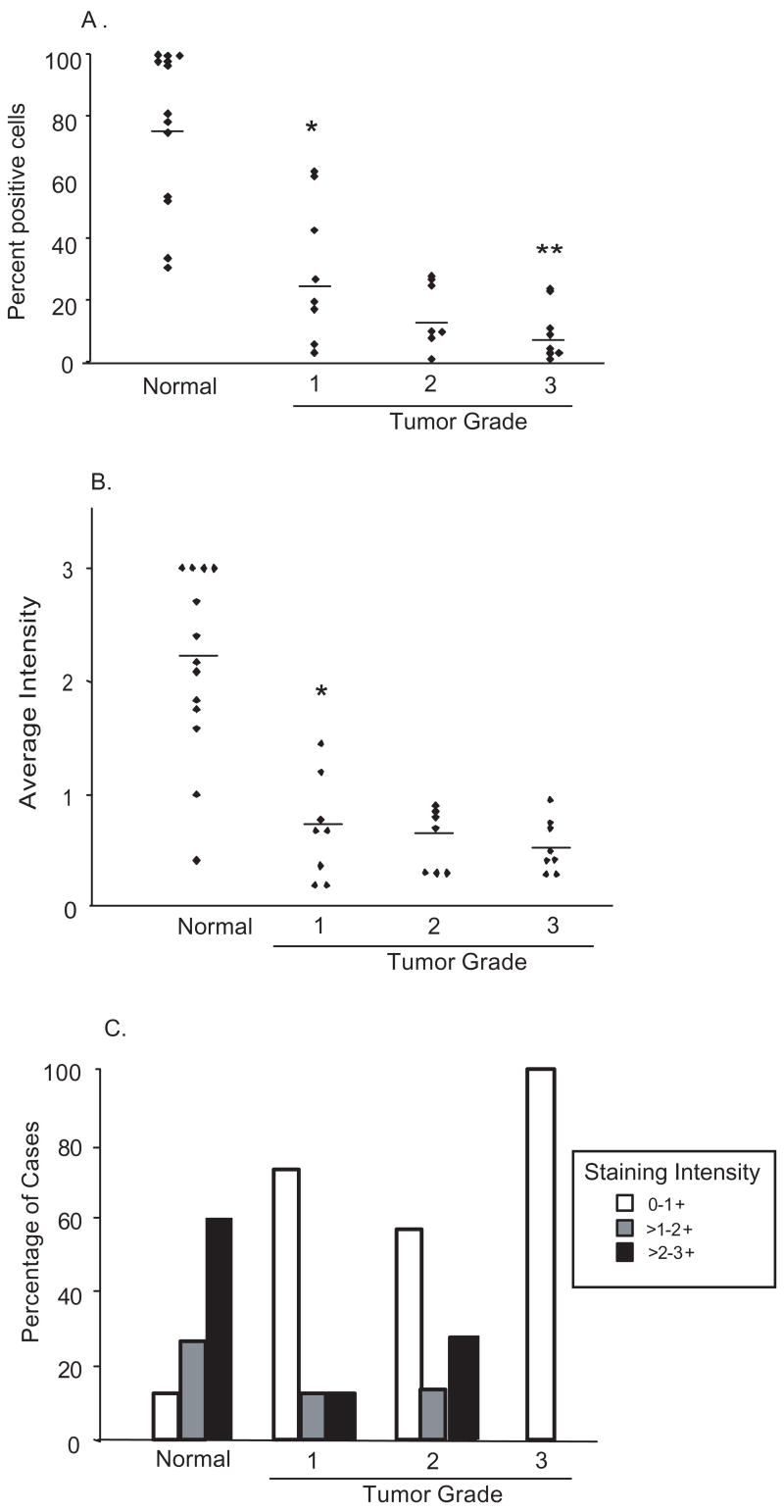
As shown in figure 4A, PKCδ was detected in almost all nuclei (99%) of atrophic endometrial glandular cells. In contrast, grade one tumors exhibit a marked reduction in the fraction of cells positive for nuclear PKCδ to only 29%. Grade two tumors were 15% positive and only 6.9% of grade three tumor cells stained for PKCδ in the nucleus, illustrating an inverse correlation between tumor grade and nuclear content of PKCδ expressing cells (Fig. 4A & Table 1).
Fig. 4.
Nuclear PKCδ expression is markedly reduced or lost in endometrial carcinomas: Tumor samples were stained and analyzed for (A) the fraction of cells per case exhibiting nuclear PKCδ staining and (B) average staining intensity in the nucleus. The mean percentage stained cells (A) and intensity (B), for each sample population of normal or increasing tumor grade, is denoted by the horizontal line. (C) The fraction of cases falling into each tertile of nuclear staining intensity, according to tumor grade. * p <0.0001. ** p <0.05.
A similar relationship was observed with respect to intensity of nuclear PKCδ staining. Tumors showed a marked reduction in nuclear PKCδ staining relative to normal tissue. In grade one carcinoma, the average nuclear staining intensity was 0.66, in grade two it decreased to 0.55 and in grade three carcinoma the average intensity dropped further to 0.44, demonstrating a progressive loss of nuclear PKCδ with increasing grade of tumor (Fig. 4B &Table 1). Analysis of the number of cases in each staining tertile revealed that, while more than 90% of normal endometrial sections showed >2+ nuclear staining, less than 30% of grade one and two tumors stained at this intensity and all grade three tumors scored in the lowest tertile (0–1+) (Fig. 4C), Indeed, in one grade three tumor, PKCδ was not detectable in the nucleus.
Tumors showed a substantial decrease in overall levels of PKCδ relative to normal endometrium and a marked reduction in both the fraction and intensity of nuclear staining, correlating with increased tumor grade and loss of differentiation (Fig. 1B). As shown in figure 2A, normal atrophic glandular tissue shows abundant cytoplasmic and nuclear staining for PKCδ, while adjacent grade 1 tumor exhibits a significant reduction in overall intensity and fraction of stained cells, particularly with respect to the nuclear compartment (Fig. 2B & C). Preferential loss of PKCδ from the nucleus may be attributable to nuclear exclusion or subcellular redistribution of the kinase.
3.5. PKCδ expression and tumor cell proliferation
To assess proliferation in tissue samples, sections were stained with antibodies to Ki-67 and quantitated. Ki-67 staining for proliferative cells exhibited considerable inter and intra sample variation, with portions of the same tumor showing wide variations in the fraction of Ki-67 positive cells. The mean proliferative index increased from zero in secretory endometrium, 4.3% in atrophic endometrium, and 13% in normal proliferative endometrium, to 30% in premenopausal grade one tumors, 35% in post menopausal grade one, 41% in grade two and 52% in grade three tumors (Table 1). Thus, as PKCδ levels decreased, the proliferative index increased. However, differences between grades were not statistically significant and no direct correlation between levels of PKCδ and proliferative index was found.
3.6. Expression of PKCδ in endometrial cancer cells
To determine if the above correlations between tumor grade and PKCδ expression observed in tumors was consistent with endometrial cancer cell lines, we evaluated relative levels of PKCδ in cells lines characteristic of type I and type II endometrioid tumors of increasing grade (19). Ishikawa cells, type I, grade1; HEC-1-A, type II, grade 2; and HEC-50, type II, grade 3, were harvested and analyzed for total PKCδ by western blotting (Fig. 5A). Membranes were reprobed for β-actin as a loading control and bands quantitated by densitometric scanning (Fig. 5B). Consistent with the tumor samples, Ishikawa cells exhibited the highest levels of PKCδ; HEC-1-A cells reduced expression and HEC-50, the lowest relative levels of PKCδ. Thus, PKCδ expression in endometrial cancer cells correlates with the type and grade of their tumor of origin.
Fig. 5.

Relative PKCδ levels in endometrial cancer cell lines: A series of endometrial cancer cell lines (Ishikawa, HEC-1-A and HEC-50) were harvested and extracts probed for PKCδ and β-actin by western blotting, as described in Materials and Methods. (A) PKCδ expression levels were quantitated by densitometry, normalized to β-actin and are expressed relative to levels in Ishikawa cells. Data are mean ± s.e.m of 3 experiments. (B) A representative western blot showing PKCδ and β-actin levels.
3.7. PKCδ undergoes nuclear translocation upon induction of apoptosis
Nuclear import of PKCδ has been shown to be necessary for induction of apoptosis in other epithelial cancer cell lines (3, 4, 16) and we have demonstrated a functional role for PKCδ in modulating apoptosis in endometrial cancer cells (14). We therefore used confocal immunofluorescence microscopy to examine changes in PKCδ subcellular location in HEC-1-A endometrial cancer cells upon induction of apoptosis. As shown in figure 6, PKCδ in untreated HEC-1-A cells is present primarily in the cytoplasm, with approximately 17% of cells exhibiting nuclear expression. Treatment of cells with etoposide, to induce apoptosis (14), results in translocation of a significant (p<0.0001) fraction of PKCδ to the nucleus (Fig. 6C & D) such that almost 92% of cells have nuclear PKCδ. These data suggest that nuclear localization of PKCδ is required for induction of apoptosis in endometrial cancer cells.
Fig. 6.
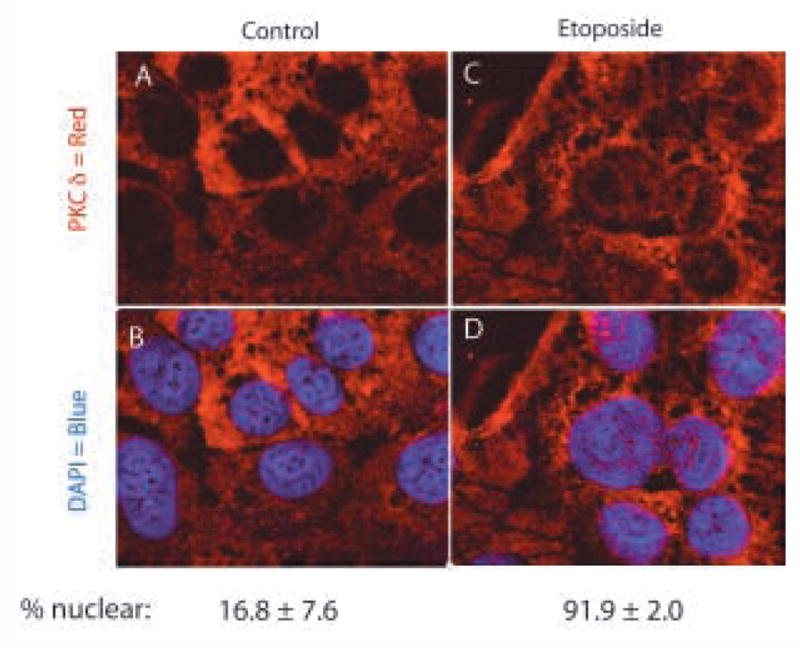
Translocation of PKCδ in response to etoposide-induced apoptosis: HEC-1-A cells were treated with 50μM etoposide or diluent (control), for 6h as indicated. Cells were fixed, permeabilized, probed with antibody to PKCδ and stained by immunofluorescent secondary antibody, as described in Materials and Methods. (A) Control cells. (B) Etoposide treated cells. (C & D) Nuclei counter stained with DAPI. Representative confocal images from two independent experiments are shown. Percent nuclear PKCδ was estimated by counting of five random fields of control or etoposide treated cells, by three independent observers. Results are mean ± s.e.m.
4. Discussion
Individual PKC isoforms are thought to mediate distinct functions within the cell, regulating mitogenesis, cell cycle, apoptosis and gene expression (20, 21) and have been implicated in neoplastic transformation and the growth and metastasis of tumors in a variety of tissues (6, 22, 23). In endometrial cancer cells, aberrant levels and/or activation of PKCs are postulated to contribute to endometrial neoplasia and transformation (7). Alterations in the expression of PKC isoforms in endometrial cancer cell lines have also been implicated in the pathogenesis of endometrial tumors and patient prognosis (24). In an analysis of PKC isoform expression in endometrial tumors by western blotting of tumor extracts, PKCα expression, as previously observed in breast cancer cells, was found to correlate inversely with estrogen receptor (ER). PKCα was more highly expressed in higher-grade endometrial tumors exhibiting lower levels of ERα (24). However, these authors failed to detect PKCδ expression in endometrial tumors, possibly due to the partial purification or western blotting procedure employed. Immunohistochemical studies of in situ expression patterns of PKC isoforms in endometrial tumors have not been reported.
Changes in PKCδ expression has been documented in a number of tumors and cancer cell lines. Consistent with our results, loss of PKCδ was observed in squamous cell carcinoma (25) and upon acquisition of a metastatic phenotype in breast cancer cells (5). High levels of PKCδ were also detected in normal bladder tissue with a reduction in expression correlating with increasing stage and grade of carcinoma (26). Decreased PKCδ protein was also observed in melanoma cells established from metastatic lesions, relative to those derived from primary tumors (27). In contrast, PKCδ levels are increased in pancreatic cancer, chronic myelogenous leukemia and hepatocellular carcinoma (12, 28, 29), suggesting that PKCδ can function as both a positive and negative regulator of cell proliferation and survival in different tissues and cell types (30).
In this study we analyzed PKCδ expression in normal endometrium and a series of tumors of increasing grade. We demonstrate abundant expression of PKCδ in epithelial glandular tissue of normal endometrium, in both nuclear and cytoplasmic compartments. Endometrioid tumors exhibit a loss of PKCδ, particularly from the nucleus. A progressive reduction in the fraction of cells expressing PKCδ and the intensity of staining was observed in with increasing grade of tumors. Accordingly, we also observed a reduction in total PKCδ expression in HEC-1-A and HEC-50 endometrial cancer cell lines, relative to the well-differentiated, Ishikawa cells. Loss of nuclear PKCδ may be in part a reflection of subcellular relocalization or nuclear exclusion.
Expression of PKCδ in NIH 3T3 cells resulted in a decrease in cell proliferation and anchorage-independent growth (31). Activation of PKCδ also induced growth arrest in thyroid and breast cancer cells (13, 32) inducing expression of the cell cycle inhibitor p27 in the latter (11). Evidence also suggests that nuclear PKCδ inhibits the cell cycle by preventing progression through the G1/S and G2/M phase checkpoints (6, 20, 30). Consistent with these observations, our data indicate that down regulation of PKCδ expression in higher-grade endometrial tumors was accompanied by an increase proliferative index (Ki-67 staining). However, other reports suggest a pro-proliferative and pro-survival role for PKCδ in human breast cancer cell lines (33, 34) and a functional role for PKCδ in regulation of growth of endometrial cells remains to be established.
PKCδ has been shown to be required for apoptosis in a variety of epithelial tumors (3, 4, 16) and we have shown that PKCδ is a critical element of a pro-apoptotic pathway in endometrial cancer cells (14). Nuclear import of PKCδ is also required for induction of apoptosis in several cell types (3, 16, 35). Accordingly, we have shown that PKCδ translocates to the nucleus in response to an apoptotic stimulus in HEC-1-A endometrial cancer cells. The observed loss of nuclear PKCδ in endometrial tumors is therefore consistent with impaired apoptosis and perhaps the acquisition of resistance to chemotherapy. In support of this hypothesis, overexpression of PKCδ enhanced cisplatin sensitivity in gastric cancer cells(36), while siRNA-mediated down regulation of PKCδ inhibited cisplatin induced apoptosis in cervical carcinoma (37). We have also shown that etoposide-induced apoptosis of endometrial cancer cells is potentiated by increased PKCδ expression (14).
In summary, we have shown using immunohistochemistry, that loss of PKCδ expression, particularly from the nuclei of malignant glandular epithelium, is a hallmark of endometrial cancer and correlates with higher grade and loss of differentiation. Our data suggest that PKCδ may function as a tumor suppressor in the endometrium.
Acknowledgments
The authors would like to thank Dr. Jim McManaman for assistance with confocal microscopy and Dr. Paul Jedlicka for critical reading of the manuscript. Dr. Dimitrova is a Colorado Women’s Reproductive Health Research Career Development Center Scholar (K12 HD001271). Supported by CA 104875 to APB.
Supported by CA 104875 to APB.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Amant F, Moerman P, Neven P, Timmerman D, Van Limbergen E, Vergote I. Endometrial cancer. Lancet. 2005;366:491–505. doi: 10.1016/S0140-6736(05)67063-8. [DOI] [PubMed] [Google Scholar]
- 2.Nishizuka Y. The protein kinase C family and lipid mediators for transmembrane signaling and cell regulation. Alcohol Clin Exp Res. 2001;25:3S–7S. doi: 10.1097/00000374-200105051-00003. [DOI] [PubMed] [Google Scholar]
- 3.Brodie C, Blumberg PM. Regulation of cell apoptosis by protein kinase c delta. Apoptosis. 2003;8:19–27. doi: 10.1023/a:1021640817208. [DOI] [PubMed] [Google Scholar]
- 4.Gutcher I, Webb PR, Anderson NG. The isoform-specific regulation of apoptosis by protein kinase C. Cell Mol Life Sci. 2003;60:1061–1070. doi: 10.1007/s00018-003-2281-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morse-Gaudio M, Connolly JM, Rose DP. Protein kinase C and its isoforms in human breast cancer cells: relationship to the invasive phenotype. Int J Oncol. 1998;12:1349–1354. doi: 10.3892/ijo.12.6.1349. [DOI] [PubMed] [Google Scholar]
- 6.Musashi M, Ota S, Shiroshita N. The role of protein kinase C isoforms in cell proliferation and apoptosis. Int J Hematol. 2000;72:12–19. [PubMed] [Google Scholar]
- 7.Bamberger AM, Bamberger CM, Wald M, Kratzmeier M, Schulte HM. Protein kinase C (PKC) isoenzyme expression pattern as an indicator of proliferative activity in uterine tumor cells. Mol Cell Endocrinol. 1996;123:81–88. doi: 10.1016/0303-7207(96)03895-6. [DOI] [PubMed] [Google Scholar]
- 8.Fujimoto J, Hori M, Ichigo S, Morishita S, Tamaya T. Clinical implication of fos and jun expressions and protein kinase activity in endometrial cancers. Eur J Gynaecol Oncol. 1995;16:138–146. [PubMed] [Google Scholar]
- 9.Connor P, Talavera F, Kang JS, Burke J, Roberts J, Menon KM. Epidermal growth factor activates protein kinase C in the human endometrial cancer cell line HEC-1-A. Gynecol Oncol. 1997;67:46–50. doi: 10.1006/gyno.1997.4828. [DOI] [PubMed] [Google Scholar]
- 10.Kiley SC, Clark KJ, Goodnough M, Welch DR, Jaken S. Protein kinase C delta involvement in mammary tumor cell metastasis. Cancer Res. 1999;59:3230–3238. [PubMed] [Google Scholar]
- 11.Vucenik I, Ramakrishna G, Tantivejkul K, Anderson LM, Ramljak D. Inositol hexaphosphate (IP6) blocks proliferation of human breast cancer cells through a PKCdelta-dependent increase in p27Kip1 and decrease in retinoblastoma protein (pRb) phosphorylation. Breast Cancer Res Treat. 2005;91:35–45. doi: 10.1007/s10549-004-6456-5. [DOI] [PubMed] [Google Scholar]
- 12.Evans JD, Cornford PA, Dodson A, Neoptolemos JP, Foster CS. Expression patterns of protein kinase C isoenzymes are characteristically modulated in chronic pancreatitis and pancreatic cancer. Am J Clin Pathol. 2003;119:392–402. doi: 10.1309/bkpc9dx98r781b87. [DOI] [PubMed] [Google Scholar]
- 13.Koike K, Fujii T, Nakamura AM, Yokoyama G, Yamana H, Kuwano M, Shirouzu K. Activation of protein kinase C delta induces growth arrest in NPA thyroid cancer cells through extracellular signal-regulated kinase mitogen-activated protein kinase. Thyroid. 2006;16:333–341. doi: 10.1089/thy.2006.16.333. [DOI] [PubMed] [Google Scholar]
- 14.Haughian JM, Jackson TA, Koterwas DM, Bradford AP. Endometrial cancer cell survival and apoptosis is regulated by protein kinase C {alpha} and {delta} Endocr Relat Cancer. 2006;13:1251–1267. doi: 10.1677/erc.1.01278. [DOI] [PubMed] [Google Scholar]
- 15.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 16.Basu A. Involvement of protein kinase C-delta in DNA damage-induced apoptosis. J Cell Mol Med. 2003;7:341–350. doi: 10.1111/j.1582-4934.2003.tb00237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tringler B, Liu W, Corral L, Torkko KC, Enomoto T, Davidson S, Lucia MS, Heinz DE, Papkoff J, Shroyer KR. B7-H4 overexpression in ovarian tumors. Gynecol Oncol. 2006;100:44–52. doi: 10.1016/j.ygyno.2005.08.060. [DOI] [PubMed] [Google Scholar]
- 18.Humphries MJ, Limesand KH, Schneider JC, Nakayama KI, Anderson SM, Reyland ME. Suppression of apoptosis in the protein kinase Cdelta null mouse in vivo. J Biol Chem. 2006;281:9728–9737. doi: 10.1074/jbc.M507851200. [DOI] [PubMed] [Google Scholar]
- 19.Albitar L, Laidler LL, Abdallah R, Leslie KK. Regulation of signaling phosphoproteins by epidermal growth factor and Iressa (ZD1839) in human endometrial cancer cells that model type I and II tumors. Mol Cancer Ther. 2005;4:1891–1899. doi: 10.1158/1535-7163.MCT-05-0274. [DOI] [PubMed] [Google Scholar]
- 20.Fishman DD, Segal S, Livneh E. The role of protein kinase C in G1 and G2/M phases of the cell cycle (Review) Int J Oncol. 1998;12:181–186. doi: 10.3892/ijo.12.1.181. [DOI] [PubMed] [Google Scholar]
- 21.Dempsey EC, Newton AC, Mochly-Rosen D, Fields AP, Reyland ME, Insel PA, Messing RO. Protein kinase C isozymes and the regulation of diverse cell responses. Am J Physiol Lung Cell Mol Physiol. 2000;279:L429–438. doi: 10.1152/ajplung.2000.279.3.L429. [DOI] [PubMed] [Google Scholar]
- 22.Goodnight J, Mischak H, Mushinski JF. Selective involvement of protein kinase C isozymes in differentiation and neoplastic transformation. Adv Cancer Res. 1994;64:159–209. doi: 10.1016/s0065-230x(08)60838-6. [DOI] [PubMed] [Google Scholar]
- 23.Gomez DE, Skilton G, Alonso DF, Kazanietz MG. The role of protein kinase C and novel phorbol ester receptors in tumor cell invasion and metastasis (Review) Oncol Rep. 1999;6:1363–1370. doi: 10.3892/or.6.6.1363. [DOI] [PubMed] [Google Scholar]
- 24.Fournier DB, Chisamore M, Lurain JR, Rademaker AW, Jordan VC, Tonetti DA. Protein kinase C alpha expression is inversely related to ER status in endometrial carcinoma: possible role in AP-1-mediated proliferation of ER-negative endometrial cancer. Gynecol Oncol. 2001;81:366–372. doi: 10.1006/gyno.2001.6164. [DOI] [PubMed] [Google Scholar]
- 25.D'Costa AM, Robinson JK, Maududi T, Chaturvedi V, Nickoloff BJ, Denning MF. The proapoptotic tumor suppressor protein kinase C-delta is lost in human squamous cell carcinomas. Oncogene. 2006;25:378–386. doi: 10.1038/sj.onc.1209065. [DOI] [PubMed] [Google Scholar]
- 26.Langzam L, Koren R, Gal R, Kugel V, Paz A, Farkas A, Sampson SR. Patterns of protein kinase C isoenzyme expression in transitional cell carcinoma of bladder. Relation to degree of malignancy Am J Clin Pathol. 2001;116:377–385. doi: 10.1309/1VKK-HWH7-YVJN-7UF7. [DOI] [PubMed] [Google Scholar]
- 27.Selzer E, Okamoto I, Lucas T, Kodym R, Pehamberger H, Jansen B. Protein kinase C isoforms in normal and transformed cells of the melanocytic lineage. Melanoma Res. 2002;12:201–209. doi: 10.1097/00008390-200206000-00003. [DOI] [PubMed] [Google Scholar]
- 28.Balasubramanian N, Advani SH, Zingde SM. Protein kinase C isoforms in normal and leukemic neutrophils: altered levels in leukemic neutrophils and changes during myeloid maturation in chronic myeloid leukemia. Leuk Res. 2002;26:67–81. doi: 10.1016/s0145-2126(01)00098-4. [DOI] [PubMed] [Google Scholar]
- 29.Tsai JH, Hsieh YS, Kuo SJ, Chen ST, Yu SY, Huang CY, Chang AC, Wang YW, Tsai MT, Liu JY. Alteration in the expression of protein kinase C isoforms in human hepatocellular carcinoma. Cancer Lett. 2000;161:171–175. doi: 10.1016/s0304-3835(00)00597-8. [DOI] [PubMed] [Google Scholar]
- 30.Jackson DN, Foster DA. The enigmatic protein kinase Cdelta: complex roles in cell proliferation and survival. Faseb J. 2004;18:627–636. doi: 10.1096/fj.03-0979rev. [DOI] [PubMed] [Google Scholar]
- 31.Mischak H, Goodnight JA, Kolch W, Martiny-Baron G, Schaechtle C, Kazanietz MG, Blumberg PM, Pierce JH, Mushinski JF. Overexpression of protein kinase C-delta and -epsilon in NIH 3T3 cells induces opposite effects on growth, morphology, anchorage dependence, and tumorigenicity. J Biol Chem. 1993;268:6090–6096. [PubMed] [Google Scholar]
- 32.Yokoyama G, Fujii T, Tayama K, Yamana H, Kuwano M, Shirouzu K. PKCdelta and MAPK mediate G(1) arrest induced by PMA in SKBR-3 breast cancer cells. Biochem Biophys Res Commun. 2005;327:720–726. doi: 10.1016/j.bbrc.2004.12.070. [DOI] [PubMed] [Google Scholar]
- 33.Kiley SC, Clark KJ, Duddy SK, Welch DR, Jaken S. Increased protein kinase C delta in mammary tumor cells: relationship to transformtion and metastatic progression. Oncogene. 1999;18:6748–6757. doi: 10.1038/sj.onc.1203101. [DOI] [PubMed] [Google Scholar]
- 34.McCracken MA, Miraglia LJ, McKay RA, Strobl JS. Protein kinase C delta is a prosurvival factor in human breast tumor cell lines. Mol Cancer Ther. 2003;2:273–281. [PubMed] [Google Scholar]
- 35.DeVries TA, Neville MC, Reyland ME. Nuclear import of PKCdelta is required for apoptosis: identification of a novel nuclear import sequence. Embo J. 2002;21:6050–6060. doi: 10.1093/emboj/cdf606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Iioka Y, Mishima K, Azuma N, Tsuchida A, Takagi Y, Aoki T, Saito I. Overexpression of protein kinase Cdelta enhances cisplatin-induced cytotoxicity correlated with p53 in gastric cancer cell line. Pathobiology. 2005;72:152–159. doi: 10.1159/000084119. [DOI] [PubMed] [Google Scholar]
- 37.Mohanty S, Huang J, Basu A. Enhancement of cisplatin sensitivity of cisplatin-resistant human cervical carcinoma cells by bryostatin 1. Clin Cancer Res. 2005;11:6730–6737. doi: 10.1158/1078-0432.CCR-05-0450. [DOI] [PubMed] [Google Scholar]