Abstract
Data from the nationally representative US NHANES III cohort were used to examine the hypothesis that socio-economic status is consistently and negatively associated with levels of biological risk, as measured by nine biological parameters known to predict health risks (diastolic and systolic blood pressure, pulse, HDL and total cholesterol, glycosylated hemoglobin, c-reactive protein, albumin and waist-hip ratio), resulting in greater cumulative burdens of biological risk among those of lower education and/or income. As hypothesized, consistent education and income gradients were seen for biological parameters reflecting cardiovascular, metabolic and inflammatory risk: those with lower education and income exhibiting greater prevalence of high risk values for each of nine individual biological risk factors. Significant education and income gradients were also seen for summary indices reflecting cumulative burdens of cardiovascular, metabolic and inflammatory risks as well as overall total biological risks. Multivariable cumulative logistic regression models revealed that the education and income effects were each independently and negatively associated with cumulative biological risks, and that these effects remained significant independent of age, gender, ethnicity and lifestyle factors such as smoking and physical activity. There were no significant ethnic differences in the patterns of association between socio-economic status and biological risks, but older age was associated with significantly weaker education and income gradients.
Keywords: biological markers, biological aging, education, ethnicity, income, Socio-economic status (SES), USA
Socio-economic status (SES) has long been a focus of interest for those seeking to elucidate the patterning of health disparities and the underlying causes for these disparities (Adler, Boyce, Chesney, Folkman, & Syme, 1993; Pincus & Callahan, 1995; Syme & Berkman, 1976). Decades of research have clearly delineated the fact that SES (as indexed by education, income and/or occupation) is a consistent and strong predictor of health differentials, with lower SES generally associated with poorer outcomes for most major types of morbidity and mortality (Adler et al., 1993; Pincus & Callahan, 1995; Crimmins & Cambois, 2003; Link & Phelan, 1995; Preston & Taubman, 1994). The data also show that these health disparities reflect a gradient of effects, rather than a threshold effect of poverty versus non-poverty, with those in the middle class experiencing better health than those below them but worse health than those above them (Marmot, 1994; Marmot et al., 1991; Adler et al., 1994; Pamuk, Makue, Heck, Reuben, & Lochner, 1998). Existing SES health gradients, however, remain incompletely understood.
A range of different factors have been postulated to contribute to these SES gradients, including differences in health behaviors, availability and quality of health care, differential exposure to environmental hazards and stressors/demands and the presence of fewer available financial and psychosocial resources with which to address life challenges (Adler et al., 1993; Pincus & Callahan, 1995; Lynch, Kaplan, Cohen, Tuomilehto, & Salonen, 1996). These (and other) SES-related differences in experiences and exposures ultimately “get under the skin” to impact on biological processes in ways that result in increased health risks. Indeed, a large and growing body of evidence documents SES gradients in major cardiovascular risk factors such as obesity, blood pressure, lipid profiles and diabetes (Lynch et al., 1996; Kaplan & Keil, 1993; Davey-Smith, 1997; Bobak, Hertzman, Skodova, & Marmot, 1999; Brunner et al., 1997; Dyer et al., 1999; Karlamangla et al., 2005) as well as markers of inflammation (Ishizaki, Martikainen, Nakagawa, & Marmot, 2000; Steptoe, Owen, Kunz-Ebrecht, & Mohamed-Ali, 2002; Koenig et al., 1999). Largely absent to date, however, has been a focus on the multi-systems nature of these risks (reflect the broad scope of SES gradients in biological risks) nor has there been much attention to the SES differentials in resulting cumulative burdens of biological risk (McEwen & Stellar, 1993; McEwen, 1998; Singer, Ryff, & Seeman, 2004).
Analyses of data from the MacArthur Studies of Successful Aging have previously shown that a cumulative index of biological risk (allostatic load) is negatively associated with SES as indexed by education, and mediates some 35 percent of the education differentials in mortality (Seeman et al., 2004). Examination of individual components of the index as well as sub-scales reflecting major systems (e.g., cardiovascular, stress hormones, inflammation) revealed consistent, though more modest effects in all cases. Parallel evidence linking lower SES to greater cumulative biological risk has also been documented in the Normative Aging Study (Kubzansky, Kawachi, & Sparrow, 1999).
The question of whether SES gradients are seen consistently within different ethnic groups in the US, however, has yet to be fully examined. Several studies suggest that income and education gradients may be stronger among whites as compared with blacks (Karlamangla et al., 2005; Watkins, Neaton, & Kuller, 1986; Kraus, Borhani, & Franti, 1980), a finding consistent with the hypothesis that minority status (and its associations with discrimination and disadvantage; [Kessler & Neighbors, 1986; Williams, Lavizzo-Mourey, & Warren, 1994; Williams & Collins, 1995; Williams, 2001]) may result in larger deficits among those of lower SES and smaller gains among those of higher SES. Recent analysis of National Health and Nutrition Examination Survey (NHANES) IV (1999–2002) data by Geronimus and colleagues (Geronimus, Hicken, Keene, & Bound, 2006) documented that blacks have significantly higher overall allostatic load scores, even after adjusting for SES (as measured by the poverty income ratio – a measure of household income relative to the US poverty threshold). Data from the NHANES III survey (1988–1994) likewise show higher overall levels of biological risk in non-whites (both blacks and Mexican Americans), though these differences are largely found among those less than 70 years of age (Crimmins, Johnston, Hayward & Seeman, 2003).
Analyses presented here use data from the National Health and Nutrition Examination Survey (NHANES III) to examine the hypothesis that education and income gradients in biological risks would be evident across a set of nine major biological parameters, resulting in significant differences in cumulative burdens of biological risk. We further hypothesized that the education gradients would be stronger than those for income for two reasons. First, while income represents the economic resources available to spend on housing, food and medical, educational attainment generally reflects both such economic resources as well as additional knowledge and skills that influence lifestyle and can facilitate coping with life’s challenges (Feinstein, 1993; Susser, Watson, Hopper, 1985; Liberator, Link, Kelsey, 1988). Second, income is subject to greater variability over time so that current household income (as reported in NHANES) may not reflect prior economic status (Williams, 1990; Smith & Kington, 1997). Indeed, as Smith & Kington (1997) have shown, currrent income is also subject to reverse effects of poor health leading to lower income. By contrast, for most adults age, education is highly stable and thus provides a better index of cumulative, longer-term SES status (Hayward, Crimmins, Miles & Yang, 2000), leading a number of researchers to conclude that education may be the best predictor of health (see Williams, 1990 and Smith, 2004 for reviews). Additional analyses tested for possible ethnic differences in the patterns of these SES-biological risk associations.
MATERIALS AND METHODS
The study population reflects adults aged 20 and older (N=18,825) in the National Health and Nutrition Examination Survey (NHANES) III (1988–1994), a nationally representative sample of the U.S. population with interview, clinical exam, and laboratory components (National Center for Health, 1994). We excluded those with no mobile examination center visit (n=2,252) (i.e., missing most biological data) and those missing education information (n=115) (our primary index of SES). The household poverty income ratio, our second index of SES, was not used as an initial exclusion criteria due to greater missing data for this measure (an additional n=1,413); rather, analyses based on the poverty income ratio were run on the subset of our general analysis sample who also had poverty income ratio data. Additional exclusion criteria included pregnancy (n=285) or excessive missing biological data (n=595; see below for details). The final analysis sample included a total of 15,578 NHANES participants.
Biological Indicators
Data based on nine biomarkers were used to create an overall summary index of multi-system risk, to reflect the cumulative effect of physiological dysregulation across multiple systems, or allostatic load (McEwen, 1998). We also created three subscales based on subsets of biomarkers reflecting inflammatory, metabolic and cardiovascular parameters. The inflammation subscale included C-reactive protein (mg/dL) and albumin (g/dL). The metabolic subscale included glycated hemoglobin (%), total cholesterol (mg/dL), HDL cholesterol (mg/dL), and waist-to-hip ratio. And, the cardiovascular subscale included systolic blood pressure (mm Hg), diastolic blood pressure (mm Hg), and resting heart rate (bt/min). Biomarkers available in the NHANES database, but excluded from the present analyses include: fibrinogen (data were only available for individuals aged 40+) and biomarkers that required fasted samples such as triglycerides, LDL cholesterol and glucose (a smaller and less representative sample was available).
For each of the variables, a dichotomous indicator was created, reflecting those with “high risk” values (assigned a score of “1”) and “lower risk” values (assigned a score of “0”). Values assigning high and low risk were based on clinically accepted “high risk” criteria (see Table 1).
Table 1.
Clinically-defined “high risk” criteria for biologic risk factors
| Variable | High Risk Clinical |
|---|---|
| Albumin (g/dL) | <3.8 g/dL (Visser et al., 2005) |
| C-reactive protein (mg/dL) | >=0.3 (Ridker, 2003) |
| Waist:Hip | >0.90 for men; >0.85 for women (Alberti & Zimmet, 1998) |
| Total cholesterol (mg/dL) | >=240 (Executive Summary of, 2001) |
| HDL (mg/dL) | <40 (Executive Summary of, 2001) |
| Glycated Hemoglobin (%) | >=6.4 (Golden et al., 2003; Osei, Rhinesmith, Gaillard, & Schuster, 2003) |
| Resting Heart Rate (bt/min) | >=90 (Seccareccia et al., 2001) |
| Systolic BP (mm Hg) | >=140 (Chobanian et al., 2003) |
| Diastolic BP (mm Hg) | >=90 (Chobanian et al., 2003) |
These indicator variables were then summed to create summary scores for each of the subscales (inflammatory, metabolic and cardiovascular). Those missing more than half of the individual components of a particular subscale (i.e., more than one for inflammation and cardiovascular subscales and more than two for the metabolic subscale) were scored as “missing” for that subscale. In addition, individuals who self-reported recent infection (e.g., cold, cough, respiratory infection), or whose white blood cell count was greater than 10,000 per μL, were assigned a missing code for C-reactive protein and/or albumin if their respective values were in the high risk range since this could reflect acute infection rather than chronic elevation. In fact, missing data were not a major problem: 81.5 percent of the cohort had no missing data and another 15 percent were missing only one measure. For those with missing data on no more than one component of a subscale (or two in the case of the metabolic indicators), summary scores were calculated by summing available data and then scaling that score by the ratio of “number of items in the subscale” to “number of items with available data”.
The summary, multi-system allostatic load score was created by summing the subscale scores. No cross-system imputations were made for missing items; summary allostatic load scores were created only for participants with a score for each of the three subscales. To allow for greater ease of comparisons across indices of SES and ethnicity, summary scores were rounded to nearest integers. Rounding affected less than five percent of the scores and comparisons of results based on raw score revealed no differences. Additional algorithms for scoring risk, including use of quartile cut-points (Seeman et al., 2004; Seeman, McEwen, Rowe, & Singer, 2001) and using reported medication use as a criterion for “high risk”, were also examined. Results were consistent with those found based on the primary scoring system outlined above using clinically defined cut-points and actual levels of biological parameters (i.e., those on medications who have “low risk” levels of a given biological parameter such as blood pressure or glucose are counted as “low risk”).
Socio-economic Status
Education was measured in terms of years completed as well as highest level completed (i.e., grade school, some high school, complete high school, some college, complete college or more). Indicator variables were created using “completed college or more” as the reference group to allow for examination of non-linear relationships to allostatic load. Income was measured based on the poverty income ratio, an index reflecting the ratio of household income to the household poverty level determined by area of residence and household size. (Centers for Disease Control, 1996). poverty income ratio values were examined both as a continuous measures and based on five categories (<1, 1–1.99, 2–2.99, 3–3.99, 4–4.99 and 5+) with those reporting incomes five (or more) times the poverty ratio serving as the reference group.
Ethnicity
Blacks and Mexican Americans were over-sampled, allowing for separate analysis of these two ethnic groups, along with whites and a fourth “other” category. Hispanics other than Mexican Americans were classified as “other” in NHANES (Centers for Disease, 1996).
Covariates
Multivariable analyses included controls for smoking and physical activity. Smoking status was coded by two indicator variables designating current and former smokers (ever smoked >=100 cigarettes), with never smokers serving as the reference group. Total physical activity was measured by summing weighted scores for all reported moderate and vigorous activities. Weighted scores for each activity reflect the intensity rating (i.e., metabolic equivalents [MET’s]) for that activity (Pate et al., 1995) multiplied by the reported monthly frequency.
Analyses
All analyses were weighted, using the NHANES final examination weight (National Center for Health Statistics [NCHS], 1996), to adjust for probabilities of selection and non-response. Initial descriptive analyses compared characteristics of those included versus excluded from the present analyses. For those included in the analyses, distributions of the various components of allostatic load were also examined by education and poverty income ratio levels.
Distributions of total allostatic load and sub-scale scores (inflammatory, metabolic and cardiovascular) were examined within groups defined by levels of education, income, and race, after standardizing the age distribution of each comparison group to the age distribution of the US at the time of the 2000 Census (as recommended by NCHS, age was categorized into 10 year units from ages 20 through 80 and older [i.e. 20–29, 30–39…80+]).
Cumulative logistic regression models were then fit to assess relative odds for increasing allostatic load by education and by poverty income ratio. For an ordinal variable like allostatic load, this approach models the log of the cumulative odds (i.e., the odds of allostatic load score k or higher against score less than k) as a linear function of predictors, fitting a single cumulative odds ratio per predictor. The cumulative OR for a predictor is the average effect of the predictor on cumulative odds at different levels k.
A series of these cumulative logistic models were fit to estimate the independent effects of education and income after: a) controlling for age and gender; b) additional controls for race (black, Mexican American and other vs. white) and c) further controls for smoking (current, former vs. never) and physical activity. Owing to secular changes in educational attainment over the last century in America, age is a major potential confounder of education associations. To minimize any residual confounding by age, we used both age-weighting and age-controls: all models were weighted to standardize the age distribution of SES groups being compared; age was also included as a covariate in the model. Squared terms were used to test for nonlinearities in age and SES effects. Where such effects were seen, categorical versions of age, education and/or income were examined to elucidate the pattern of the non-linearity. Possible age differences in SES effects were also examined through tests of age-by-SES interactions along with examination of possible race-by-SES interactions..
To account for the complex survey design, we used the SVY procedures in Stata (version 9) to fit the cumulative logistic regression models and to obtain p-values for trends for the overall sample. For ethnic-stratified analyses, where data in some primary sampling units were sparse, we ran regression models (without the SVY command), accounting for the complex survey design by using sampling weights and robust variance estimation with clustering at the primary sampling unit level, using Stata’s cluster option.
RESULTS
Table 2 provides descriptive information comparing those included in the analyses to the overall NHANES sample aged 20+ and to those excluded from the current analyses due to missing data, no mobile examination center exam, or pregnancy. As shown, the analysis sample is representative of the overall NHANES sample with respect to age, gender, race/ethnicity, and education and poverty income ratio. The analysis sample had a median age of 42, with 48.7 percent male, 76.9 percent white, 10.4 percent black, 5 percent Mexican American and 7.7 percent “other”. A majority of the participants had completed high school or more, and reported household incomes twice the poverty level or more.
Table 2.
Characteristics of the NHANES III and study samples, based on data for adults aged 20 or more years from the National Health and Nutrition Survey, 1988–1994*
A. Demographic Characteristics
B. Biological Indicators
| Variables | NHANES aged 20+ (n=18,825) | Study Sample (n=15,578) | Excluded Sample (n=3,247) |
|---|---|---|---|
| % | % | % | |
| Age (Median) | 41.0 | 42.0 | 32.0 |
| Age Groups | |||
| 20–29 | 22.2 | 21.3 | 40.2 |
| 30–39 | 24.0 | 23.8 | 29.1 |
| 40–49 | 18.7 | 19.1 | 10.5 |
| 50–59 | 12.4 | 12.8 | 6.1 |
| 60–69 | 11.4 | 11.7 | 5.7 |
| 70–79 | 7.8 | 8.0 | 4.9 |
| 80+ | 3.4 | 3.4 | 3.5 |
| Male | 47.6 | 48.7 | 26.7 |
| Race/Ethnicity | |||
| White | 76.2 | 76.9 | 63.0 |
| Black | 10.9 | 10.4 | 21.6 |
| Mexican American | 5.1 | 5.0 | 7.3 |
| Other | 7.7 | 7.7 | 8.1 |
| Education | |||
| Grade School | 11.6 (11.6)† | 11.6 | 10.9 (12.0) † |
| Some High School | 13.3 (13.3) | 13.4 | 11.4 (12.6) |
| Complete High School | 33.5 (33.7) | 33.6 | 32.1 (35.5) |
| Some College | 20.6 (20.7) | 20.7 | 19.3 (21.4) |
| >=Complete College | 20.6 (20.7) | 20.8 | 16.8 (18.6) |
| Missing | 0.5 | 9.5 | |
| Poverty Income Ratio (median) | 2.9 | 2.9 | 2.8 |
| Poverty Income Ratio | |||
| <1.0 | 11.8 | 11.5 | 17.4 |
| 1.0–1.99 | 19.8 | 19.7 | 22.3 |
| 2.0–2.99 | 19.8 | 20.1 | 13.5 |
| 3.0–3.99 | 16.8 | 17.0 | 13.0 |
| 4.0–4.99 | 9.9 | 10.2 | 5.3 |
| >=5.0 | 15.4 | 15.4 | 16.1 |
| Missing | 6.5 | 6.2 | 12.4 |
| Allostatic Summary Score (Mean (Median)) | 1.6 (1.0) | 1.6 (1.0) | 0.91 (1.0) |
| (range: 0.–8) | |||
| Inflammatory Score (Mean (Median)) | 0.25 (0) | 0.25 (0) | 0.60 (0) |
| (range: 0 –2) | |||
| Metabolic Score (Mean (Median)) | 1.1 (1.0) | 1.1 (1.0) | 0.97 (1.0) |
| (range: 0–4) | |||
| Cardiovascular Score (Mean (Median)) | 0.28 (0) | 0.28 (0) | 0.31 (0) |
| (range: 0–3) | |||
| C-Reactive Protein | |||
| High Risk (>=0.3 mg/dL) | 17.7 | 17.6 | 21.5 |
| Low Risk (<0.3 mg/dL) | 71.3 | 71.6 | 57.0 |
| High Risk and Infection | 11.0 | 10.8 | 21.5 |
| Missing | [4.8]‡ | [2.1] ‡ | [60.3] ‡ |
| Albumin | |||
| High Risk (<3.8 g/dL) | 6.8 | 6.3 | 28.9 |
| Low Risk (>=3.8 g/dL) | 89.3 | 90.2 | 42.6 |
| High Risk and Infection | 4.0 | 3.5 | 28.6 |
| Missing | [5.1] ‡ | [2.4] ‡ | [60.3] ‡ |
| Glycosylated Hemoglobin | |||
| High Risk (>=6.4% ) | 5.8 | 5.9 | 0.6 |
| Low Risk (<6.4%) | 94.2 | 94.1 | 99.4 |
| Missing | [3.2] ‡ | [0.5] ‡ | [58.6] ‡ |
| Total Cholesterol | |||
| High Risk (>=240 mg/dL ) | 19.7 | 19.6 | 23.5 |
| Low Risk (<240 mg/dL) | 80.3 | 80.4 | 76.5 |
| Missing | [4.2] ‡ | [1.4] ‡ | [59.8] ‡ |
| HDL Cholesterol | |||
| High Risk (<40 mg/dL) | 23.5 | 23.8 | 6.7 |
| Low Risk (>=40 mg/dL) | 76.5 | 76.2 | 93.3 |
| Missing | [5.0] ‡ | [2.2] ‡ | [60.2] ‡ |
| Waist to-Hip Ratio | |||
| High Risk (men >0.9, women>0.85) | 62.8 | 63.1 | 56.8 |
| Low Risk (men<=0.9, women<=0.85) | 37.2 | 36.9 | 43.2 |
| Missing | [4.1] ‡ | [3.6] ‡ | [13.2] ‡ |
| Systolic Blood Pressure | |||
| High Risk (>=140 mm Hg ) | 14.5 | 14.6 | 11.0 |
| Low Risk (<140 mm Hg) | 85.6 | 85.4 | 89.1 |
| Missing | [0.2] ‡ | [0.2] ‡ | [0.4] ‡ |
| Diastolic Blood Pressure | |||
| High Risk (>=90 mm Hg) | 6.6 | 6.6 | 6.4 |
| Low Risk (<90 mm Hg) | 93.4 | 93.4 | 93.7 |
| Missing | [0.3] ‡ | [0.3] ‡ | [0.5] ‡ |
| Pulse Rate | |||
| High Risk (>=90 bt/min ) | 6.6 | 6.2 | 13.4 |
| Low Risk (<90 bt/min) | 93.5 | 93.8 | 86.6 |
| Missing | [2.7] ‡ | [2.6] ‡ | [3.8] ‡ |
Weighted by NHANES mobile examination center weight (WTPFEX6)
Percentage excludes those missing.
Number in brackets indicates percent with missing data; percentages for remaining groups total to 100%
Examination of the individual biological measures revealed that high waist-hip-ratio was the most prevalent high risk factor with 63.1 percent of the cohort having waist-hip-ratios above the high-risk threshold. Next most frequent high-risk values were for low HDL cholesterol (23.8 percent), high total cholesterol (19.6 percent) and high C-reactive protein (17.6 percent). Each of the individual biomarkers exhibited the expected age-related trend toward less optimal values: age was significantly and positively correlated with HgA1c (0.27), total cholesterol (0.33), waist-hip-ratio (0.42), C-reactive protein (0.11), systolic blood pressure (0.55), diastolic blood pressure (0.06) and HDL cholesterol (0.02) and negatively correlated with albumin (−0.25) and pulse (−0.02).
The expected education and income gradients were also seen for each of the individual biomarkers (with the exception of albumin and diastolic blood pressure), with increasing prevalence of “high risk” values with lower education (see Table 3A). Parallel analyses based on the poverty income ratio revealed generally similar results, with the exception of total cholesterol which no longer exhibited a gradient.
Table 3.
Table 3a. Percent with “high-risk” values for individual biomarkers by levels of education and poverty income ratio, based on data for adults aged 20 or more years from the National Health and Nutrition Survey, 1988–1994*
Table 3b. Age-adjusted means and inter-quartile ranges and percent with high cumulative biological risk scores by levels of education and poverty income ratio, based on data for adults aged 20 or more years from the National Health and Nutrition Survey, 1988–1994*
| C-reactive protein | Albumin | Glyco. Hemo globin. | Total Chol. | HDL Chol | waist-hip-ratio | systolic blood pressure | diastolic blood pressure | Pulse | |
|---|---|---|---|---|---|---|---|---|---|
| % with “high-risk” values | |||||||||
| Education | |||||||||
| Grade School | 22.3% | 6.4% | 10.6% | 23.3% | 29.7% | 75.4% | 16.9% | 6.3% | 8.6% |
| Some High School | 24.4% | 7.9% | 8.5% | 21.2% | 27.8% | 71.8% | 16.9% | 7.1% | 9.9% |
| Complete High School | 22.7% | 7.3% | 6.0% | 21.5% | 24.5% | 65.9% | 16.1% | 7.0% | 5.9% |
| Some College | 20.3% | 6.5% | 5.6% | 20.1% | 21.8% | 62.2% | 15.7% | 6.7% | 5.5% |
| >=Complete College | 14.8% | 6.6% | 3.6% | 17.3% | 20.7% | 57.6% | 13.7% | 7.2% | 3.9% |
| P-value for Trend | 0.003 | 0.9 | <0.0001 | 0.02 | <0.0001 | <0.0001 | 0.004 | 0.4 | <0.0001 |
| Poverty Income Ratio | |||||||||
| <1.0 | 28.1% | 9.7% | 10.1% | 20.6% | 26.4% | 71.0% | 19.9% | 7.4% | 8.4% |
| 1.0–1.99 | 25.1% | 7.4% | 7.7% | 20.2% | 25.7% | 66.8% | 16.4% | 7.3% | 8.4% |
| 2.0–2.99 | 19.1% | 6.4% | 6.4% | 21.5% | 25.8% | 66.4% | 14.8% | 7.0% | 6.5% |
| 3.0–3.99 | 18.2% | 5.4% | 5.0% | 19.8% | 22.5% | 64.6% | 14.5% | 6.2% | 5.5% |
| 4.0–4.99 | 17.9% | 6.2% | 4.5% | 20.4% | 18.8% | 62.8% | 15.3% | 5.7% | 5.0% |
| >=5.0 | 16.6% | 8.1% | 4.7% | 18.3% | 21.8% | 56.7% | 14.6% | 6.9% | 3.7% |
| P-value for Trend | <0.0001 | 0.06 | <0.0001 | 0.3 | 0.003 | <0.0001 | <0.0001 | 0.3 | <0.0001 |
| Total allostatic load (AL) | Inflammation | Metabolism | Cardiovascular | % AL = 2+ | % Inflammation = 1+ | % Metabolism = 1+ | % Cardiovascular = 1+ | ||
| Mean (25th, 75th percentiles) | % high scores | ||||||||
| Education | |||||||||
| Grade School | 1.93 (1.00, 3.00) | 0.26 (0.00, 0.00) | 1.37 (1.00, 2.00) | 0.32 (0.00, 0.00) | 58.1 | 22.9 | 81.7 | 24.3 | |
| Some High School | 1.89 (1.00, 3.00) | 0.29 (0.00, 0.00) | 1.28 (1.00, 2.00) | 0.34 (0.00, 1.00) | 57.8 | 24.6 | 76.5 | 27.9 | |
| Complete High School | 1.72 (1.00, 3.00) | 0.28 (0.00, 0.00) | 1.17 (0.00, 2.00) | 0.29 (0.00, 0.00) | 52.0 | 24.2 | 73.1 | 24.0 | |
| Some College | 1.60 (1.00, 2.00) | 0.25 (0.00, 0.00) | 1.09 (0.00, 2.00) | 0.28 (0.00, 0.00) | 47.2 | 21.8 | 68.9 | 23.1 | |
| >=Complete College | 1.43 (0.00, 2.00) | 0.21 (0.00, 0.00) | 0.99 (0.00, 2.00) | 0.25 (0.00, 0.00) | 41.5 | 17.9 | 64.7 | 19.7 | |
| P-value for Trend | <0.0001 | 0.03 | <0.0001 | 0.001 | <0.0001 | 0.04 | <0.0001 | 0.001 | |
| Poverty Income Ratio | |||||||||
| <1.0 | 1.95 (1.00, 3.00) | 0.34 (0.00, 1.00) | 1.27 (1.00, 2.00) | 0.36 (0.00, 1.00) | 57.6 | 29.0 | 76.6 | 27.9 | |
| 1.0–1.99 | 1.79 (1.00, 3.00) | 0.30 (0.00, 1.00) | 1.19 (0.00, 2.00) | 0.33 (0.00, 1.00) | 53.2 | 25.9 | 73.1 | 26.0 | |
| 2.0–2.99 | 1.70 (1.00, 3.00) | 0.24 (0.00, 0.00) | 1.19 (0.00, 2.00) | 0.28 (0.00, 0.00) | 51.5 | 20.6 | 72.1 | 23.9 | |
| 3.0–3.99 | 1.59 (1.00, 2.00) | 0.22 (0.00, 0.00) | 1.11 (0.00, 2.00) | 0.27 (0.00, 0.00) | 47.1 | 19.3 | 71.4 | 21.9 | |
| 4.0–4.99 | 1.53 (0.00, 2.00) | 0.23 (0.00, 0.00) | 1.05 (0.00, 2.00) | 0.26 (0.00, 0.00) | 44.9 | 20.7 | 68.2 | 21.5 | |
| >=5.0 | 1.48 (0.00, 2.00) | 0.23 (0.00,0.00) | 1.00 (0.00, 2.00) | 0.25 (0.00,0.00) | 43.0 | 19.3 | 64.9 | 19.9 | |
| P-value for Trend | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |
All values are weighted by NHANES mobile examination center weight: WTPFEX6 and age standardized to the 2000 US Census population.
P-values for trend were determined based on regression models that considered education and poverty as ordinal variables in the models.
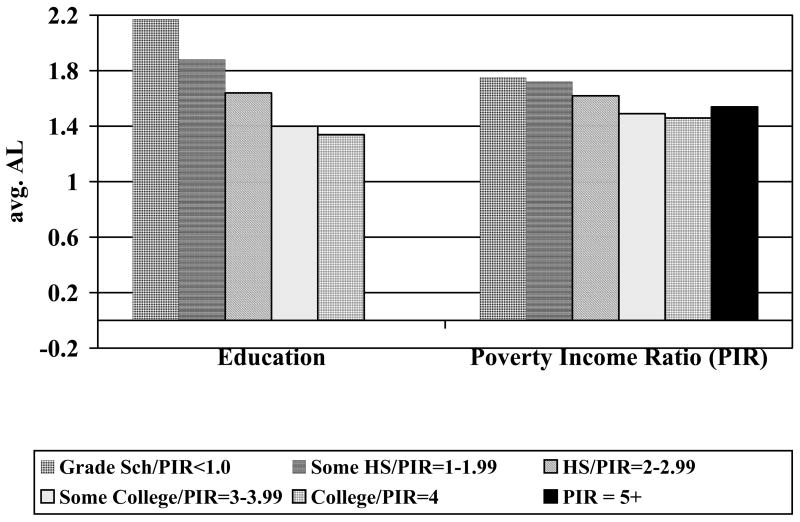
Education and income gradients were also found for the three subscales and the overall allostatic load index. Due to the skew of the various biological indices (with expected lower frequencies with higher scores), we examined both the scale means as well as “percent with higher scores”. Each of the subscales revealed the expected education gradients with respect to both mean scores and “percent with high scores” – those with less education having higher scores (see Table 3B). Analyses based on the poverty income ratio revealed similar, though less steep, gradients. For the overall cumulative risk index of allostatic load, a gradient of increasing cumulative allostatic load with decreasing level of education was evident (see Figure 1); a similar gradient was also seen for the poverty income ratio (See Figure 1).
Figure 1.
Mean Overall Biological Risk (Allostatic Load) Scores by Education and Income Groups
Stratification by ethnicity revealed parallel education gradients for low overall allostatic load (i.e. scores of 0 or 1; see Figure 2A) and for higher allostatic load (i.e., scores of 2 or more; see Figure 2B) and formal tests for ethnicity-by-education interactions were non-significant. Parallel gradients were also found by poverty income ratio within each ethnic group (data not shown). Significant education and income gradients were also seen for higher risk scores on most of the subscales (see Tables 4 & 5). For whites and blacks, the strongest gradients were seen for indices of metabolic and cardiovascular risks. For Mexican Americans, the strongest gradients were seen for the inflammation subscale.
Figure 2.
Figure 2A. Age-adjusted percentages with Low Overall Biological Risk (Allostatic Load) (scores=0–1) by Education and Ethnicity: NHANES III
Figure 2B. Age-adjusted percentages with Higher Total Biological Risk (Allostatic Load) (scores=2+) by Education and Ethnicity: NHANES III
Table 4.
Ethnic-specific gradients in inflammation, metabolic risk and cardiovascular subscales by education, based on data for adults aged 20 or more years from the National Health and Nutrition Survey, 1988–1994*
| Inflammation | Metabolic Risk | Cardiovascular Risk | ||||
|---|---|---|---|---|---|---|
| 0 | 1+ | 0 | 1+ | 0 | 1+ | |
| Whites – N’s | 4,728 | 1,507 | 2,317 | 4,212 | 4,517 | 2,003 |
| % | % | % | % | % | % | |
| Grade School | 80.6 | 19.4 | 24.2 | 75.8 | 77.3 | 22.7 |
| Some HS | 78.9 | 21.1 | 22.8 | 77.2 | 71.9 | 28.1 |
| HS | 77.4 | 22.6 | 26.3 | 73.7 | 77.0 | 23.0 |
| Some College | 79.5 | 20.5 | 31.3 | 68.7 | 77.2 | 22.9 |
| >=College | 83.2 | 16.8 | 36.0 | 64.0 | 80.9 | 19.1 |
| P-Value | 0.2 | <0.0001 | 0.005 | |||
| Blacks- N’s | 2,662 | 1,268 | 2,037 | 2,188 | 2,900 | 1,318 |
| % | % | % | % | % | % | |
| Grade School | 66.0 | 34.0 | 24.2 | 75.8 | 60.9 | 39.1 |
| Some HS | 67.0 | 33.0 | 26.7 | 73.3 | 63.4 | 36.6 |
| HS | 65.1 | 34.9 | 33.9 | 66.1 | 66.8 | 33.3 |
| Some College | 67.5 | 32.5 | 32.1 | 67.9 | 72.7 | 27.3 |
| >=College | 66.6 | 33.4 | 37.1 | 62.9 | 71.5 | 28.5 |
| P-Value | 0.9 | <0.0001 | <0.0001 | |||
| Mexicans – N’s | 3,069 | 959 | 1,525 | 2,676 | 3,233 | 959 |
| % | % | % | % | % | % | |
| Grade School | 73.0 | 27.1 | 14.2 | 85.8 | 74.5 | 25.6 |
| Some HS | 74.2 | 25.8 | 18.5 | 81.5 | 77.1 | 22.9 |
| HS | 76.3 | 23.8 | 18.5 | 81.5 | 72.7 | 27.4 |
| Some College | 82.3 | 17.7 | 21.6 | 78.4 | 70.5 | 29.5 |
| >=College | 87.7 | 12.3 | 23.4 | 76.6 | 77.2 | 22.8 |
| P-Value | 0.001 | <0.0001 | 0.08 | |||
| Others - N’s | 465 | 130 | 260 | 362 | 486 | 136 |
| % | % | % | % | % | % | |
| Grade School | 80.6 | 19.4 | 14.2 | 85.8 | 76.0 | 24.0 |
| Some HS | 67.2 | 32.8 | 25.4 | 74.6 | 83.3 | 16.7 |
| HS | 76.8 | 23.2 | 23.8 | 76.3 | 77.6 | 22.5 |
| Some College | 84.9 | 15.1 | 27.2 | 72.8 | 88.8 | 11.2 |
| >=College | 75.8 | 24.2 | 31.0 | 69.0 | 82.1 | 18.0 |
| P-Value | 0.9 | 0.006 | 0.1 | |||
All values are weighted by NHANES mobile examination center weight: WTPFEX6 and age standardized to the 2000 US Census population. P-values for trend were determined based on regression models that considered education as an ordinal variable in the model.
Table 5.
Ethnic-specific gradients in inflammation, metabolic risk and cardiovascular subscales by poverty income ratio, based on data for adults aged 20 or more years from the National Health and Nutrition Survey, 1988–1994*
| Inflammation | Metabolic Risk | Cardiovascular Risk | ||||
|---|---|---|---|---|---|---|
| 0 | 1+ | 0 | 1+ | 0 | 1+ | |
| Whites – N’s | 4,413 | 1,385 | 2,189 | 3,877 | 4,224 | 1,834 |
| % | % | % | % | % | % | |
| <1.0 | 73.4 | 26.6 | 24.5 | 75.5 | 75.0 | 25.0 |
| 1.0–1.99 | 76.5 | 23.5 | 26.3 | 73.7 | 75.5 | 24.5 |
| 2.0–2.99 | 80.7 | 19.3 | 28.4 | 71.7 | 76.5 | 23.6 |
| 3.0–3.99 | 81.9 | 18.1 | 28.1 | 72.0 | 78.4 | 21.6 |
| 4.0–4.99 | 80.1 | 19.9 | 32.1 | 67.9 | 78.7 | 21.3 |
| >=5.0 | 81.0 | 19.0 | 35.2 | 64.9 | 80.1 | 19.9 |
| P-Value | 0.02 | <0.0001 | 0.004 | |||
| Blacks – N’s | 2,411 | 1,154 | 1,870 | 1,963 | 2,641 | 1,186 |
| % | % | % | % | % | % | |
| <1.0 | 62.2 | 37.8 | 29.6 | 70.5 | 61.9 | 38.1 |
| 1.0–1.99 | 66.3 | 33.7 | 32.7 | 67.3 | 65.7 | 34.4 |
| 2.0–2.99 | 68.2 | 31.8 | 29.8 | 70.2 | 67.0 | 33.0 |
| 3.0–3.99 | 66.4 | 33.6 | 36.3 | 63.7 | 67.7 | 32.3 |
| 4.0–4.99 | 71.2 | 28.8 | 33.2 | 66.8 | 75.7 | 24.4 |
| >=5.0 | 68.0 | 32.0 | 36.5 | 63.5 | 74.1 | 25.9 |
| P-Value | 0.03 | 0.02 | <0.0001 | |||
| Mexican-Americans – N’s | 2,716 | 836 | 1,368 | 2,332 | 2,874 | 818 |
| % | % | % | % | % | % | |
| <1.0 | 71.9 | 28.1 | 15.1 | 84.9 | 76.2 | 23.8 |
| 1.0–1.99 | 74.7 | 25.4 | 18.6 | 81.5 | 76.8 | 23.2 |
| 2.0–2.99 | 78.0 | 22.1 | 19.6 | 80.4 | 72.1 | 27.9 |
| 3.0–3.99 | 79.0 | 21.0 | 17.6 | 82.4 | 70.8 | 29.2 |
| 4.0–4.99 | 76.4 | 23.6 | 19.9 | 80.1 | 72.2 | 27.8 |
| >=5.0 | 83.5 | 16.5 | 23.6 | 76.4 | 76.0 | 24.0 |
| P-Value | 0.02 | 0.005 | 0.1 | |||
| Others – N’s | 423 | 118 | 242 | 323 | 441 | 124 |
| % | % | % | % | % | % | |
| <1.0 | 73.2 | 26.8 | 19.2 | 80.8 | 71.9 | 28.2 |
| 1.0–1.99 | 72.4 | 27.6 | 29.4 | 70.6 | 73.7 | 26.4 |
| 2.0–2.99 | 82.0 | 18.0 | 26.0 | 74.0 | 80.1 | 19.9 |
| 3.0–3.99 | 78.9 | 21.1 | 29.1 | 70.9 | 81.1 | 18.9 |
| 4.0–4.99 | 81.2 | 18.8 | 33.8 | 66.3 | 87.3 | 12.7 |
| >=5.0 | 89.1 | 10.9 | 49.0 | 51.0 | 97.0 | 3.0 |
| P-Value | 0.1 | 0.04 | <0.0001 | |||
All values are weighted by NHANES mobile examination center weight: WTPFEX6 and age standardized to the 2000 US Census population. P-values for trend were determined based on regression models that considered poverty as an ordinal variable in the model.
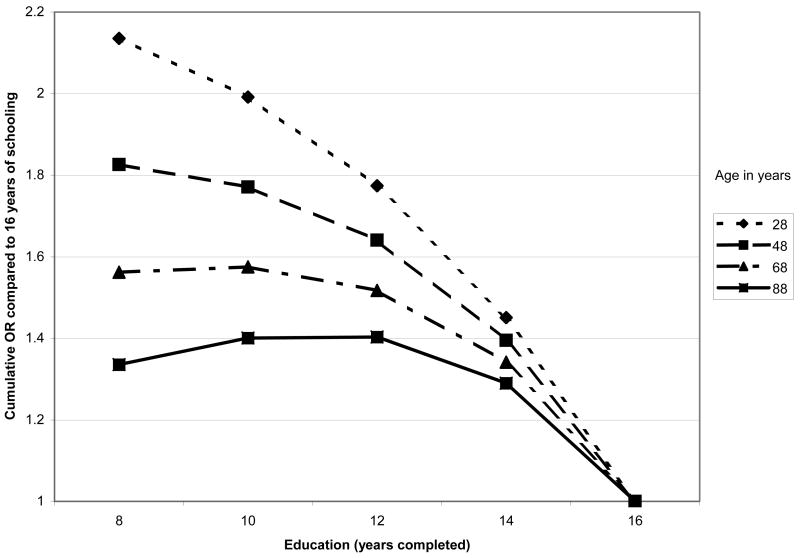
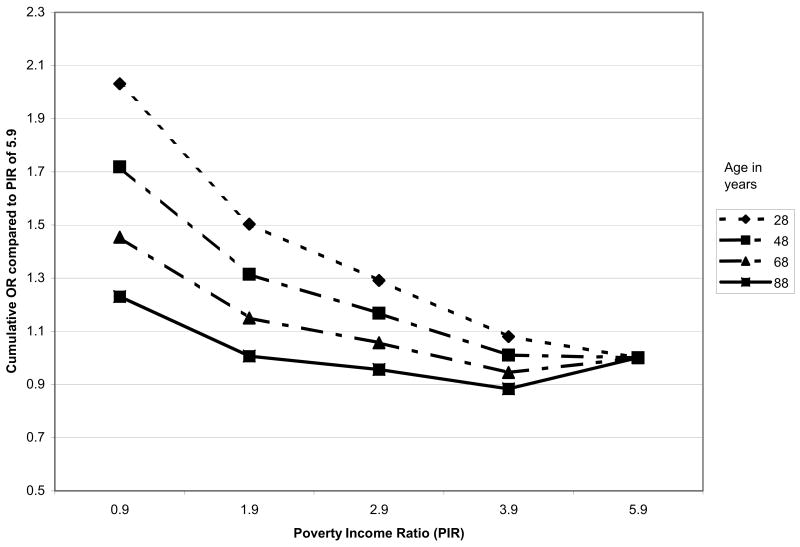
Multivariable cumulative logistic regression models were examined next to assess education and income gradients in allostatic load with controls for age and other potential confounders. Consistent with previous analyses of age trends in allostatic load (Crimmins, Johnston, Hayward, & Seeman, 2003), significant non-linearities were seen, with age-related increases in allostatic load seen to flatten at the oldest ages. These effects were well captured by a 7-category age covariate (reflecting decades) beginning with 20–29 years, and continuing to a top category of 80+ years. As shown in Table 6, cumulative logistic regression models including age and gender, along with both education and poverty income ratio, confirmed that education and income gradients in cumulative biological risk scores each remained significant. Significant non-linearities in both the education and poverty income ratio/income gradients were also seen (see Model 1 “continuous SES model”, Table 6). Examination of the categorical designations for education and income revealed that for education the non-linearity reflected a steeper gradient at higher levels of education with a flatter gradient at the lower end while the reverse was true for income (poverty income ratio) (see Model 2, Table 6). Furthermore, tests for possible age differences in the patterns of these associations revealed significant interactions for age with both education p=0.007) and income (p=0.02). As shown in Figures 3a & 3b, the interactions indicated a weakening of the education and income gradients with older age. Models that included both age interactions simultaneously suggested that the age differences are more marked for education where the age interaction remained marginally significant (p=0.06; See Model 3, Table 6) while the income interaction was reduced to non-significance. Further adjustments for race/ethnicity and lifestyle factors (i.e., smoking and physical activity) did not alter the education and income effects substantially (see Table 6, Models 4 & 5). Tests for possible education or income interactions with ethnicity were all non-significant. Main effects for ethnicity indicated significantly increased risks among blacks and marginally increased risks for Mexican-Americans as compared with whites, independent of education and income.
Table 6A.
Estimated relative cumulative odds (95% confidence intervals) of higher total allostatic load score (AL) by education and income from ulti-nomial ordinal logistic regression models, based on data for adults aged 20 or more years from the National Health and Nutrition Survey, 1988–1994*
| Model 1 Continuous SES | Model 2 Categorical SES | Model 3 Combined Education, Income model w/age interactions | Model 4 = Model 3 + Adjusted for Ethnicity † | Mode 5= Model 4 Adjusted for Lifestyle Factors‡ | |
|---|---|---|---|---|---|
| Education (years) | 0.08 (p=0.05) | ||||
|
| |||||
| Education (squared) | −0.006 (p<0.0001) | ||||
|
| |||||
| Grade School | 1.8 (1.5–2.3) | 1.8 (1.5–2.3) | 1.8 (1.4–2.3) | 1.6 (1.3–2.1) | |
| Some High School | 1.8 (1.4–2.2) | 1.8 (1.4–2.2) | 1.8 (1.4–2.2) | 1.6 (1.3–2.0) | |
| Complete High School | 1.6 (1.4–1.9) | 1.6 (1.4–1.9) | 1.6 (1.4–1.9) | 1.5 (1.3–1.8) | |
| Some College | 1.4 (1.2–1.6) | 1.4 (1.2–1.6) | 1.4 (1.2–1.6) | 1.4 (1.2–1.6) | |
| >=Complete College | Reference | Reference | Reference | Reference | |
| P-value for Trend | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |
| Age* Education (Centered) | 0.00076 (p=0.07) | 0.00075 (p=0.08) | 0.0007 (p=0.09) | ||
| Poverty Income Ratio (PIR) | −0.223 (p<0.0001) | ||||
| PIR - squared | 0.019 (p=0.03) | ||||
| <1.0 | 1.7 (1.4–2.1) | 1.7 (1.4–2.2) | 1.6 (1.3–2.1) | 1.6 (1.2–2.0) | |
| 1.0–1.99 | 1.3 (1.0–1.6) | 1.3 (1.1–1.6) | 1.3 (1.0–1.6) | 1.2 (1.0–1.5) | |
| 2.0–2.99 | 1.1 (1.0–1.4) | 1.2 (1.0–1.4) | 1.1 (1.0–1.4) | 1.1 (0.9–1.3) | |
| 3.0–3.99 | 1.0 (0.8–1.1) | 1.0 (0.9–1.1) | 1.0 (0.8–1.1) | 1.0 (0.8–1.1) | |
| 4.0–4.99 | 1.0 (0.8–1.3) | 1.0 (0.8–1.3) | 1.0 (0.8–1.3) | 1.0 (0.8–1.3) | |
| >=5.0 | Reference | Reference | Reference | Reference | |
| P-value for Trend | <0.0001 | <0.0001 | <0.0001 | 0.001 | |
| Age* PIR (Centered) | 0.00099 (p=0.2) | 0.00087 (p=0.3) | 0.0009 (p=0.3) | ||
Adjusted for age (using age-standardized weights, based on the US 2000 Census Population distribution, as well as age as a categorical variable) and gender.
Additional adjustment for race/ethnic groups (black, Mexican American, other vs. white)
Additional adjustment for smoking (now, former vs. never) and physical activity (overall moderate and vigorous activity score).
Figure 3.
Figure 3a. Age-specific Education Gradients in Cumulative Relative Odds of Higher Allostatic Load: NHANES III
Figure 3b. Age-specific Poverty Income Ratio Gradients in Cumulative Relative Odds of Higher Allostatic Load: NHANES III.
DISCUSSION
Analyses of NHANES III, a nationally representative sample of the US, provide evidence of education and income gradients in biological risks. Indeed, gradients were seen for each of the individual biomarkers comprising our index of biological risk, along with consistent gradients for each of the subscales reflecting major systems of biological activity such as inflammation, metabolism and cardiovascular activity. Not surprisingly in light of the foregoing, cumulative biological risk profiles (allostatic load) showed significant gradients as well. This pattern of findings is consistent with the proposition that SES is an enduring characteristic of individuals’ lives with broad contextual implications in terms of the conditions of one’s life, including both types and frequency of “stressors” as well as resources available to deal with such stressors and that such SES-related differences in life conditions are likely to result in “wear and tear” on many (if not all) of the body’s physiological regulatory systems (McEwen & Seeman, 1999).
In contrast to some previous studies (Karlamangla et al., 2005; Watkins et al., 1986) suggesting that SES gradients in biological risk profiles were shallower in blacks, gradients based on the NHANES data were found to be similar across whites, Mexican Americans and blacks as well as an undifferentiated group of “others”. The strength of the NHANES data are their national representation and the over-sampling of blacks and Mexican Americans which allows for greater statistical power in assessments of SES gradients within these two important ethnic groups. By contrast, earlier findings from the Coronary Artery Risk Development in Young Adults (CARDIA) study (Karlamangla et al., 2005) reflect blacks and whites aged only 18–30 at baseline and drawn from four specific cities (Oakland, CA; Chicago, IL; Birmingham, allostatic load; and Minneapolis, MN) while the Watkins study (Watkins et al., 1986), based on data from the Multiple Risk Factor Intervention Trial (MRFIT), included only black and white men at risk for cardiovascular disease. The weaker SES gradients found in these earlier studies could reflect effects of enrollment selection criteria (i.e., risk for heart disease in the case of the MRFIT study and, in the case of the CARDIA cohort, over-sampling of black men and women with high school education or more; [Friedman et al., 1988]) and, at least for the CARDIA cohort, a focus on younger ages (i.e., oldest participants were only 40 at final follow-up in Karlamangla et al, 2005) when cumulative differences in biological risk are unlikely yet to be fully manifest.. The NHANES data provide a much broader and representative picture of the overall adult US population and the present analyses indicate that education and income gradients in biological risk are similar across major ethnic groups. Indeed, the lack of significant ethnic variation in these SES gradients of biological risk suggests that, regardless of ethnic/minority status, those achieving higher education and income appear to reap benefits with respect to lower cumulative biological risk profiles – an encouraging pattern to the extent that social policies and programs can be further targeted to foster achievement of higher levels of education and income for all members of society.
Interestingly, controls for two major health behaviors (smoking and exercise) did not alter the SES gradients substantially, indicating that such lifestyle factors, while contributors to health disaparities, do not explain observed differentials in biological risk profiles – a finding consistent with the hypothesis that such biological risk profiles reflect more global effects of SES in terms of greater versus lesser “wear and tear” on physiological regulatory systems over the life course. Evidence that ethnic differences in such biological risk profiles remain, independent of SES, also suggests that there are other non-SES related differences in the life experiences of those of different ethnicities living in the US that contribute to differential rates of accumulating biological risk.
Evidence of non-linearities in these SES gradient in biological risk are also worth noting as they point to potentially greater benefits from educational gains at the lower ends of the education gradient. If substantiated through replication in additional studies, such findings have important policy implication with respect to where investments might yield maximal returns with respect to reductions in ultimate health disparities through reductions in the underlying patterns of biological risk profiles.
Strengths of the present analyses include the nationally representative nature of the NHANES III sample and the over-sampling of blacks and Mexican Americans, which allowed for more detailed examination of possible ethnic variation in SES-related gradients in biological risks. The range of available biomarkers also allowed for examination of the consistency of SES gradients across multiple categories of known biological risk factors, including inflammation, metabolism and cardiovascular risk profiles.
Limitations that should be acknowledged include the fact that the NHANES data represent cross-sectional data such that we cannot evaluate temporal trends in biological risk accumulation or the degree to which this is different by SES. Also, available biomarker data provide unequal assessments across different physiological systems, ranging from two indicators of inflammation to four indicators of metabolic profiles. Like many large population-based studies, the NHANES also provides biomarker data based solely on a single, static assessment; no information is available on possible differences in system dynamics, nor can we assess the degree to which the available information reflects individuals’ “usual” status. Our use of a scoring system based on a simple count of whether or not the individual’s value for a given biomarker was in a clinically defined “higher risk” category likely provides a relatively crude index of cumulative biological risks as information on actual levels is lost, as is the potential variability in the contributions of different individual biomarkers to overall risk. Indeed, in previous work, we have demonstrated that more complex and sensitive scoring algorithms that include individual biomarker scores and that allow for unequal weighting of the different biomarkers do result in stronger relationships to health outcomes (Karlamangla, Singer, McEwen, Rowe, & Seeman, 2002). Nonetheless, the available data do provide information on a range of biomarkers known to influence risks for major health outcomes and the indices created for the current analyses clearly show evidence for consistent education- and income-related gradients in cumulative risks. A final possible limitation is our use of NHANES III data (collected 1988–1994) rather than the more recent NHANES IV data (1999–2005). We elected to use the NHANES III data because these data offer the opportunity to link our current findings on SES and biological risks to subsequent mortality risks (the focus of separate analyses). Several lines of evidence, including our own previous work showing consistent SES gradients in indivdiual biological risk factors in NHANES III and IV (Crimmins, Alley, Reynolds, Johnston, Karlamangla, & Seeman, 2005) as well as the fact that consistent SES gradients in health have been observed for decades if not longer, suggest that it is highly unlikely that patterns of association between SES and biological risk profiles documented here using NHANES III data would differ if later NHANES IV data had been examined.
The findings presented here underscore the consistent and widespread impact of SES on major physiologic systems that affect our health and functioning. Using both educational and income-related indices of SES, consistent patterns of increasing prevalence of higher biological risk at lower levels of SES were found for individual biological parameters, summary indices reflecting burdens of inflammation, metabolic and cardiovascular risk and for a summary index of overall cumulative biological risk. The fact that these same gradients were seen within all ethnic groups further highlights the degree to which SES plays a significant role in the development of differential profiles of biological risk – profiles that can be seen as warning signs for the increased risks for major disease, disability and mortality outcomes known to be associated with such biological risks.
While examination of individual biomarkers and even individual systems or processes such as metabolism provides important information on that system or biomarker’s potential role as a mediator of SES differences in health, it fails to convey the full picture of biological consequences associated with lower SES. Evidence presented here provides a more complete picture, highlighting the wide range of biological consequences of lower SES and suggesting the need to consider this multiplicity of biological consequences in our efforts to both better understand how SES “gets under the skin” and to develop more effective approaches to primary (and secondary) interventions to reduce and/or prevent SES-related health disparities.
Acknowledgments
This research was supported by grants R01 AG023347, K12AG01004 and P30 AG17265 from the National Institute on Aging (NIA) and by the MacArthur Research Network on SES and Health through grants from the John D. and Catherine T. MacArthur Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Dr. Teresa Seeman, UCLA School of Medicine Los Angeles, CA UNITED STATES
Sharon S Merkin, UCLA School of Medicine, smerkin@mednet.ucla.edu
Eileen Crimmins, University of Southern California, crimmin@usc.edu
Brandon Koretz, UCLA School of Medicine, bkoretz@mednet.ucla.edu
Susan Charette, UCLA School of Medicine, scharette@mednet.ucla.edu
Arun Karlamangla, UCLA School of Medicine, akarlamangla@mednet.ucla.edu
References
- Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) The Journal of the American Medical Association. 2001;285(19):2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- Adler NE, Boyce T, Chesney MA, Cohen S, Folkman S, Kahn RL, et al. Socioeconomic status and health. The challenge of the gradient. The American Psychologist. 1994;49(1):15–24. doi: 10.1037//0003-066x.49.1.15. [DOI] [PubMed] [Google Scholar]
- Adler NE, Boyce WT, Chesney MA, Folkman S, Syme SL. Socioeconomic inequalities in health. No easy solution. Journal of the American Medical Association. 1993;269(24):3140–3145. [PubMed] [Google Scholar]
- Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part I: Diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabetic Medicine. 1998;15:539–553. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Bobak M, Hertzman C, Skodova Z, Marmot M. Socioeconomic status and cardiovascular risk factors in the Czech Republic. International Journal of Epidemiology. 1999;28(1):46–52. doi: 10.1093/ije/28.1.46. [DOI] [PubMed] [Google Scholar]
- Brunner EJ, Marmot MG, Nanchahal K, Shipley MJ, Stansfeld SA, Juneja M, et al. Social inequality in coronary risk: central obesity and the metabolic syndrome. Evidence from the Whitehall II study. Diabetologia. 1997;40(11):1341–1349. doi: 10.1007/s001250050830. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC) National Center for Health Statistics (CHS) National Health and Nutrition Examination Survey III: Household Adult Data File Documentation. [Accessed, 8-10-06];1996 http://www.cdc.gov/nchs/data/nhanes/nhanes3/ADULT-acc.pdf.
- Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42(6):1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- Crimmins EM, Cambois E. Social inequalities in health expectancy. In: Robine JM, Jagger C, Mathers CD, Crimmins EM, Suzman RM, editors. Determining Health Expectancies. England: John Wiley & Sons; 2003. pp. 111–125. [Google Scholar]
- Crimmins EM, Seeman TE. Integrating biology into the study of health disparities. In: Waite LJ, editor. Aging, health, and public policy: Demographic and economic perspectives. New York: Population Council; 2004. pp. 89–107. [Google Scholar]
- Crimmins EM, Alley D, Reynolds SL, Johnston M, Karlamangla AS, Seeman TE. Changes in Biological Markers of Health: Older Americans in the 1990s. The Journals of gerontology. Series A, Biological sciences and medical sciences. 2005;60(11):1409–1413. doi: 10.1093/gerona/60.11.1409. [DOI] [PubMed] [Google Scholar]
- Crimmins EM, Hayward MD, Seeman TE. Race/Ethnicity, Socioeconomic Status and Health. In: Anderson NB, Bulatao RA, Cohen B, editors. Critical Perspectives On Racial and Ethnic Differences In Health In Late Life. National Research Council of the National Academies; 2004. pp. 310–352. [PubMed] [Google Scholar]
- Crimmins EM, Johnston M, Hayward M, Seeman T. Age differences in allostatic load: an index of physiological dysregulation. Experimental Gerontology. 2003;38(7):731–734. doi: 10.1016/s0531-5565(03)00099-8. [DOI] [PubMed] [Google Scholar]
- Davey-Smith G. Socioeconomic differentials. In: Kuh D, Ben-Shlomo Y, editors. A life course approach to chronic disease epidemiology. New York: Oxford University Press; 1997. [Google Scholar]
- Dyer AR, Liu K, Walsh M, Kiefe C, Jacobs DR, Jr, Bild DE. Ten-year incidence of elevated blood pressure and its predictors: The CARDIA Study. Coronary Artery Risk Development in (Young) Adults. Journal of Human Hypertension. 1999;13(1):13–21. doi: 10.1038/sj.jhh.1000740. [DOI] [PubMed] [Google Scholar]
- Friedman GD, Cutter GR, Donahue RP, Hughes GH, Hulley SB, Jacobs DR, Jr, et al. CARDIA: Study design, recruitment, and some characteristics of the examined subjects. Journal of Clinical Epidemiology. 1988;41(11):1105–1116. doi: 10.1016/0895-4356(88)90080-7. [DOI] [PubMed] [Google Scholar]
- Geronimus AT, Hicken M, Keene D, Bound J. “Weathering” and age patterns of allostatic load scores among blacks and whites in the United States. American Journal of Public Health. 2006;96(5):826–833. doi: 10.2105/AJPH.2004.060749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden S, Boulware LE, Berkenblit G, Brancati F, Chander G, Marinopoulos S, et al. Use of glycated hemoglobin and microalbuminuria in the monitoring of diabetes mellitus. Evidence Report/Technology Assessment. 2003;(84):1–6. [PMC free article] [PubMed] [Google Scholar]
- Hayward MD, Crimmins EM, Miles T, Yang Y. The Significance of socioeconomic status is explaining the race gap in chronic health conditions. American Sociological Review. 2000;65:910–930. [Google Scholar]
- Ishizaki M, Martikainen P, Nakagawa H, Marmot M. The relationship between employment grade and plasma fibrinogen level among Japanese male employees. Atherosclerosis. 2000;151(2):415–421. doi: 10.1016/s0021-9150(99)00414-1. [DOI] [PubMed] [Google Scholar]
- Kaplan GA, Keil JE. Socioeconomic factors and cardiovascular disease: a review of the literature. Circulation. 1993;88(4):1973–1998. doi: 10.1161/01.cir.88.4.1973. [DOI] [PubMed] [Google Scholar]
- Karlamangla AS, Singer BH, McEwen BS, Rowe JW, Seeman TE. Allostatic load as a predictor of functional decline. MacArthur studies of successful aging. Journal of Clinical Epidemiology. 2002;55(7):696–710. doi: 10.1016/s0895-4356(02)00399-2. [DOI] [PubMed] [Google Scholar]
- Karlamangla A, Singer BS, Williams DR, Schwartz J, Matthews K, Kiefe CI, et al. Impact of socio-economic status on longitudinal accumulation of cardiovascular risk in young adults: The CARDIA Study. Social Science & Medicine. 2005;60:999–1015. doi: 10.1016/j.socscimed.2004.06.056. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Neighbors HW. A new perspective on the relationships among race, social class, and psychological distress. Journal of Health and Social Behavior. 1986;27(2):107–115. [PubMed] [Google Scholar]
- Koenig W, Sund M, Frohlich M, Fischer HG, Lowel H, Doring A, et al. C-Reactive protein, a sensitive marker of inflammation, predicts future risk of coronary heart disease in initially healthy middle-aged men: results from the MONICA Augsburg Cohort Study, 1984 to 1992. Circulation. 1999;99(2):237–242. doi: 10.1161/01.cir.99.2.237. [DOI] [PubMed] [Google Scholar]
- Kraus JF, Borhani NO, Franti CE. Socioeconomic status, ethnicity, and risk of coronary heart disease. American Journal of Epidemiology. 1980;111(4):407–414. doi: 10.1093/oxfordjournals.aje.a112915. [DOI] [PubMed] [Google Scholar]
- Kubzansky L, Kawachi I, Sparrow D. Socioeconomic status and risk factor clustering in the normative aging study: Any help from the concept of allostatic load? Annals of Behavioral Medicine. 1999;21(4):330–338. doi: 10.1007/BF02895966. [DOI] [PubMed] [Google Scholar]
- Link BG, Phelan J. Social conditions as fundamental causes of disease. Journal of Health & Social Behavior. 1995;(Spec No 80–94) [PubMed] [Google Scholar]
- Lynch JW, Kaplan GA, Cohen RD, Tuomilehto J, Salonen JT. Do cardiovascular risk factors explain the relation between socioeconomic status, risk of all-cause mortality, cardiovascular mortality, and acute myocardial infarction? American Journal of Epidemiology. 1996;144(10):934–942. doi: 10.1093/oxfordjournals.aje.a008863. [DOI] [PubMed] [Google Scholar]
- Marmot MG. Social differentials in health within and between populations. Health and wealth. Daedalus. 1994;123:197–216. [Google Scholar]
- Marmot MG, Smith GD, Stansfeld S, Patel C, North F, Head J, et al. Health inequalities among British civil servants: the Whitehall II study. Lancet. 1991;337(8754):1387–1393. doi: 10.1016/0140-6736(91)93068-k. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Protective and damaging effects of stress mediators. The New England Journal of Medicine. 1998;338(3):171–179. doi: 10.1056/NEJM199801153380307. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Seeman TE. Protective and damaging effects of mediators of stress. In: Adler NE, Marmot M, McEwen BS, editors. Socioeconomic status and health in industrial nations: Social, psychological and biological pathways. New York: Academic of Sciences; 1999. pp. 30–47. [Google Scholar]
- McEwen BS, Stellar E. Stress and the individual. Mechanisms leading to disease. Archives of Internal Medicine. 1993;153(18):2093–2101. [PubMed] [Google Scholar]
- National Center for Health Statistics. Plan and operation of the Third National Health and Nutrition Examination Survey, 1988–94. Series 1: programs and collection procedures. Vital and Health Statistics. Series 1, Programs and Collection Procedures. 1994;(32):1–407. [PubMed] [Google Scholar]
- National Center for Health Statistics. National Health and Nutrition Examination Survey III: Weighting and estimation methodology. Executive Summary. Hyattsville, MD: National Center for Health Statistics; 1996. [Google Scholar]
- Osei K, Rhinesmith S, Gaillard T, Schuster D. Is glycosylated hemoglobin A1c a surrogate for metabolic syndrome in nondiabetic, first-degree relatives of African-American patients with type 2 diabetes? The Journal of Clinical Endocrinology and Metabolism. 2003;88(10):4596–4601. doi: 10.1210/jc.2003-030686. [DOI] [PubMed] [Google Scholar]
- Pamuk E, Makue D, Heck K, Reuben C, Lochner K. Socioeconomic status and health chartbook: health, United States, 1998. Hyattsville, MD: National Center for Health Statistics; 1998. [Google Scholar]
- Pate RR, Pratt M, Blair SN, Haskell WL, Macera CA, Bouchard C, et al. Physical activity and public health. A recommendation from the Centers for Disease Control and Prevention and the American College of Sports Medicine. The Journal of the American Medical Association. 1995;273(5):402–407. doi: 10.1001/jama.273.5.402. [DOI] [PubMed] [Google Scholar]
- Pincus T, Callahan LF. What explains the association between socioeconomic status and health: Primarily access to medical care or mind-body variables? Advances. 1995;11:4–36. [Google Scholar]
- Preston SE, Taubman P. Socioeconomic differences in adult mortality and health status. In: Martin LG, Preston SH, editors. Demography of Aging. Washington, DC: National Academy Press; 1994. pp. 279–318. [Google Scholar]
- Ridker PM. C-Reactive protein: A simple test to help predict risk of heart attack and stroke. Circulation. 2003;108:81–85. doi: 10.1161/01.CIR.0000093381.57779.67. [DOI] [PubMed] [Google Scholar]
- Seccareccia F, Pannozzo F, Dima F, Minoprio A, Menditto A, Lo Noce C, et al. Heart rate as a predictor of mortality: the MATISS project. American Journal of Public Health. 2001;91(8):1258–1263. doi: 10.2105/ajph.91.8.1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeman TE, Crimmins E, Huang MH, Singer B, Bucur A, Gruenewald T, et al. Cumulative biological risk and socio-economic differences in mortality: MacArthur studies of successful aging. Social Science & Medicine. 2004;58(10):1985–1997. doi: 10.1016/S0277-9536(03)00402-7. [DOI] [PubMed] [Google Scholar]
- Seeman TE, McEwen BS, Rowe JW, Singer BH. Allostatic load as a marker of cumulative biological risk: MacArthur studies of successful aging. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(8):4770–4775. doi: 10.1073/pnas.081072698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer B, Ryff C, Seeman TE. Allostasis, homeostasis, and the cost of physiological adaptation. In: Schulkin J, editor. Allostatic load. Operationalizing allostatic load. Cambridge, UK: Cambridge University Press; 2004. pp. 113–149. [Google Scholar]
- Smith JP. Unraveling the SES-health connection. In: Waite LJ, editor. Aging, health, and public policy: demographic and economic perspectives. New York: Population Council, Inc; 2004. pp. 108–132. [Google Scholar]
- Steptoe A, Owen N, Kunz-Ebrecht S, Mohamed-Ali V. Inflammatory cytokines, socioeconomic status, and acute stress responsivity. Brain, Behavior, and Immunity. 2002;16(6):774–784. doi: 10.1016/s0889-1591(02)00030-2. [DOI] [PubMed] [Google Scholar]
- Syme SL, Berkman LF. Social class, susceptibility and sickness. American Journal of Epidemiology. 1976;104(1):1–8. doi: 10.1093/oxfordjournals.aje.a112268. [DOI] [PubMed] [Google Scholar]
- Visser M, Kritchevsky SB, Newman AB, Goodpaster BH, Tylavsky FA, Nevitt MC, et al. Lower serum albumin concentration and change in muscle mass: the Health, Aging and Body Composition Study. The American Journal of Clinical Nutrition. 2005;82(3):531–537. doi: 10.1093/ajcn.82.3.531. [DOI] [PubMed] [Google Scholar]
- Watkins LO, Neaton JD, Kuller LH. Racial differences in high-density lipoprotein cholesterol and coronary heart disease incidence in the usual-care group of the multiple risk factor intervention trial. The American Journal of Cardiology. 1986;57:538–545. doi: 10.1016/0002-9149(86)90831-3. [DOI] [PubMed] [Google Scholar]
- Williams DR. Racial variations in adult health status: Patterns, paradoxes and prospects. In: Smelser NJ, Wilson WJ, Mitchell F, editors. America becoming: Racial trends and their consequences. Washington, DC: National Academy Press; 2001. pp. 371–410. [Google Scholar]
- Williams DR, Collins C. US socioeconomic and racial differences in health: Patterns and explanations. Annual Review of Sociology. 1995;21:349–386. [Google Scholar]
- Williams DR, Lavizzo-Mourey R, Warren RC. The concept of race and health status in America. Public Health Reports. 1994;109:26–41. [PMC free article] [PubMed] [Google Scholar]