Abstract
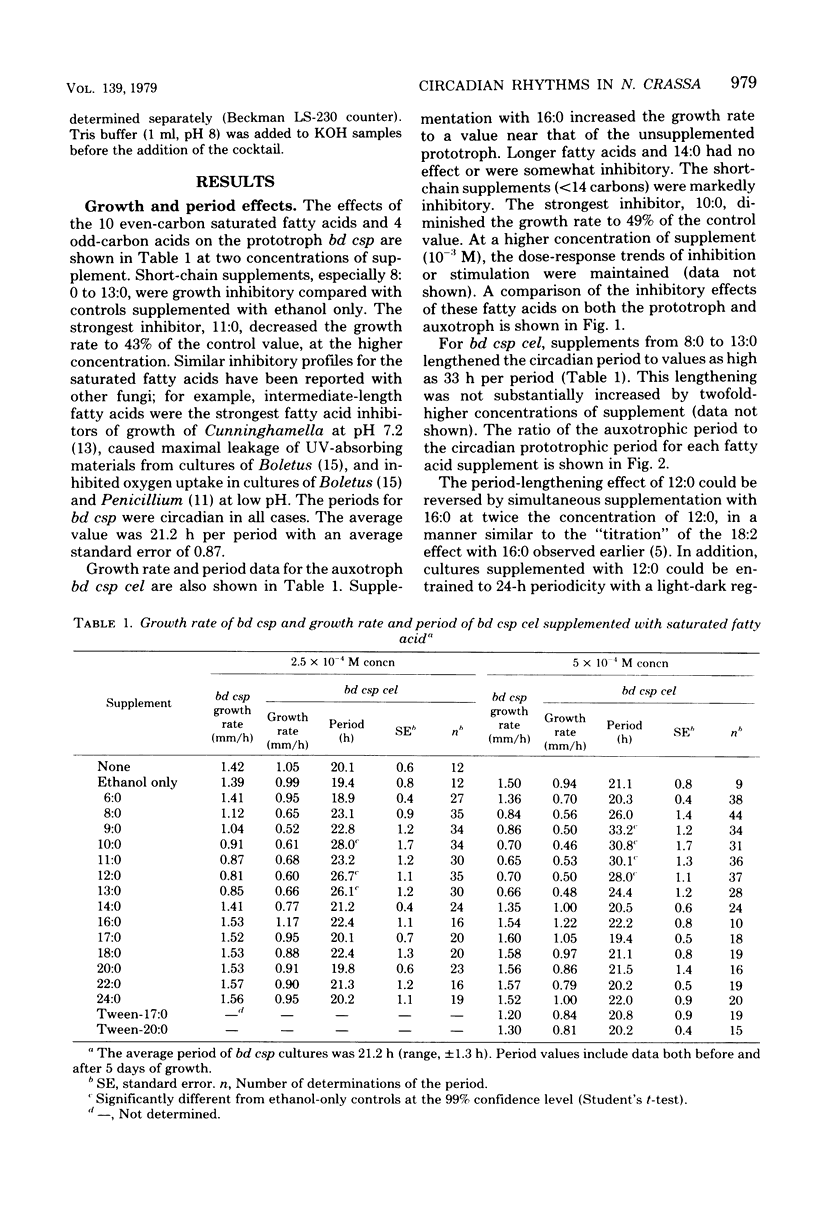
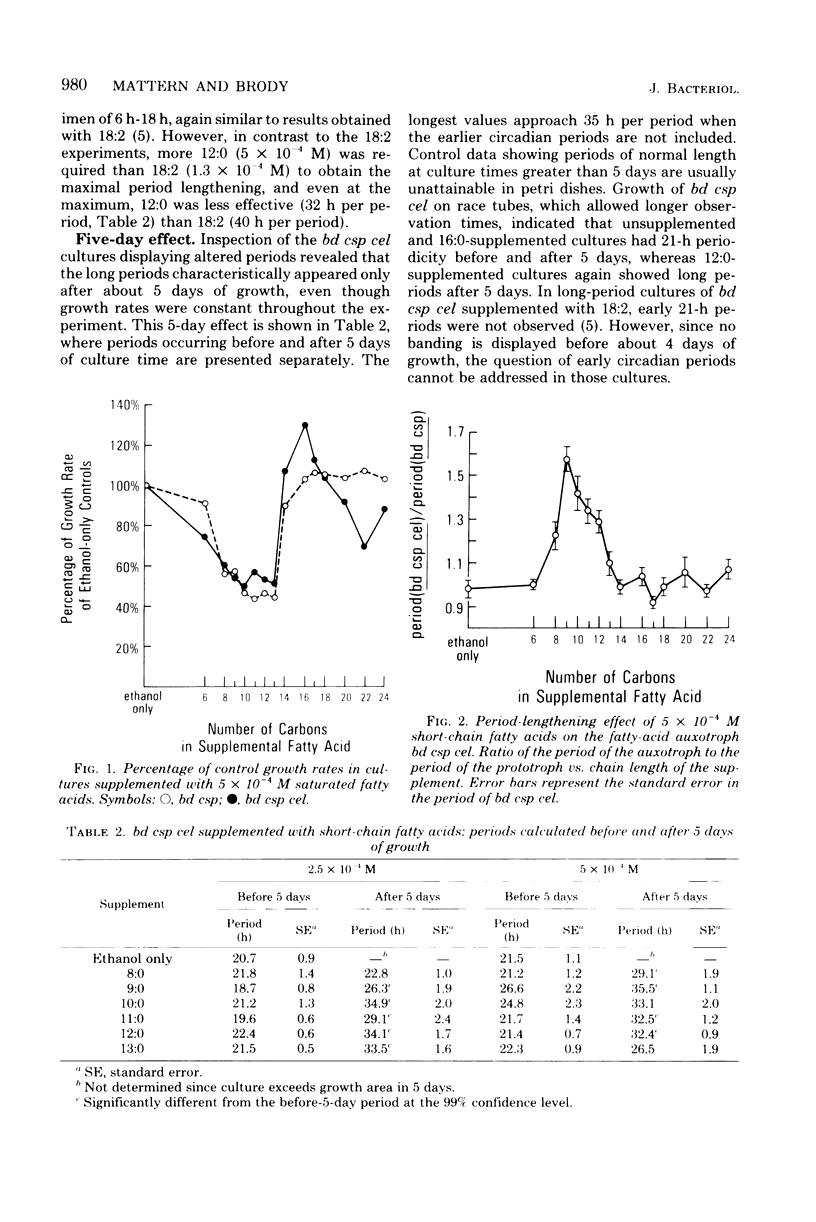
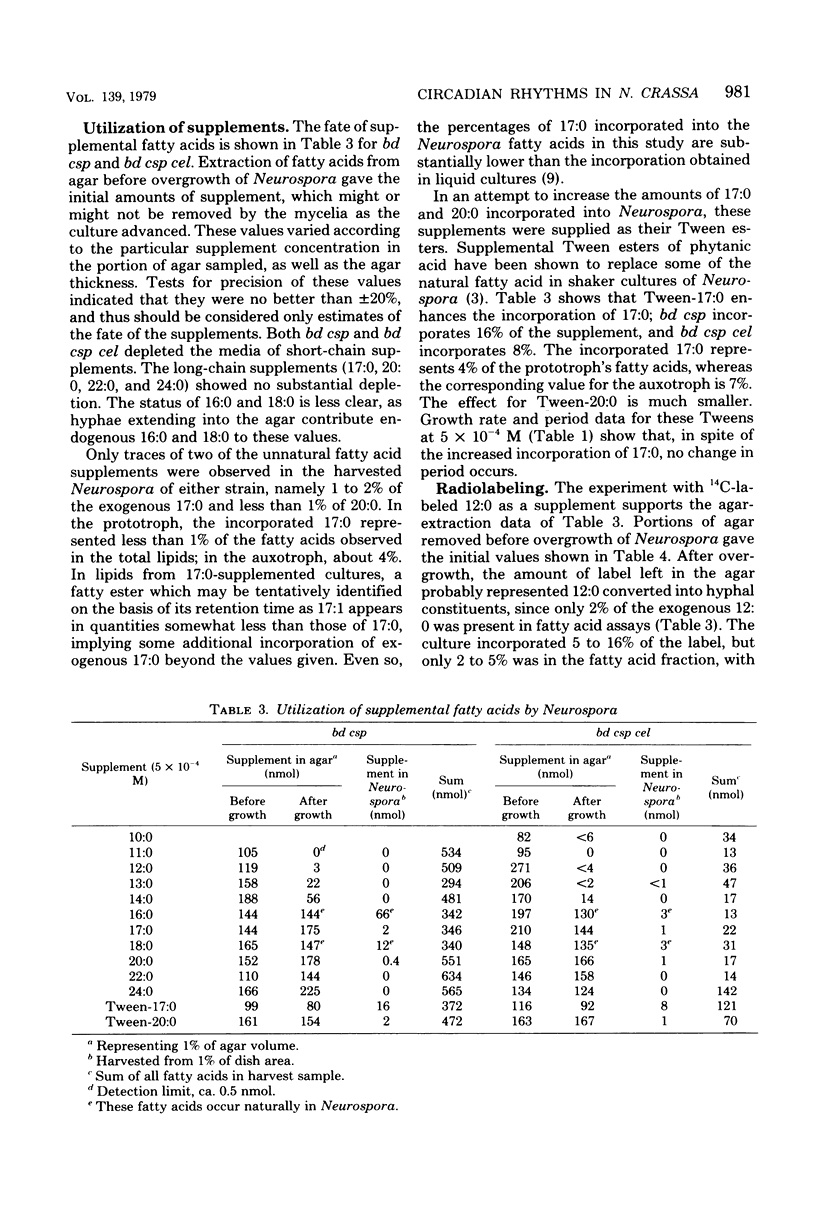
To assess their effects on the conidiation rhythm in Neurospora, 14 saturated fatty acids from 6 to 24 carbons long were used to supplement the bd csp and bd csp cel strains. Both strains express a circadian spore-forming rhythm when grown on solid media; the cel mutation confers a partial fatty acid requirement. Fatty acid supplements from 8 to 13 carbons long lengthened the free-running period of bd csp cel compared with the control value of 21 h; the maximal effect (33 h) was obtained with nonanoic acid (9:0) at a concentration of 5 × 10−4 M. In contrast, the period of bd csp remained unchanged under all experimental conditions. The short-chain fatty acids (<14 carbons) reduced the rate of advance of the growth front in both strains, compared with unsupplemented controls. However, this inhibition did not appear to be responsible for the lengthened periods in bd csp cel. Nor was direct incorporation of the short-chain (period-lengthening) fatty acids into mycelial total lipids responsible, since such incorporation was not observed. In fact, extensive metabolic conversion of these supplements by both strains was indicated by the disappearance of short-chain fatty acids from the agar media coupled with their absence in mycelial lipids, and by the liberation of 14CO2 from cultures supplemented with [1-14C]lauric acid (12:0).
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barrow A., Griffiths L. A. Liquid scintillation counting of aqueous solutions of potassium salts in a Triton X-100/toluene scintillant. Anal Biochem. 1976 Jul;74(1):246–250. doi: 10.1016/0003-2697(76)90329-8. [DOI] [PubMed] [Google Scholar]
- Brody S., Allen B. The effects of branched chain fatty acid incorporation into Neurospora crassa membranes. J Supramol Struct. 1972;1(2):125–134. doi: 10.1002/jss.400010205. [DOI] [PubMed] [Google Scholar]
- Brody S., Harris S. Circadian rhythms in neurospora: spatial differences in pyridine nucleotide levels. Science. 1973 May 4;180(4085):498–500. doi: 10.1126/science.180.4085.498. [DOI] [PubMed] [Google Scholar]
- Brody S., Martins S. A. Circadian rhythms in Neurospora crassa: effects of unsaturated fatty acids. J Bacteriol. 1979 Feb;137(2):912–915. doi: 10.1128/jb.137.2.912-915.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elovson J. Purification and properties of the fatty acids synthetase complex from Neurospora crassa, and the nature of the fas-mutation. J Bacteriol. 1975 Oct;124(1):524–533. doi: 10.1128/jb.124.1.524-533.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry S. A., Keith A. D. Saturated fatty acid requirer of Neurospora crassa. J Bacteriol. 1971 Apr;106(1):174–182. doi: 10.1128/jb.106.1.174-182.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson B. S., Kannangara C. G., Stumpf P. K. The elongation of medium chain trienoic acids to -linolenic acid by a spinach chloroplast stroma system. Biochem Biophys Res Commun. 1973 Jun 19;52(4):1190–1198. doi: 10.1016/0006-291x(73)90626-8. [DOI] [PubMed] [Google Scholar]
- LEIN J., LEIN P. S. The production of acetate from fatty acids by Neurospora. J Bacteriol. 1950 Aug;60(2):185–190. doi: 10.1128/jb.60.2.185-190.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence R. C., Hawke J. C. The oxidation of fatty acids by mycelium of Penicillium roqueforti. J Gen Microbiol. 1968 Apr;51(2):289–302. doi: 10.1099/00221287-51-2-289. [DOI] [PubMed] [Google Scholar]
- Lewis H. L., Johnson G. T. Growth and oxygen-uptake responses of Cunninghamella echinulata on even-chain fatty acids. Mycologia. 1967 Sep-Oct;59(5):878–887. [PubMed] [Google Scholar]
- Njus D., Sulzman F. M., Hastings J. W. Membrane model for the circadian clock. Nature. 1974 Mar 8;248(5444):116–120. doi: 10.1038/248116a0. [DOI] [PubMed] [Google Scholar]
- Richards R. L., Quackenbush F. W. Alternate pathways of linolenic acid biosynthesis in growing cultures of Penicillium chrysogenum. Arch Biochem Biophys. 1974 Dec;165(2):780–786. doi: 10.1016/0003-9861(74)90307-5. [DOI] [PubMed] [Google Scholar]
- Sargent M. L., Briggs W. R., Woodward D. O. Circadian nature of a rhythm expressed by an invertaseless strain of Neurospora crassa. Plant Physiol. 1966 Oct;41(8):1343–1349. doi: 10.1104/pp.41.8.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargent M. L., Kaltenborn S. H. Effects of medium composition and carbon dioxide on circadian conidiation in neurospora. Plant Physiol. 1972 Jul;50(1):171–175. doi: 10.1104/pp.50.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargent M. L., Woodward D. O. Genetic determinants of circadian rhythmicity in Neurospora. J Bacteriol. 1969 Feb;97(2):861–866. doi: 10.1128/jb.97.2.861-866.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selitrennikoff C. P., Nelson R. E., Siegel R. W. Phase-specific genes for macroconidiation in Neurospora crassa. Genetics. 1974 Oct;78(2):679–690. doi: 10.1093/genetics/78.2.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Gier J., Mandersloot J. G., van Deenen L. L. Lipid composition and permeability of liposomes. Biochim Biophys Acta. 1968 Jun 11;150(4):666–675. doi: 10.1016/0005-2736(68)90056-4. [DOI] [PubMed] [Google Scholar]



