Abstract
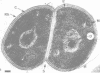
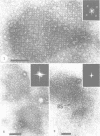
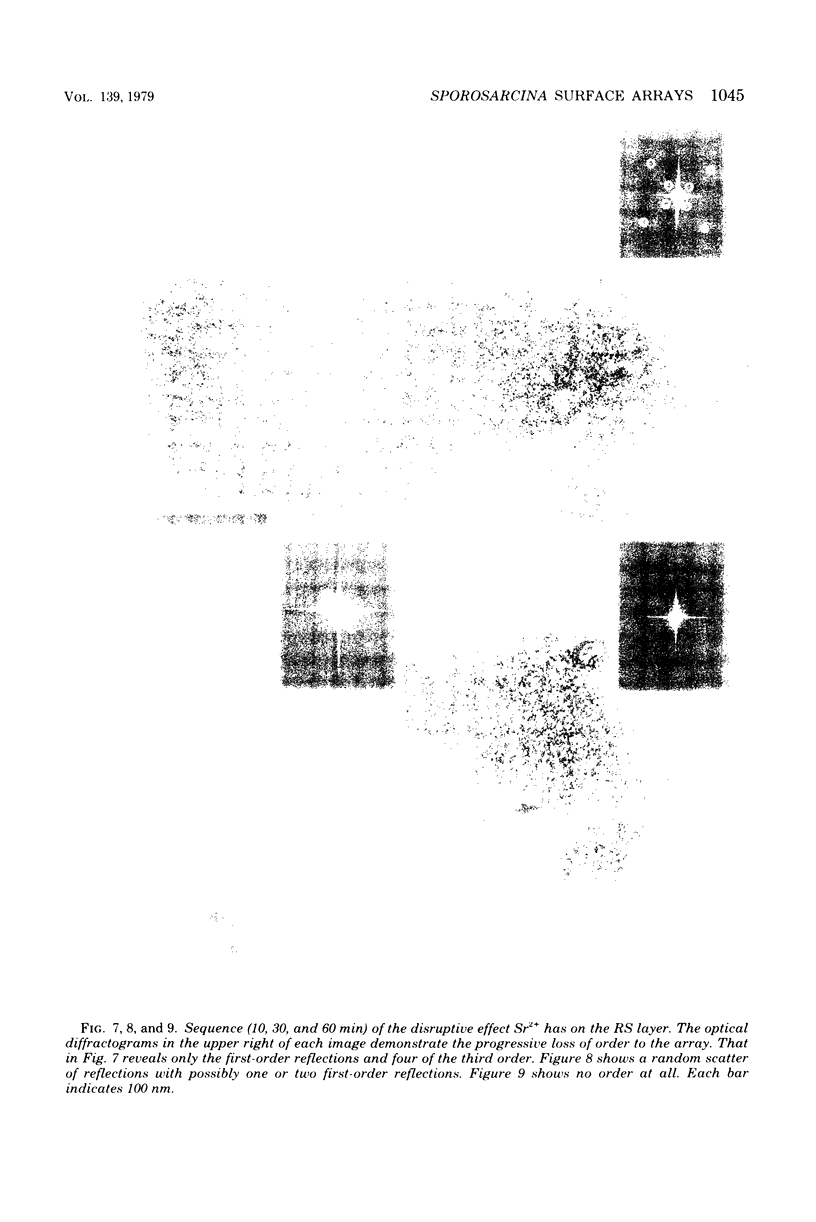
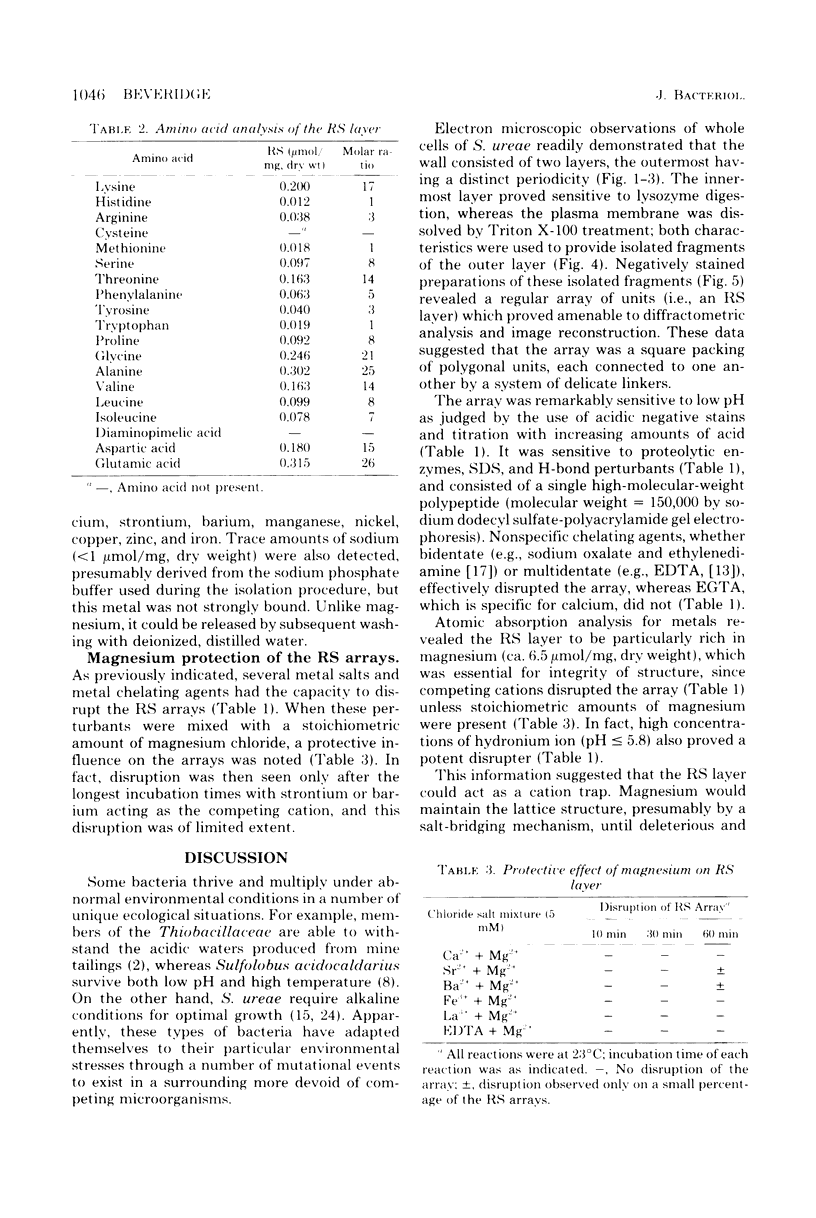
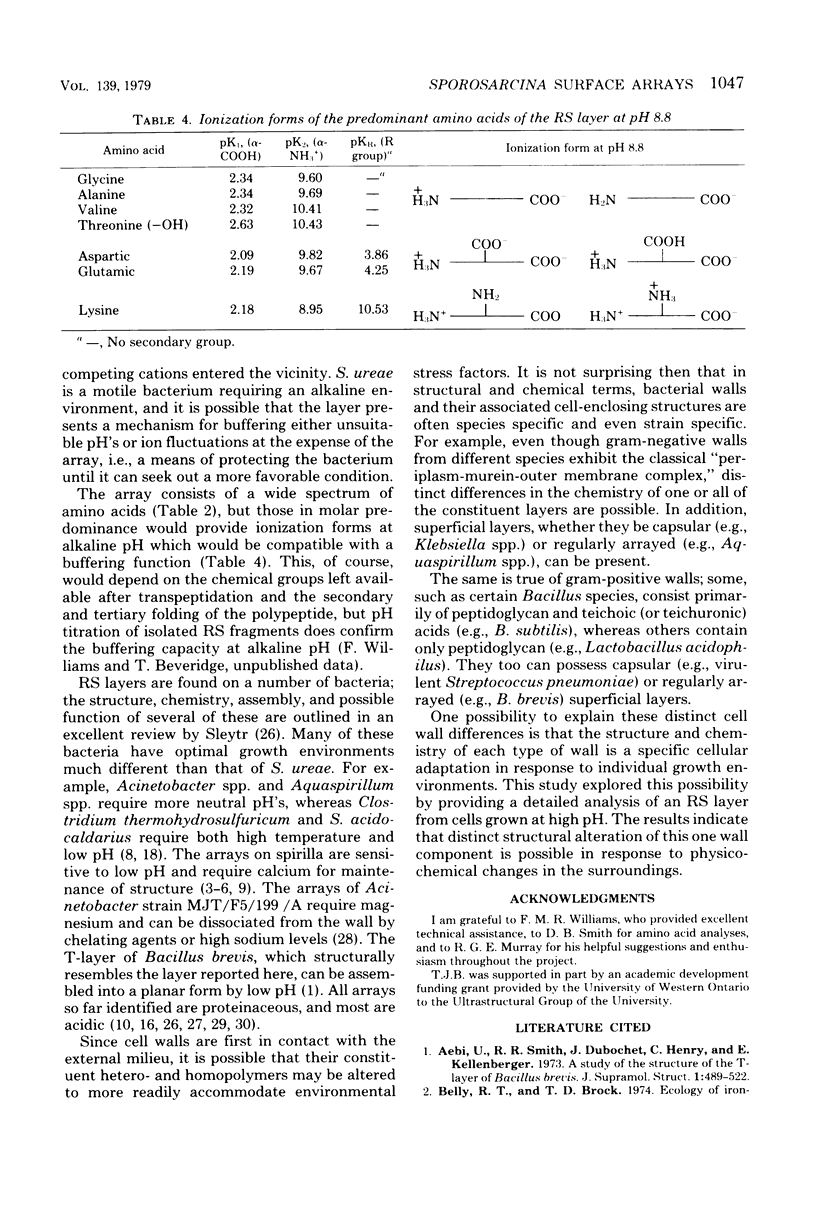
Thin sections of the cell wall of Sporosarcina ureae revealed two structurally distinct layers: a continuous amorphous zone, approximately 15 nm thick, which was adjacent to the plasma membrane, and an overlying periodic zone, approximately 16 nm thick. Sequential Triton X-100 and lysozyme treatment of isolated walls produced small fragments of the outer regular structure which allowed high-resolution, negatively stained images suitable for optical diffractometric analysis. These data suggested a tetragonal array of complex polygonal units of C-C spacing = 12 nm, with each unit joined to another by two delicate linkers. The array was entirely proteinaceous, consisting of a 150,000-dalton polypeptide which had a high affinity for Mg2+. It proved to be sensitive to chelating agents, 5 mM concentrations of Ca2+, Sr2+, or Ba2+, proteases, heat greater than or equal to 45 degrees C, sodium dodecyl sulfate, and pH greater than or equal to 5.8, but magnesium offered protection against the chelating agents and the deleterious salts.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aebi U., Smith P. R., Dubochet J., Henry C., Kellenberger E. A study of the structure of the T-layer of Bacillus brevis. J Supramol Struct. 1973;1(6):498–522. doi: 10.1002/jss.400010606. [DOI] [PubMed] [Google Scholar]
- Beveridge T. J., Murray R. G. Reassembly in vitro of the superficial cell wall components of Spirillum putridiconcyhylium. J Ultrastruct Res. 1976 Apr;55(1):105–118. doi: 10.1016/s0022-5320(76)80086-x. [DOI] [PubMed] [Google Scholar]
- Beveridge T. J., Murray R. G. Superficial cell-wall layers on Spirillum "Ordal" and their in vitro reassembly. Can J Microbiol. 1976 Apr;22(4):567–582. doi: 10.1139/m76-085. [DOI] [PubMed] [Google Scholar]
- Beveridge T. J., Murray R. G. Surface arrays on the cell wall of Spirillum metamorphum. J Bacteriol. 1975 Dec;124(3):1529–1544. doi: 10.1128/jb.124.3.1529-1544.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckmire F. L., Murray R. G. Studies on the cell wall of Spirillum serpens. 1. Isolation and partial purification of the outermost cell wall layer. Can J Microbiol. 1970 Oct;16(10):1011–1022. doi: 10.1139/m70-171. [DOI] [PubMed] [Google Scholar]
- Buckmire F. L., Murray R. G. Studies on the cell wall of Spirillum serpens. II. Chemical characterization of the outer structured layer. Can J Microbiol. 1973 Jan;19(1):59–66. doi: 10.1139/m73-009. [DOI] [PubMed] [Google Scholar]
- Hollaus F., Sleytr U. On the taxonomy and fine structure of some hyperthermophilic saccharolytic Clostridia. Arch Mikrobiol. 1972;86(2):129–146. doi: 10.1007/BF00413368. [DOI] [PubMed] [Google Scholar]
- Holt S. C., Leadbetter E. R. Comparative ultrastructure of selected aerobic spore-forming bacteria: a freeze-etching study. Bacteriol Rev. 1969 Jun;33(2):346–378. doi: 10.1128/br.33.2.346-378.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horne R. W., Ronchetti I. P. A negative staining-carbon film technique for studying viruses in the electron microscope. I. Preparative procedures for examining icosahedral and filamentous viruses. J Ultrastruct Res. 1974 Jun;47(3):361–383. doi: 10.1016/s0022-5320(74)90015-x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Osborn M. J., Gander J. E., Parisi E. Mechanism of assembly of the outer membrane of Salmonella typhimurium. Site of synthesis of lipopolysaccharide. J Biol Chem. 1972 Jun 25;247(12):3973–3986. [PubMed] [Google Scholar]
- Sleytr U. B., Thorne K. J. Chemical characterization of the regularly arranged surface layers of Clostridium thermosaccharolyticum and Clostridium thermohydrosulfuricum. J Bacteriol. 1976 Apr;126(1):377–383. doi: 10.1128/jb.126.1.377-383.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornley M. J., Thorne K. J., Glauert A. M. Detachment and chemical characterization of the regularly arranged subunits from the surface of an Acinetobacter. J Bacteriol. 1974 May;118(2):654–662. doi: 10.1128/jb.118.2.654-662.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss R. L. Subunit cell wall of Sulfolobus acidocaldarius. J Bacteriol. 1974 Apr;118(1):275–284. doi: 10.1128/jb.118.1.275-284.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]