Abstract
Proper septation and valvulogenesis during cardiogenesis depend on interactions between the myocardium and the endocardium. By combining use of a hypomorphic Bone morphogenetic protein 4 (Bmp4) allele with conditional gene inactivation, we here identify Bmp4 as a signal from the myocardium directly mediating atrioventricular septation. Defects in this process cause one of the most common human congenital heart abnormalities, atrioventricular canal defect (AVCD). The spectrum of defects obtained through altering Bmp4 expression in the myocardium recapitulates the range of AVCDs diagnosed in patients, thus providing a useful genetic model with AVCD as the primary defect.
Keywords: Bmp4, atrioventricular septation, cardiogenesis
Congenital heart malformation is the most common human birth defect and the leading cause of death in the first year of life (Hoffman 1995). One of the most frequently diagnosed disorders is atrioventricular canal defect (AVCD), which accounts for 7.3% of all congenital heart abnormalities (Pierpont et al. 2000). During normal development, septation of the AV canal (AVC) is initiated with the formation of the inferior and superior endocardial cushions through epithelial-mesenchymal-transformation (EMT) by some endocardial cells invading into cardiac jelly, the extracellular matrix between the endocardium and myocardium (Nakajima et al. 2000; Markwald and Wessels 2001). Subsequent growth and fusion of the AV cushions produce the central mesenchymal mass, which further develops into the mature AV septum and valves through complex remodeling processes. The central mesenchymal mass also fuses with the atrial septum primum (ASP) and the inlet portion of the ventricular septum to prevent abnormal blood flow between chambers (Marino and Digilio 2000; Markwald and Wessels 2001). AVCD covers a spectrum of abnormalities, from the partial form with defects in the lower part of the ASP to the complete form in which absence of the AV septum results in a single common AVC (Marino and Digilio 2000).
The complex cushion morphogenesis during AV septation depends both on signals released from the overlying myocardium and on proper responses of the endocardial and mesenchymal cells (Nakajima et al. 2000; Markwald and Wessels 2001). The molecular pathways for the initiation of cushion formation (EMT) have been studied extensively. In both chicken and mouse explant culture assays, transforming growth factor β2 (TGFβ2) is able to replace the overlying myocardium to activate EMT, and inhibition of TGFβ2 activity blocks EMT (Boyer et al. 1999; Nakajima et al. 2000; Camenisch et al. 2002a). Consistent with these in vitro studies, TGFβ2-deficient mice show defects in valvulogenesis (Bartram et al. 2001). In addition to the TGFβ signaling pathway, the Hyaluronic acid (Ha) synthase 2 (Has2)/ErbB2,ErbB3/Ras pathway has been recently shown to play essential roles in EMT (Camenisch et al. 2000, 2002b). Ha is a prominent component of cardiac jelly in embryonic day 9.5 (E9.5) embryos and its production depends on Has2. In addition to serving as a substrate for migration, Ha is responsible for phosphorylation of ErbB2 and ErbB3 through unknown mechanisms to activate the Ras pathway. Expression of dominant-negative Ras blocks EMT in wild-type embryonic hearts, suggesting that activation of the Ras pathway is required for EMT (Lakkis and Epstein 1998; Camenisch et al. 2000, 2002b).
Although knowledge about the onset of EMT is accumulating, little is known about mechanisms governing morphogenic events after cushions have formed. A recent study shows that myocardial-specific inactivation of a Bmp type I receptor gene, Alk3, causes abnormal AV cushion morphogenesis, although the similarity between the defects in these mice and human patients is not clearly defined (Gaussin et al. 2002; Schneider et al. 2003). Because the receptor gene is specifically deleted in muscle cells, the AV cushion defect in these mice is primarily caused by cardiomyocyte abnormalities, including increased apoptosis and reduced TGFβ2 expression (Gaussin et al. 2002; Schneider et al. 2003). Other studies have also demonstrated a requirement for myocardial integrity for proper AV septation, including analysis of Fog2-/- (Tevosian et al. 2000) and Cx40-/-; Cx43+/ mutants (Kirchhoff et al. 2000). These results further strengthen the idea that communication between the myocardium and mesenchyme/endocardium is essential for normal morphogenesis even after cushions have formed. However, no signaling molecule from the myocardium that directly mediates later stages of AV septation has been identified. Resolution of this problem is key to understanding the molecular pathology of clinically observed AVCDs.
Bmp4 belongs to the TGFβ superfamily of cytokines, and has been implicated in numerous developmental processes (Hogan 1996; von Bubnoff and Cho 2001). The activity of Bmp4 is mediated through heterodimeric complexes of type I and type II serine/threonine kinase receptors (von Bubnoff and Cho 2001). Previous gene expression and in vitro explant studies suggested that Bmp4 is a key myocardial signal for activating EMT (Markwald and Wessels 2001, and references therein). In this study, we circumvent the early lethality of Bmp4 null embryos (Lawson et al. 1999; Fujiwara et al. 2002) by combining use of a hypomorphic Bmp4 allele with a Cre/loxp approach to manipulate levels of Bmp4 expression specifically in cardiomyocytes. Contrary to previous hypotheses, our results show that Bmp4 is dispensable for the initiation of cushion formation but is specifically required for proper AV septation after cushions have formed.
Results and Discussion
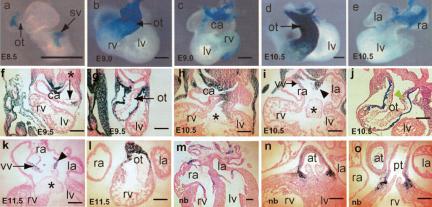
Despite extensive literature on the subject (Jones et al. 1991; Ikeda et al. 1996; Abdelwahid et al. 2001), the expression of Bmp4 during cardiogenesis has not been thoroughly characterized. We therefore examined embryos heterozygous for the Bmp4lacZ null reporter allele (Lawson et al. 1999) from E8.5 to birth (Fig. 1). Expression of Bmp4 in the outflow tract (OT) is detectable at E8.5 (Fig. 1a), and expression in the muscle layer of the OT and its derivatives (aorta/pulmonary trunk) is maintained until birth. In the inflow region, Bmp4 expression was first detected in the sinus venosus at E8.5 (Fig. 1a). At E9.0, Bmp4 signals are detected in the dorsal midline of the common atrium and weakly in the AVC. Significantly, these cells mark the position where formation of the ASP and inferior endocardial cushion (IEC) is initiated. Later, Bmp4 is expressed in cardiomyocytes overlying the IEC from E9.5 to E12.5 and in the muscular portion of the ASP from E10.5 to birth (Fig. 1; data not shown).
Figure 1.
Expression of Bmp4 during cardiogenesis. (a-e) Embryonic hearts of Bmp4lacZ/+ embryos on ICR background were collected at stages labeled in each panel, and were stained with X-gal. In the Bmp4lacZ allele, the lacZ reporter gene was knocked into the Bmp4 locus, and lacZ expression faithfully recapitulates endogenous Bmp4 transcription in Bmp4lacZ/+ embryos. (f-o) Transverse sections of whole embryos (f-g) or frontal sections of hearts (h-o) after whole-mount X-gal staining. All sections were counterstained with eosin. In addition to expression in the muscle layer of the OT (g,j,l,n,o) in cardiomyocytes overlying the IEC (f,h) and in muscular portions of ASP (i,k) described in the text, positive signals were also detected in the mesenchyme of the truncus cushion at E10.5 (green arrowhead in j), in the dorsal wall of the atrium (f,h,i), in venous valves (i,k), and in the annulus of mitral and tricuspid valves (m). The Bmp4 expression pattern was confirmed with in situ hybridization analysis (data not shown). (at) Aorta trunk; (ca) common atrium; (la) left atrium; (lv) left ventricle; (nb) newborn; (ot) outflow tract; (pt) pulmonary trunk; (ra) right atrium; (rv) right ventricle; (vv) venous valve; (*) AV endocardial cushions; (black arrow) atrial septum primum. Bars, 0.2 mm.
Expression of Bmp4 in cardiomyocytes in regions where septa are formed suggests that the gene plays critical roles during septation and valvulogenesis. To test this hypothesis, we applied a Cre/loxp system to specifically inactivate Bmp4 in cardiomyocytes. We first generated a transgenic line in which transcription of Cre recombinase is driven by the rat cardiac TroponinT (cTnT) promoter (Wang et al. 2000, 2001). By crossing these mice with ROSA26 reporter mice (Soriano 1999), we showed that cTnT-Cre induces recombination early in the cardiomyocyte lineage, starting from E7.5 and that recombination is restricted to the hearts until E10.5 (Fig. 2). High levels of recombination occur in the myocardium of all heart segments at E10.5 (Fig. 2d,e). Reverse transcriptase PCR (RT-PCR) analysis (Fig. 2f) confirmed that cTnT-Cre efficiently deletes Bmp4 from embryonic hearts between E9.5 and 10.5.
Figure 2.
Recombination induced by cTnT-Cre. (a,b) The cTnT Cre;R26R+/- embryos at E7.5 (a) and E10.5 (b) were stained with X-gal. (c) The heart dissected from the embryo in panel b. (d,e) Sagittal sections of a cTnT-Cre; R26R+/- embryo at E10.5 stained with X-gal. Panel d represents the view in bright field and panel e corresponds to the dark-field view. X-gal staining products appear pink, and are more easily detected in the dark field. Sections were counterstained with eosin. (f) Embryonic hearts were collected from Bmp4loxp-lacZ/tm1 (-Cre) or Bmp4cre;loxp-lacZ/tm1 (+Cre) embryos from stages E9.5 to E11.5. RT-PCR analysis was performed to examine the expression of Bmp4, and β-Actin was used as a positive control. No PCR products were obtained if reverses transcriptase was omitted (data not shown). (H) Heart; (hf) head fold; (la) left atrium; (ot) outflow tract; (pc) precardiac crescent; (a) atrium; (ra) right atrium; (v) ventricle; (lv) left ventricle. Bars, 0.4 mm.
We previously constructed the floxed Bmp4 allele (Bmp4loxp-lacZ), which has the advantage that recombination at the Bmp4 locus leads to expression of lacZ under the control of Bmp4 regulatory elements (Kulessa and Hogan 2002). Although Bmp4loxp-lacZ/loxp-lacZ mice develop normally, mice that are compound mutant for the Bmp4loxp-lacZ allele and the Bmp4tm1 null allele (Bmp4loxp-lacZ/tm1) lack normal eyes and die between day 1 and day 7 after birth, indicating that Bmp4loxp-lacZ is a hypomorphic allele. To examine the potential cardiac defects caused by deficiency (but not absence) of Bmp4 expression in all cells, we examined Bmp4loxp-lacZ/tm1 neonatal hearts histologically (n = 7). Figure 3d reveals a severe ASP abnormality with normal AV and ventricular septa, resulting in an ostium primum atrial septation defect or a partial AVCD. In humans, this defect, unless corrected by surgery, will lead to excessive intra-atrial shunting, right ventricle overload, and, ultimately, pulmonary hypertension. To specifically delete Bmp4 in the cardiomyocytes, we first crossed cTnT-Cre; Bmp4loxp-lacZ/+ mice with Bmp4loxp-lacZ/loxp-lacZ mice. The cTnT-Cre; Bmp4loxp-lacZ/loxp-lacZ (Bmp4cre;loxp-lacZ/loxp-lacZ) neonates (n = 5) are grossly normal, but also die perinataly, recapitulating a similar ASP defect seen in Bmp4loxp-lacz/tm1 mice (Fig. 3f).
Figure 3.
Dosage requirement of Bmp4 for AV septation. (a-f) Neonatal hearts from cTnT-Cre;Bmp4loxp-lacZ/+ (a,b), Bmp4loxp-lacZ/tm1 (c,d), and Bmp4cre;loxp-lacZ/loxp-lacZ (e,f) mice were stained with X-gal. cTnT-Cre;Bmp4loxp-lacZ/+ mice are indistinguishable from wild-type mice (data not shown) and are used as positive controls. Bmp4loxp-lacZ/tm1 and Bmp4cre;loxp-lacZ/loxp-lacZ hearts display partial AVCD with normal AV and ventricular septa. (g-h) Sagittal sections of an E11.0 Bmp4cre;loxp-lacZ/tm1i (Cko) embryo (g) or an E11.0 cTnT-Cre;Bmp4loxp-lacZ/+ (Contr) embryo (h) stained with X-gal. (i-q) Frontal sections of Bmp4cre;loxp-lacZ/tm1 (Cko) embryonic hearts (i,k,m,n,p,q) and cTnT-Cre;Bmp4loxp-lacZ/+ (Contr) embryonic hearts (j,l,o) at stages labeled in each panel. Panels n, p, and q are from the same heart shown in panel m. Panel n shows the defect in ASP, which is not obvious in panel m. Panels p and q show that both aorta and pulmonary trunk are connected to the right ventricle. (r) An ultrasound scan of a heart from a human patient with the complete form of AVCD. The + indicates the common AVC. All sections were counterstained with eosin except sections of panels m, n, p, and q, which were stained with hematoxylin. (a) Atrium; (at) aorta trunk; (la) left atrium; (lv) left ventricle; (ot) outflow tract; (pt) pulmonary trunk; (ra) right atrium; (rv) right ventricle; (v) ventricle; (arrowhead) atrial septum primum; (*) AV septum in panels b, d, and f, and AV cushions in panels g, i, and j. Bars, 0.2 mm.
We next crossed cTnT-Cre;Bmp4+/tm1 mice with Bmp4loxp-lacZ/loxp-lacZ miceto generate cTnT-Cre;Bmp4lacZ-loxp/tm1 (Bmp4cre;loxp-lacZ/tm1) embryos with only one floxed Bmp4 allele to be recombined. Complete deletion of Bmp4 in the heart would therefore be expected to be achieved more efficiently than in Bmp4cre;loxp-lacZ/loxp-lacZ embryos, which have two floxed alleles to be recombined. We did not identify any Bmp4cre;loxp-lacZ/tm1 neonates from a total of 58 pups (nine litters), indicating embryonic lethality. We therefore recovered Bmp4cre;loxp-lacZ/tm1 embryos (n = 10) at E15.5-E16.5, when septation is normally completed. These embryos have normal body and heart sizes, excluding a growth retardation defect. Histological examination revealed that all Bmp4cre;loxp-lacZ/tm1 embryonic hearts have the complete AVCD, a single AV junction with a common valve, which is identical to the phenotype of a human patient observed by ultrasound scan (Fig. 3m,r). In the outflow regions, 80% (8/10) of the embryos display double-outlet-right-ventricle (Fig. 3p,q). Examination of Bmp4cre;loxp-lacZ/tm1 hearts at earlier stages reveals that, although AV cushions are properly formed at E11.0 (Fig. 3g,h), their size is invariably reduced at E12.5 (Fig. 3i,j). The more severe malformation in Bmp4cre;loxp-lacZ/tm1 hearts compared with Bmp4cre;loxp-lacZ/loxp-lacZ hearts indicates that AV septation is very sensitive to the dosage of Bmp4 activity in vivo. The difference in Bmp4 expression in the localized region of the AVC of Bmp4cre;loxp-lacZ/tm1 versus Bmp4cre;loxp-lacZ/loxp-lacZ embryos is likely to be very subtle (see following) and difficult to quantitate at the cellular level using current available techniques. Rather, the variable malformations summarized in Table 1 provide a sensitive biological readout of the different phenotype/genotype correlations.
Table 1.
Summary of the cardiac malformations observed in Bmp4 mutants of different genotypes between E15.5 and PO
| Genotype | Defects in OT | Defects in AVC |
|---|---|---|
| Bmp4loxp-lacZ/tm1 | None observed | ASD, partial AVCD |
| cTnT-Cre; Bmp4loxp-lacZ/loxp-lacZ | None observed | ASD, partial AVCD |
| cTnT-Cre; Bmp4cre;loxp-lacZ/tm1 | DORV (80%) | Complete AVCD |
(OT) Outflow tract; (AVC) atrioventricular canal; (ASD) atrial septal defect; (AVCD) AVC defect; (DORV) double outlet right ventricle.
Partial AVCD is caused by a deficiency in ASP formation, whereas the complete form is due to the underdevelopment of AV cushions (Marino and Digilio 2000; Pierpont et al. 2000). In humans, both forms are most commonly associated with Down syndrome (DS; Marino and Digilio 2000; Pierpont et al. 2000). Mice with trisomy 16 (Ts16), though serving as a classic model for studying DS, have several differences in cardiac phenotype compared with patients with DS (Webb et al. 1997; Waller et al. 2000). All Ts16 mice show defects in their OTs, and 50% show unbalanced AV junction. In contrast, all Bmp4cre;loxp-lacZ/tm1 mice display a balanced AV junction between ventricles, and 20% of them show normal OT development, indicating that the etiology of AVCDs is separable from that of OT defects. Thus, Bmp4cre;loxp-lacZ/tm1 mice have cardiac defects that more closely resemble those of DS than do Ts16 mice. Because Bmp4 is on chromosome 14, it is likely that genetic interactions between Bmp4 and gene(s) on chromosome 21 are contributing factors in AVCD in DS patients.
The lack of defects in the initiation of cushion formation (EMT) in Bmp4cre;loxp-lacZ/tm1 embryos is surprising given in vitro studies showing that Bmps act synergistically with TGFβ in the initiation of EMT (Nakajima et al. 2000). This discrepancy may come from insufficient excision of Bmp4 before E9.5. To address this possibility, we took advantage of the fact that some Bmp4lacZ/lacZ embryos on ICR (Institute for Cancer Research) background survive to the ∼30-somite stage with grossly normal hearts (Fig. 4a). Both the OT (data not shown) and AV cushions (Fig. 4b,c) in these hearts are normally initiated (extracellular matrix production and EMT) even when Bmp4 is completely deleted, supporting the conclusion that Bmp4 is dispensable for the initiation of cushion formation. Many other BMPs (BMP2, BMP5, BMP6, BMP7, BMP10) are expressed in the embryonic heart (Lyons et al. 1990, 1995; Dudley and Robertson 1997; Neuhaus et al. 1999) and may compensate for the loss of Bmp4. However, after cushions have formed, Bmp4 is uniquely required for the proper septation of the AVC.
Figure 4.
Phenotype of AV region of embryos of different Bmp4 genotypes. (a,b,c) Normal formation of AV cushions in Bmp4lacZ/lacZ embryonic hearts. A heart from a Bmp4lacZ/lacZ embryo with 30 somites is grossly normal when compared with a Bmp4lacZ/+ heart at the same stage (a). Formation of AV cushions in a Bmp4lacZ/lacZ embryo (b) parallels that of a Bmp4lacZ/+ embryonic heart (c). (d,e) Results of section in situ hybridization using a TGFβ2 probe. Expression of TGFβ2 in the AVC of a Bmp4cre;loxp-lacZ/tm1 embryonic heart at E12.5 (d) is not altered when compared with the results observed from a wild-type embryonic heart at the same stage (e). (f-i) Frontal sections of Bmp4cre;loxp-lacZ/tm1 (f,g) and cTnTCre;Bmp4loxp-lacZ/+ (h,i) embryonic hearts at E12.5 are stained with DAPI to visualize the nuclei (f,h) and with anti-Ki67 antibody to visualize proliferating cells (g,i). Panels f and g correspond to the boxed regions of Figure 3c; panels h and i correspond to the boxed regions of Figure 3d. (j) The number of Ki67-positive nuclei as a percentage of total nuclei (mean ± S.E.) of Bmp4cre;loxp-lacZ/tm1 embryonic hearts (Cko) is reduced to about 80% of cTnTCre;Bmp4loxp-lacZ/+ embryonic hearts (Contr). Data were averaged from four embryonic hearts of each strain, and at least 1500 nuclei were counted for each heart. (ca) Common atrium; (v) ventricle; (*) AV cushions; (#) P = 0.047 < 0.05.
Myocardial-specific inactivation of Alk3 causes malformation in the AV cushions due to increased myocyte apoptosis and down-regulation of TGFβ2 expression in the myocardium surrounding the AVC (Gaussin et al. 2002; Schneider et al. 2003). Bmp4cre;loxp-lacZ/tm1 hearts do not show any morphological defects, aberrant apoptosis, or abnormal proliferation in their myocardium (see following), suggesting that Bmp4 is dispensable for myocardial development. We further showed that TGFβ2 expression is unaltered in Bmp4cre;loxp-lacZ/tm1 hearts (Fig. 4d,e), suggesting that the AVCD in Bmp4cre;loxp-lacZ/tm1 hearts is not due to impaired Bmp signaling in the myocardium. We directly tested whether alteration in cell proliferation and/or apoptosis in AV cushions contributes to the AVCD in Bmp4cre;loxp-lacZ/tm1 hearts. Anti-Ki67 staining showed that the cell proliferation rate in AV cushions, but not in other cardiac regions at E12.5, is reduced by ∼20% compared with controls (Fig. 4f-j). Although moderate, the significant growth reduction is sufficient to cause a dramatic cumulative effect over several generations of cell division. Consistent with this result, inactivation of Smad6 (a Bmp-specific nuclear inhibitor) causes hyperplasia of cardiac valves (Galvin et al. 2000). We did not observe enhanced apoptosis in Bmp4cre;loxp-lacZ/tm1 hearts (data not shown). The moderate growth rate reduction of mesenchymal cells in AV cushions provides a potential mechanism accounting for the abnormal AV septum formation associated with normal valvulogenesis, as observed in the Bmp4cre;loxp-lacZ/tm1 embryos and patients with AVCDs (like DS patients). A severe growth rate reduction in AV cushions is expected to block both the septation and valvulogenesis processes, whereas a moderate reduction may sufficiently block the AV septum formation but has little effect on valvulogenesis.
This study reveals Bmp4 as the only known signaling factor from the myocardium that directly regulates AV septation after cushions have formed. As summarized in Table 1, a modest reduction in Bmp4 gene expression (in Bmp4loxp-lacZ/tm1 or Bmp4cre;loxp-lacZ/loxp-lacZ mice) results in the partial AVCD, and a more severe reduction (Bmp4cre;loxp-lacZ/tm1) causes the complete form of AVCD. We thus provide a unique genetic model with AVCD as the primary defect.
Materials and methods
Mouse and embryo manipulations
All procedures are approved by the Institutional Animal Care and Use Committee at Vanderbilt University. Bmp4lacZ/+ mice on the (129/SvEvxBlack Swiss) background have been outcrossed with ICR mice for at least 10 generations. Bmp4lacZ/lacZ embryos were obtained by intercrossing ICR Bmp4lacZ/+ mice (Weaver et al. 1999). The nls-Cre-hGH cassette [a gift from Dr. M. Magnuson (Vanderbilt University Medical Center, Nashville, TN)] was fused with the rat cTnT promoter [Wang et al. 2000, 2001; a gift from Dr. J. Lin (University of Iowa, Iowa City, IA)], and the DNA construct was injected into the pronuclei of one cell (B6D2)F1 embryos. A total of five independent cTnT-Cre founders were obtained. The #5 line, maintained by intercross with (B6D2)F1 mice, was selected because it induced the highest level of recombination, as determined by crossing with Rosa26R reporter mice, which express β-galactosidase only when recombination has been effected by Cre (Soriano 1999). Embryo dissection, sectioning, and X-gal staining were performed as described (Lawson et al. 1999). Section in situ hybridization was performed as described previously (Jiao et al. 2002) using a TGFβ2 probe [a gift from Dr. H. Moses (Vanderbilt University Medical Center, Nashville, TN)].
Proliferation assay
Immunostaining was performed using anti-Ki67 nuclear antigen (Novocastra Laboratories), following the manufacturer's instructions. A Texas red conjugated anti-rabbit body was used as the secondary antibody and slides were also stained with DAPI to visualize all nuclei.
RT-PCR analysis
RT-PCR analysis was performed as described previously (Jiao et al. 2002). The primer sequences for amplifying Bmp4 and β-Actin mRNAs will be provided on request.
Acknowledgments
We thank Drs. J. Lin, M. Magnuson, and H. Moses for providing regents; Dr. J. Barnett for suggestions on the project; and Dr. E. Myers for commenting on the manuscript. K.J. was an Associate and B.L.M.H. an Investigator of the Howard Hughes Medical Institute. This project is currently supported by the NIH grant (HL62177) to H.S.B.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Article published online ahead of print. Article and publication date are at http://www.genesdev.org/cgi/doi/10.1101/gad.1124803.
References
- Abdelwahid E., Rice, D., Pelliniemi, L.J., and Jokinen, E. 2001. Overlapping and differential localization of Bmp-2, Bmp-4, Msx-2 and apoptosis in the endocardial cushion and adjacent tissues of the developing mouse heart. Cell Tissue Res. 305: 67-78. [DOI] [PubMed] [Google Scholar]
- Bartram U., Molin, D.G., Wisse, L.J., Mohamad, A., Sanford, L.P., Doetschman, T., Speer, C.P., Poelmann, R.E., and Gittenberger-de Groot, A.C. 2001. Double-outlet right ventricle and overriding tricuspid valve reflect disturbances of looping, myocardialization, endocardial cushion differentiation, and apoptosis in TGF-β(2)-knockout mice. Circulation 103: 2745-2752. [DOI] [PubMed] [Google Scholar]
- Boyer A.S., Ayerinskas I.I., Vincent, E.B., McKinney, L.A., Weeks, D.L, and Runyan, R.B. 1999. TGFβ2 and TGFβ3 have separate and sequential activities during epithelial-mesenchymal cell transformation in the embryonic heart. Dev. Biol. 208: 530-545. [DOI] [PubMed] [Google Scholar]
- Camenisch T.D., Spicer, A.P., Brehm-Gibson, T., Biesterfeldt, J., Augustine, M.L., Calabro Jr., A., Kubalak, S., Klewer, S.E., and McDonald, J.A. 2000. Disruption of hyaluronan synthase-2 abrogates normal cardiac morphogenesis and hyaluronan-mediated transformation of epithelium to mesenchyme. J. Clin. Invest. 106: 349-360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camenisch T.D., Molin, D.G., Person, A., Runyan, R.B., Gittenberger-de Groot, A.C., McDonald, J.A., and Klewer, S.E. 2002a. Temporal and distinct TGFβ ligand requirements during mouse and avian endocardial cushion morphogenesis. Dev. Biol. 248: 170-181. [DOI] [PubMed] [Google Scholar]
- Camenisch T.D., Schroeder, J.A., Bradley, J., Klewer, S.E., and McDonald, J.A. 2002b. Heart-valve mesenchyme formation is dependent on hyaluronan-augmented activation of ErbB2-ErbB3 receptors. Nat. Med. 8: 850-855. [DOI] [PubMed] [Google Scholar]
- Dudley A.T. and Robertson, E.J. 1997. Overlapping expression domains of bone morphogenetic protein family members potentially account for limited tissue defects in BMP7 deficient embryos. Dev. Dyn. 208: 349-362. [DOI] [PubMed] [Google Scholar]
- Fujiwara T., Dehart, D.B., Sulik, K.K., and Hogan, B.L. 2002. Distinct requirements for extra-embryonic and embryonic bone morphogenetic protein 4 in the formation of the node and primitive streak and coordination of left-right asymmetry in the mouse. Development 129: 4685-4696. [DOI] [PubMed] [Google Scholar]
- Galvin K.M., Donovan, M.J., Lynch, C.A., Meyer, R.I., Paul, R.J., Lorenz, J.N., Fairchild-Huntress, V., Dixon, K.L., Dunmore, J.H., Gimbrone Jr., M.A., et al. 2000. A role for smad6 in development and homeostasis of the cardiovascular system. Nat. Genet. 24: 171-174. [DOI] [PubMed] [Google Scholar]
- Gaussin V., Van de Putte, T., Mishina, Y., Hanks, M.C., Zwijsen, A., Huylebroeck, D., Behringer, R.R., and Schneider, M.D. 2002. Endocardial cushion and myocardial defects after cardiac myocyte-specific conditional deletion of the bone morphogenetic protein receptor ALK3. Proc. Natl. Acad. Sci. 99: 2878-2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman J.I. 1995. Incidence of congenital heart disease: II. Prenatal incidence. Pediatr. Cardiol. 16: 155-165. [DOI] [PubMed] [Google Scholar]
- Hogan B.L. 1996. Bone morphogenetic proteins: multifunctional regulators of vertebrate development. Genes & Dev. 10: 1580-1594. [DOI] [PubMed] [Google Scholar]
- Ikeda T., Takahashi, H., Suzuki, A., Ueno, N., Yokose, S., Yamaguchi, A., and Yoshiki, S. 1996. Cloning of rat type I receptor cDNA for bone morphogenetic protein-2 and bone morphogenetic protein-4, and the localization compared with that of the ligands. Dev. Dyn. 206: 318-329. [DOI] [PubMed] [Google Scholar]
- Jiao K., Zhou, Y., and Hogan, B.L. 2002. Identification of mZnf8, a mouse Kruppel-like transcriptional repressor, as a novel nuclear interaction partner of Smad1. Mol. Cell. Biol. 22: 7633-7644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones C.M., Lyons, K.M., and Hogan, B.L. 1991. Involvement of Bone Morphogenetic Protein-4 (BMP-4) and Vgr-1 in morphogenesis and neurogenesis in the mouse. Development 111: 531-542. [DOI] [PubMed] [Google Scholar]
- Kirchhoff S., Kim, J.S., Hagendorff, A., Thonnissen, E., Kruger, O., Lamers, W.H., and Willecke, K. 2000. Abnormal cardiac conduction and morphogenesis in connexin40 and connexin43 double-deficient mice. Circ. Res. 87: 399-405. [DOI] [PubMed] [Google Scholar]
- Kulessa H. and Hogan, B.L. 2002. Generation of a loxP flanked bmp4loxP-lacZ allele marked by conditional lacZ expression. Genesis 32: 66-68. [DOI] [PubMed] [Google Scholar]
- Lakkis M.M. and Epstein, J.A. 1998. Neurofibromin modulation of ras activity is required for normal endocardial-mesenchymal transformation in the developing heart. Development 125: 4359-4367. [DOI] [PubMed] [Google Scholar]
- Lawson K.A., Dunn, N.R., Roelen, B.A., Zeinstra, L.M., Davis, A.M., Wright, C.V., Korving, J.P., and Hogan, B.L. 1999. Bmp4 is required for the generation of primordial germ cells in the mouse embryo. Genes & Dev. 13: 424-436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons K.M., Pelton, R.W., and Hogan, B.L. 1990. Organogenesis and pattern formation in the mouse: RNA distribution patterns suggest a role for bone morphogenetic protein-2A (BMP-2A). Development 109: 833-844. [DOI] [PubMed] [Google Scholar]
- Lyons K.M., Hogan, B.L., and Robertson, E.J. 1995. Colocalization of BMP 7 and BMP 2 RNAs suggests that these factors cooperatively mediate tissue interactions during murine development. Mech. Dev. 50: 71-83. [DOI] [PubMed] [Google Scholar]
- Marino B. and Digilio, M.C. 2000. Congenital heart disease and genetic syndromes: Specific correlation between cardiac phenotype and genotype. Cardiovasc. Pathol. 9: 303-315. [DOI] [PubMed] [Google Scholar]
- Markwald R.R. and Wessels, A. 2001. Overview of heart development. In Formation of the heart and its regulation (eds. R.J. Tomanek and R.B. Runyan), pp. 1-22. Birkhauser, Boston.
- Nakajima Y., Yamagishi, T., Hokari, S., and Nakamura, H. 2000. Mechanisms involved in valvuloseptal endocardial cushion formation in early cardiogenesis: Roles of transforming growth factor (TGF)-β and bone morphogenetic protein (BMP). Anat. Rec. 258: 119-127. [DOI] [PubMed] [Google Scholar]
- Neuhaus H., Rosen, V., and Thies, R.S. 1999. Heart specific expression of mouse BMP-10 a novel member of the TGF-β superfamily. Mech. Dev. 80: 181-184. [DOI] [PubMed] [Google Scholar]
- Pierpont M.E., Markwald, R.R., and Lin, A.E. 2000. Genetic aspects of atrioventricular septal defects. Am. J. Med. Genet. 97: 289-296. [PubMed] [Google Scholar]
- Schneider M.D., Gaussin, V., and Lyons, K.M. 2003. Tempting fate: BMP signals for cardiac morphogenesis. Cytokine Growth Factor Rev. 14: 1-4. [DOI] [PubMed] [Google Scholar]
- Soriano P. 1999. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat. Genet. 21: 70-71. [DOI] [PubMed] [Google Scholar]
- Tevosian S.G., Deconinck, A.E., Tanaka, M., Schinke, M., Litovsky, S.H., Izumo, S., Fujiwara, Y., and Orkin, S.H. 2000. FOG-2, a cofactor for GATA transcription factors, is essential for heart morphogenesis and development of coronary vessels from epicardium. Cell 101: 729-739. [DOI] [PubMed] [Google Scholar]
- von Bubnoff A. and Cho, K.W. 2001. Intracellular BMP signaling regulation in vertebrates: Pathway or network? Dev. Biol. 239: 1-14. [DOI] [PubMed] [Google Scholar]
- Waller B.R., McQuinn, T., Phelps, A.L., Markwald, R.R., Lo, C.W., Thompson, R.P., and Wessels, A. 2000. Conotruncal anomalies in the trisomy 16 mouse: An immunohistochemical analysis with emphasis on the involvement of the neural crest. Anat. Rec. 260: 279-293. [DOI] [PubMed] [Google Scholar]
- Wang Q., Sigmund, C.D., and Lin, J.J. 2000. Identification of cis elements in the cardiac troponin T gene conferring specific expression in cardiac muscle of transgenic mice. Circ. Res. 86: 478-484. [DOI] [PubMed] [Google Scholar]
- Wang Q., Reiter, R.S., Huang, Q.Q., Jin, J.P., and Lin, J.J. 2001. Comparative studies on the expression patterns of three troponin T genes during mouse development. Anat. Rec. 263: 72-84. [DOI] [PubMed] [Google Scholar]
- Weaver M., Yingling, J.M., Dunn, N.R., Bellusci, S., and Hogan, B.L. 1999. Bmp signaling regulates proximal-distal differentiation of endoderm in mouse lung development. Development 126: 4005-4015. [DOI] [PubMed] [Google Scholar]
- Webb S., Brown, N.A., and Anderson, R.H. 1997. Cardiac morphology at late fetal stages in the mouse with trisomy 16: Consequences for different formation of the atrioventricular junction when compared to humans with trisomy 21. Cardiovasc. Res. 34: 515-524. [DOI] [PubMed] [Google Scholar]