Abstract
The histone code guides many aspects of chromosome biology including the equal distribution of chromosomes during cell division. In the chromatin domains surrounding the centromere, known as pericentric heterochromatin, histone modifications, particularly deacetylation and methylation, appear to be essential for proper chromosome segregation. However, the specific factors and their precise roles in this highly orchestrated process remain under active investigation. Here, we report that germ-line or somatic deletion of mSds3, an essential component of the functional mSin3/HDAC corepressor complex, generates a cell-lethal condition associated with rampant aneuploidy, defective karyokinesis, and consequently, a failure of cytokinesis. mSds3-deficient cells fail to deacetylate and methylate pericentric heterochromatin histones and to recruit essential heterochromatin-associated proteins, resulting in aberrant associations among heterologous chromosomes via centromeric regions and consequent failure to properly segregate chromosomes. Mutant mSds3 molecules that are defective in mSin3 binding fail to rescue the mSds3 null phenotypes. On the basis of these findings, we propose that mSds3 and its associated mSin3/HDAC components play a central role in initiating the cascade of pericentric heterochromatin-specific modifications necessary for the proper distribution of chromosomes during cell division in mammalian cells.
Keywords: Sin3 complex, histone modification, pericentric heterochromatin, chromosome segregation
Covalent modification of histone tails impacts on numerous physiological processes including DNA packaging and replication, gene transcriptional control, chromosome segregation, cytoskeletal dynamics. The patterns of covalent modifications—acetylation, methylation, phosphorylation, and ubiquitylation impart distinct functionalities consistent with the emerging concept of a histone code (for review, see Turner 2000, 2002; Jenuwein and Allis 2001). Histone acetylation is itself a dynamic process driven by the opposing actions of acetyl transferases (HATs) and deacetylases (HDACs). The HDACs are integral components of several gene regulatory complexes, most notably mSin3/HDAC. The mammalian mSin3A and mSin3B molecules were identified as mediators of gene-specific transcriptional repression via their capacity to interact with sequence-specific transcription factors (Ayer et al. 1995; Schreiber-Agus et al. 1995) and to recruit chromatin modification factors such as the highly related Class I HDACs (HDAC1 and HDAC2; for review, see Knoepfler and Eisenman 1999) and histone methyltransferase (Wysocka et al. 2003; Yang et al. 2003). Recent genetic and biochemical studies have identified another key component of the Sin3/HDAC complex, Sds3 (Dorland et al. 2000; Lechner et al. 2000; Alland et al. 2002), which, upon deletion in yeast (Lechner et al. 2000) or siRNA-mediated neutralization in mammalian cells (Alland et al. 2002), leads to decreased Sin3-associated HDAC enzymatic activity. In mammalian cells, the extent to which the mSin3 complex participates in processes other than the regulation of gene expression has not been explored. Analysis of fission yeast deficient for the putative mSin3 ortholog, Pst1p, has pointed to impaired cell division, abnormal acetylation of histones within the centromeric region, and defective centromeric cohesion (Silverstein et al. 2003).
During mitosis, structural integrity of the centromeric and of the flanking pericentric chromatin regions is essential for proper assembly of the kinetochore and ultimately equal distribution of genetic material to daughter cells (Dobie et al. 1999; Sullivan 2001; Sharp and Kaufman 2003). Centromeric and pericentric nucleosomes are subject to numerous post-translational modifications (Van Hooser et al. 1999; Sullivan et al. 2001). One specific feature of pericentric nucleosomes is their rapid deacetylation after chromatin assembly, resulting in a local hypoacetylated state (Jeppesen et al. 1992; O'Neill and Turner 1995; Sobel et al. 1995; Taddei et al. 1999). Accordingly, in fission yeast and in mammalian cells, pharmacological inhibition of HDACs is associated with alterations in pericentric heterochromatin-modifications—specifically hypoacetylation of histone H4 Lys 5 and 12 and methylation of histone H3 Lys 9—and the consequent inability to recruit HP1 proteins (Ekwall et al. 1997; Taddei et al. 2001). Whereas these pharmacological studies point to the importance of histone deacetylation in the formation of pericentric heterochromatin, the identity of the HDAC-containing complexes involved in this regional modification has not been identified. Systematic genetic analysis of this process is complicated by HDAC family functional redundancy and by the expanding number of complexes possessing HDAC activity. The recent observation of an obligate role for mSds3 in the functional mSin3/HDAC complex prompted us to explore the consequences of loss of mSds3 function in normal development with a specific focus on chromosome segregation dynamics.
Results
A conditional knockout allele of mSds3 was engineered in the mouse germ line using standard technology (Fig. 1). Flp recombinase-mediated removal of an FRT-flanked neomycin-resistance cassette generated a functional mSds3 allele, designated mSds3L (L for lox) and subsequent Cre-mediated recombination of intronic loxP sites was shown to delete critical coiled-coil domain sequences and produce a nonsense frameshift, generating a null allele (designated mSds3-). mSds3+/- intercrosses failed to produce live mSds3-/- offspring or normal embryos at E6.5 or later (Fig. 1D). In contrast, mSds3-/- blastocysts were identified, implying either rescue from mSds3 maternal stores or mSds3 dispensability during preimplantation development. When cultured in vitro, 6 of 20 of the blastocysts generated from mSds3+/- intercrosses failed to hatch and ultimately died whereas 16 of 17 blastocysts generated from mSds3+/+ × mSds3+/- matings hatched and produced blastocyst outgrowths (Fig. 1E; data not shown). Together, these studies point to an essential role for mSds3 during peri-implantation development.
Figure 1.
mSds3 deficiency leads to early embryonic lethality. (A) Targeting strategy and mutant alleles of mSds3. The neomycin resistance cassette is flanked by FRT sites (diamonds), and the neomycin cassette plus the exon 6 region to be deleted (hatched bars) are flanked by LoxP sites (triangles). The external probe used for Southern screening is depicted in gray. Restriction sites used are EcoRI (E) and SalI (S). Exon 5 corresponds to nucleotides 341-360 starting at the initiating ATG; exon 6, nucleotides 361-517; exon 7, nucleotides 518-613. (B) Southern analysis of ES cell clones showing targeting of the mSds3 locus. (C) Genotyping by PCR of mice bearing a lox allele (top) or a null allele (bottom) for mSds3. (D) Genotype distribution of offspring and embryos resulting from mSds3 heterozygote mice intercrosses. (R) Resorbed. (E) In vitro outgrowth of blastocysts from mSds3 heterozygote intercrosses harvested at 3.5 dpc (=d0). The two bottom panels show putative mSds3 null blastocysts undergoing death. (ICM) Inner cell mass; (TE) trophectoderm.
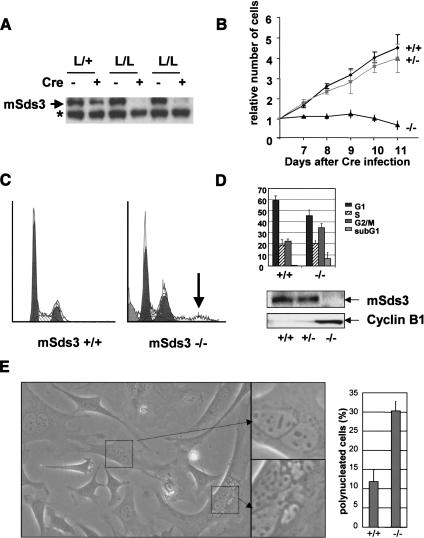
To assess the phenotype of somatic mSds3 inactivation, early passage mSds3+/+, mSds3L/+, mSds3L/L mouse embryonic fibroblast (MEF) cultures were harvested and infected with a retrovirus encoding constitutively active Cre recombinase. These cells sustained deletion of the mSds3L allele by PCR (data not shown), and complete loss of mSds3 protein was observed in the mSds3L/L cultures only (Fig. 2A). Continued growth and survival and normal cellular morphology was observed in the post-infection mSds3+/+ and mSds3+/- cultures, whereas mSds3-/- cultures experienced dramatic growth arrest and diminished survival (Fig. 2B). Flow cytometric analyses of many independent cultures revealed increased G2/M (average 35% vs. 21%) and decreased G1 (average 46% vs. 59%) fractions in mSds3-/- cells relative to mSds3+/+ and mSds3+/- control cultures (Fig. 2C,D; mSds3+/- not shown). That mSds3 deficiency was associated with a G2/M arrest was reinforced by the marked increase cyclin B1 levels, a specific marker of the G2/M phase (Pines and Hunter 1989; Fig. 2D). The emergence of a subG1 population only in the mSds3-/- cultures mirrored increased AnnexinV-positive cells in the mSds3-/- cultures relative to mSds3+/+ controls (average 22% vs. 8%), consistent with increased apoptosis associated with mSds3 deficiency. Finally, mSds3-/- cultures showed a prominent increase in polyploidy (Fig. 2C), paralleling emergence of a high proportion of polynucleated cells upon microscopic inspection (30% vs. 11%; p < 0.001; Fig. 2E). Within this polynucleated cells population, the ratio between di- and multinucleated cells is about 2:3 at day 4 after infection, and 1:3 at day 8 (data not shown). Interestingly, apoptosis was not observed at early time points after Cre-mediated deletion of mSds3 (day 4), perhaps pointing to the need to achieve a threshold level of abnormal DNA content (see below) incompatible with cell viability and/or the abnormalities in expression of essential genes subject to Sin3 complex regulation. The above findings establish an essential role for mSds3 in cell growth and survival and imply a link to the control of cell division and proper distribution of DNA content to daughter cells. As numerous studies have asserted the importance of establishment and maintenance of pericentric heterochromatin structure in processes of chromosomal segregation and cytokinesis/karyokinesis (Sharp and Kaufman 2003), we used a variety of assays to assess the impact of mSds3 deficiency on these structures. Histones H4 are known to be deposited as hyperacetylated forms on Lys 5 and Lys 12 within pericentric heterochromatin domains during late S phase, but are rapidly deacetylated to exclude acetylated histone forms in pericentric loci (Taddei et al. 1999). In mSds3-/- cells, H4 histones are inappropriately maintained in a hyperacetylated form in pericentric heterochromatin domains, indicating that mSds3 is required for histone H4 deacetylation in pericentric heterochromatin regions (Fig. 3a, panels A,B). Despite the strong reduction in mSin3-associated histone deacetylase activity in mSds3 null cells (average reduction of 52% in three independent assays; data not shown), there were no detectable differences in total cellular H4 acetylation among the various genotypes (Fig. 3b), suggesting that mSds3 functions primarily to direct and/or enable active deacetylation complexes at a limited subset of chromatin loci. As expected, hypoacetylation of histone H4 in pericentric heterochromatin was restored in mSds3-/-cells infected with a retrovirus encoding wild-type mSds3 prior to Cre-mediated deletion (data not shown).
Figure 2.
mSds3 deficiency results in cell cycle defects. (A) Western blot analysis with an anti-mSds3 antibody of MEFs, 8 d after Cre recombinase infection. The asterisk marks a nonspecific band. (B) Growth curve of MEFs wild-type, heterozygous or null for mSds3 after Cre recombinase infection. For each genotype, six independent cell lines were tested for this assay in duplicate. Empty vector-infected mSds3L/L MEFs grew similar to wild-type MEFs (data not shown). (C) Flow cytometry analysis of mSds3 MEFs 8 d after Cre recombinase infection. Shown is a representative result obtained for mSds3+/+ and mSds3-/- cells. The arrow points to polyploid cells in mSds3-/- cell population. (D, top) Cell cycle repartition average on two independent cell lines for each genotype in duplicate 8 d after Cre recombinase infection. (Bottom) Western Blots performed on the indicated cell lines with an anti-mSds3 antibody or an anti-cyclin-B1 antibody 8 d after Cre recombinase infection. (E, left) Bright field picture of representative mSds3-/- MEFs culture 8 d after Cre recombinase infection. (Right) Quantification of polynucleated cells in three independent primary MEFs of each genotype (at least 200 cells were counted for each cell line).
Figure 3.
mSds3 deficiency impairs pericentric heterochromatin structure. (a) Immunofluorescence performed on mSds3+/+ (panels A,C,E) or mSds3L/L (panels B,D,F) immortalized cells 8 d after Cre recombinase infection. Primary MEFs null for mSds3 exhibited the same pattern for each of the antibodies used in this study (data not shown). Left column is immunofluorescence performed using an anti-acetylated H4 on Lys 12 antibody (panels A,B), an anti-methylated H3 on Lys 9 antibody (panels C,D) or an anti-HP1α antibody (panels E,F). DAPI is visualized in the middle column and merge is shown in the right column. Shown is a representative cell for each genotype and antibody used. No differences were observed between mSds3+/+ and mSds3+/- cells. Images were acquired using a deconvolution microscope. Bar, 10 μm. (b) Western blot performed on matched cell lines to those shown in a, with the indicated antibodies. The bottom right gel represent a Comassie stain of the relevant molecular weight region.
Acetylation of histone H3 in pericentric heterochromatin was also investigated, as it has been shown that histones H3 are, like histones H4, deposited as acetylated forms in mammalian cells (Verreault et al. 1996). Using an antibody against histone H3 acetylated on Lys 9, no difference was observed between wild-type and mSds3-/- cells. In both cases, pericentric heterochromatin displayed exclusion of acetylated Lys 9 histone H3 (Supplementary Fig. S1). This result is in agreement with a previous study that failed to demonstrate acetylation of Lys 9 in histone H3 during deposition in mammalian cells (Sobel et al. 1995). It remains formally possible that, in the absence of mSds3, pericentric histone H3 is hyperacetylated at other residues.
In addition to persistent histone H4 acetylation, mSds3-deficient cells showed a loss in H3 histone methylation on Lys 9 in these heterochromatin domains, which normally exhibit a speckled pattern characteristic of pericentric domains (Peters et al. 2001; Maison et al. 2002; Fig. 3a, cf. panels C and D). Again, the loss in the concentrated methylated H3 signal in pericentric loci was not associated with a global reduction in methylation on Lys 9 histone H3 (Fig. 3b). The known reciprocal and interdependent pattern of methylation on histone H3 Lys 9 versus 4 (for review, see Fischle et al. 2003) prompted us to determine whether loss of Lys 9 methylation in mSds3-/- cells provoked Lys 4 methylation. No increase in mono-, di-, or trimethylation of histone H3 Lys 4 was detected, suggesting that the failure of mSds3-/- cells to methylate Lys 9 is not sufficient to induce methylation on Lys 4 (Supplementary Fig. S2). This impaired pericentric methylation is associated with a failure to recruit HP1α to pericentric domains in mSds3-/- cells (Fig. 3a, cf. panels E and F), consistent with previous studies showing that methylation of Lys 9 in histone H3 establishes a binding interface for HP1 proteins (Bannister et al. 2001; Lachner et al. 2001; Nakayama et al. 2001). Together, these results demonstrate that mSds3 plays an initiating role in the formation of pericentric heterochromatin. Finally, mSds3-/- cells show formation of micronuclei (Supplementary Fig. S3) as well as enlargement of DAPI-bright structures consistent with reorganization of pericentric heterochromatin domains (Fig. 3a, cf. panels B′ and A′; data not shown). Notably, these large DAPI-bright structures still colocalized with centromeric structural proteins, as revealed by immunofluorescence with anti-CENP-B (data not shown). Together, the findings here support a role for mSds3 and its mSin3/HDAC components in the subnuclear organization and post-translational modification of pericentric heterochromatin.
As mentioned above, these findings are reminiscent of the pericentric chromatin anomalies and micronuclei phenotype elicited by transient pharmacological inhibition of histone deacetylases by Trichostatin A (TSA; Taddei et al. 2001; Maison et al. 2002). Moreover, they are consistent with the essential role of mSds3 in the preservation of mSin3/HDAC-associated activity (Alland et al. 2002). Our previous efforts defined a large region within mSds3 capable of binding mSin3 (so-called mSin3 interacting domain, SID; Alland et al. 2002). To further define the SID and assess the relevance of the mSds3-mSin3 interaction to the phenotype reported here, we performed complementation studies utilizing a series of small deletions that spanned the region proximal to and within the SID. Each mSds3 mutant was engineered with an in-frame 10 amino acid deletion (Fig. 4A) and tested for its capacity to bind to mSin3A and to rescue the cellular and cytogenetic phenotypes elicited by loss of mSds3 function. As shown in Figure 4B, deletions encompassing the N-terminal SID did not affect the binding to mSin3A, whereas mutants deleted for amino acids 201-210 (Δ5), 211-220 (Δ6), or 221-230 (Δ7) showed markedly reduced mSin3A binding activity (Fig. 4B). Mutants competent for mSin3 binding were comparable with wild-type mSds3 in their ability to rescue cell growth and viability in an mSds3L/- immortalized cell line subjected to Cre-mediated deletion of the remaining wild-type mSds3 allele (Fig. 4C; data not shown). In contrast, mSds3L/- immortalized cells failed to grow followed Cre-mediated deletion of the wild-type allele when transduced with empty retrovirus or retroviruses encoding mSds3 mutant possessing poor mSin3-binding activity. Mutants with an intermediate level of mSin3-binding activity (e.g., mutants with C-terminal deletions in the SID, Δ5, Δ6, and Δ7) displayed an intermediate capacity to rescue cell survival (Fig. 4C). In addition to these biological correlations, the mSds3-mSin3A interaction profile matched well with the capacity to restore pericentric heterochromatin-specific modifications, as scored by HP1α localization to pericentric heterochromatin domains (Fig. 4D). Together, these results strongly support the view that the mSds3 null phenotypes likely depend upon mSds3 activities executed in the context of a functional mSin3/HDAC complex.
Figure 4.
mSds3 binding to mSin3A is necessary to rescue cell viability and pericentric heterochromatin defects. (A) Schematic representation of mSds3 and mSds3 mutants. The coiled coil is encompassed by amino acids 59-170, and the SID (mSin3 interaction domain) by amino acids 188-226. The region deleted in each of the mutants is depicted as a white area. Δ1 corresponds to mSds3Δ161-170, Δ2 to mSds3Δ171-180, Δ3 to mSds3Δ181-190, Δ4 to mSds3Δ191-200, Δ5 to mSds3Δ201-210, Δ6 to mSds3Δ211-220, and Δ7 to mSds3Δ221-230. (B) Western blot documenting the interaction between mSin3A and mSds3 or mSds3 deletion mutants in 293T cells. (Top) Western blot with an anti-Flag antibody detecting transfected Flag-tagged mSds3 or Flag-tagged mSds3 deletion mutants in whole-cell extracts. Ten percent of the amount of proteins used in the immunoprecipitation were loaded. (Bottom) Western blot with an anti-Flag antibody detecting Flag-tagged mSds3 or Flag-tagged mSds3 deletion mutants pulled down with an anti-mSin3A antibody. As reported previously, mSds3 runs as a doublet when overexpressed. mSin3AFlag was transfected to increase the amount of pulled-down mSds3. (C) Growth curve of immortalized mSds3L/- MEFS transduced with retrovirus encoding the mSds3 mutants, and subsequently infected with a retrovirus encoding Cre recombinase. For each genotype, two independent cell lines were tested. The growth of mSds3L/+ cell lines expressing the different mutants was not affected after Cre infection (data not shown). (D) Table representing the percentage of colocalization of HP1α with pericentric heterochromatin identified as DAPI-bright domains in the cell lines described in C, 8 d after Cre-mediated infection.
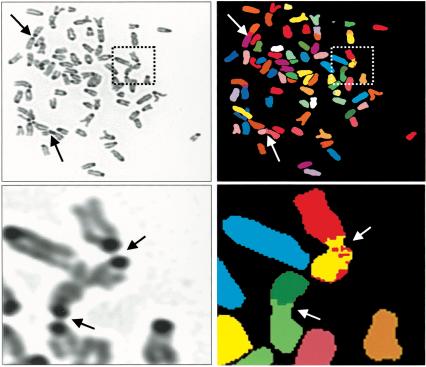
Alterations of pericentric heterochromatin-specific modifications have been linked to defects in chromosome segregation over a large phylogenetic distance. Cytogenetic analysis of metaphases derived from primary mSds3+/+ and mSds3-/- revealed marked aneuploidy in mSds3-/- cultures, with chromosome counts ranging from 8 to 172 compared with euploidy in the majority of mSds3+/+ controls (Fig. 5A). Aneuploidy was evident as early as 4 d following infection with a Cre-encoding, but not empty, retrovirus (data not shown), arguing against nonspecific effects relating to culture conditions or cell growth defects associated with mSds3 deficiency. DAPI staining and FISH using a centromere-specific probe showed that, in mSds3-/- cells, chromosomes remained associated through their centromeric or pericentric regions, but not throughout their arms (Fig. 5B,C). These aberrant associations were observed at a significantly higher frequency in mSds3 null cells than in wild-type cells (average 4.2 per metaphase in mSds3-/- cells compared with 0.75 per metaphase in mSds3+/+ cells 4 d after deletion, p < 0.001). The low, but measurable chromosome associations in mSds3+/+ metaphases might relate to Cre-recombinase effects and/or technical limitations of metaphase spreading. Regardless, the significantly greater number of associated chromosomes detected in mSds3-/- cells strongly supports the link between mSds3 deficiency and abnormal chromosomal associations. It is notable that only in mSds3-/- metaphases, some chromosomes exhibited a multibranched pattern (Fig. 5B), in which there were more than two chromosomes associated through their pericentric regions. These chromosomal structures are reminiscent of those encountered in the ICF syndrome (for immunodeficiency, centromere instability and facial anomalies; Brown et al. 1995; see below). To characterize more definitively these aberrant chromosomal structures, spectral karyotyping (SKY) was performed on metaphases derived from mSds3-/- primary MEF cultures 4 d after Cre retrovirus infection. As shown in Figure 6, these complex multiradiate structures involve heterologous chromosomes in most of the cases, although occasional homologous chromosome associations are observed. Finally, to assess the status of telomeres in mSds3-/- primary MEFs, double fluorescent in situ hybridization using a centromeric-specific probe and a telomeric-specific probe was performed. This study revealed no acute loss of telomeric sequences in all metaphases examined. Moreover, q-q or p-q chromosomal fusions were not observed (Fig. 5D), further excluding the possibility that chromosomal associations were related to a generalized telomere-capping defect. Collectively, these findings support the view that defective pericentric heterochromatin formation leads to a cascade of events that promote abnormal chromosomal association and missegregation.
Figure 5.
mSds3 deficiency results in genomic instability. (A) Chromosomal counts of metaphases from mSds3 wild-type primary MEFs (left) or mSds3 null primary MEFs (right), 8 d after Cre infection. Each bar represents one metaphase. Ten metaphase were counted on three independent cell lines for each genotype. (B) DAPI (blue) and centromeric-specific FISH (red) staining of one representative metaphase from mSds3+/+ primary MEFs (top) or mSds3-/- primary MEFs (bottom). The arrows point to associated centromeric regions. (C) Chromosomes from mSds3 null primary MEFs metaphase stained with DAPI (blue) and centromeric-specific FISH (red). (D) Double FISH with centromeric-specific probe (red) and telomeric-specific probe (green) of a portion of a representative metaphase from mSds3 null primary MEFs. Arrows indicate associated centromeric regions.
Figure 6.
DAPI-stained representation (left) and corresponding spectral karyotyping (SKY) analysis (right) of a representative metaphase from primary mSds3-/- MEFs 4 d after Cre-mediated deletion. The arrows point to the associated chromosomes. (Bottom) A close-up of the aberrant chromosomal structures designated by the dotted windows at top.
Discussion
We report here the organismal and cellular consequences of the deletion of an essential component of the mSin3/HDAC complex, mSds3. Genetic inactivation of mSds3 results in early embryonic lethality and in impaired somatic cell growth and survival. On the molecular level, loss of mSds3 function results in the failure of pericentric histones to be deacetylated, thereby preventing the cascade of histone modification events required for the establishment of a functional pericentric heterochromatin structure. By establishing a tight correlation between these mSds3-associated phenotypes and the mSds3-mSin3 interaction, we suggest that the functions of mSin3/HDAC complex can be expanded beyond its classical role as a regulator of gene expression when tethered to sequence-specific transcription factors. Although the lack of an antibody recognizing mSds3 by immunofluorescence precludes a definitive physical link between mSds3 and the pericentric heterochromatin regions during replication, several lines of evidence support the view that the reported phenotypes are a direct consequence of loss of this complex at the time of histone deposition. First, HDAC2, a component of the mSin3 complex, has been reported to localize at replication foci during late S phase, the period during which pericentric heterochromatin domains are replicated (Rountree et al. 2000). Second, the Sin3 ortholog in fission yeast, Pst1p shows colocalization with the centromeric regions (Silverstein et al. 2003). Finally, the specific alteration of the mSin3 complex-associated histone deacetylase activity, together with the histones modifications defects in mSds3 null cells, suggests that the HDAC activity responsible for deacetylation of histone H4 at pericentric domains lies within the mSin3/mSds3 complex.
Several phenotypic consequences of mSds3 inactivation resemble those that have been reported in cells treated with the pan histone deacetylase inhibitor, TSA (Ekwall et al. 1997; Taddei et al. 2001). As mentioned above, these include the alteration of pericentric heterochromatin-specific modifications and HP1 proteins association, the formation of micronuclei, and aberrant cell cycle profiles. There are, however, a number of notable distinctions between these pharmacological manipulations and our genetic approach. First, mSds3 deletion is not associated with TSA-induced relocalization of centromeric structures (Taddei et al. 2001). Second, there is a lack of TSA-induced cell death, although this may relate to the transient nature of TSA. Notably, the presumed diverse actions of mSds3 are also suggested by significant differences in the cellular phenotype brought about by genetic inactivation of HDAC1 in the mouse that is not associated with loss of cell viability and increased apoptosis, perhaps due to compensation by HDAC2 (Lagger et al. 2002). Along these lines, it is worth noting that fibroblasts derived from mice lacking Suv39h proteins, responsible for H3 methylation at pericentric regions, are viable despite genomic instability (Peters et al. 2001). This observation suggests that hypomethylation of histone 3 Lys 9 is not sufficient to induce a cell-lethal condition, and argue in favor of an additional role of the mSin3/HDAC complex in regulating the transcription of diverse genes essential to maintain cell viability.
Chromosome behavior during mitosis is a highly regulated process. The above cytogenetic profiles suggest that aneuploidy associated with mSds3 deficiency stems primarily from defective chromosome segregation. Defective mitosis is also evident in cells treated with histone deacetylase inhibitors (Ekwall et al. 1997; Taddei et al. 2001). Together, these observations raised the possibility that the spindle checkpoint may be compromised in the setting of impaired HDAC activity. In the setting of mSds3 deficiency, this possibility appears to be remote as evidenced by markedly increased cyclin B1 levels. Cyclin B1 is normally degraded by the APC at the onset of anaphase, and APC activity is inhibited upon activation of the spindle checkpoint. Finally, mSds3-/- cells show proper kinetochore localization of several components of the spindle checkpoint, including Bub1 and BubR1 (data not shown). Because the spindle checkpoint does not seem to be inactivated upon mSds3 depletion, a possible mechanism driving aneuploidy in mSds3-/- cell may relate to the identification of multibranched chromosome structures observed in Figure 6. As shown by SKY analysis, the chromosomes that appear associated through their centromeric/pericentric regions are not homologs in most cases. This is highly reminiscent of what is observed in lymphocytes derived from patients afflicted with ICF syndrome. In ICF, chromosomes 1, 16, and less often, chromosome 9 are involved in multiradial structures linked through their pericentric regions that appear to sustain breakage and rejoining at the level of satellite DNA within the pericentric regions (Tuck-Muller et al. 2000). Interestingly, multibranched chromosomes can associate heterologous chromosomes, mainly chromosomes 1 and 16, which in human are the only chromosomes with long regions of heterochromatin adjacent to the centromere. The mutation responsible for the ICF syndrome has been identified, and affects the activity of a DNA methyltransferase, DNMT3b (Xu et al. 1999). It has been shown that the DNA methyl-binding protein MeCP2 localizes to pericentric heterochromatin, and that this localization is dependent upon DNA methylation at these sites (Nan et al. 1996). Significantly, MeCP2 is able to recruit the mSin3/HDAC complex (Jones et al. 1998; Nan et al. 1998). It is therefore plausible that the mSin3/HDAC complex is normally recruited to pericentric heterochromatin regions, and that loss of DNA methylation through the inactivation of DNMT3b as seen in ICF and loss of mSin3/HDAC complex activity as reported here result in overlapping phenotypes, including the appearance of micronuclei and aberrant multibranched chromosome structures.
In summary, this study establishes a role for the essential mSin3 component, mSds3, in the pericentric chromatin modifications needed for proper chromosome separation and segregation. The essential role of mSds3 in the maintenance of genome stability raises the possibility that the tumor suppressor activities of the mSin3 complex extend beyond its classical role as a regulator of gene expression.
Materials and methods
Targeting construct, colony generation, and genotyping
We cloned and mapped the mSds3 locus from a bacterial artificial chromosome library. The targeting vector carried a negative selection marker for diptheria toxin (DT), a positive selection marker for neomycin acetyltransferase (Neo), Frt sites (diamonds) and loxP sites (triangles). An EcoRI site was introduced in the 3′ LoxP site for Southern genotyping. TC1 embryonic stem (ES) cells were electroporated and selected transformed cells by standard techniques. We screened 90 clones by Southern analysis using the described external to the targeting construct (Fig. 1A) to identify four recombinants. Blastocyst injections were carried out with two independent targeted clones, and transmitting chimaeric mice were bred to CAGG-Flpe and EIIa-Cre transgenic mice to generate the mSds3L and mSds3- alleles, respectively. Mice were genotyped by Southern analysis and multiplex PCR (Fig. 1C; primers and conditions are available from R.A. DePinho on request). The mice and cells analyzed in this report were negative for the Flp and Cre transgenes.
Cellular analysis
We generated MEFs from 13.5 d post-coitus embryos and grew them in DMEM medium plus 10% fetal calf serum, 50 μM β-mercaptoethanol, penicillin, and streptomycin Excision of the mSds3lox was obtained in vitro using a Cre recombinase expressing retrovirus, using standard infection protocol. Cells infected were selected using puromycin (2.5 μg/mL). For primary MEFs analysis, cultures were exposed to Cre retrovirus at passage 2. Immortalized MEFs were generated from embryos resulting from crosses with p53 null animals. Rescue experiments were performed on immortalized MEFs, infected with the corresponding mutants expressing retrovirus (details of the constructs available upon request), selected with blasticidin S for 7 d, and infected subsequently with Cre-expressing retrovirus. To perform growth curve analysis, 20,000 cells were plated on day 0 in 12-well plates, and were fixed on the indicated day, stained with crystal violet, extracted with 10% acetic acid, and we measured the relative cell number at an absorbance of 595 nm. FACS analysis was performed 8 d after infection. Annex-inV-PI was performed according to the manufacturer's instructions (BD Biosciences).
Protein analysis
Cell lysates from MEFs were prepared using RIPA buffer and resolved on polyacrylamide gels. Western blots were carried out using a rabbit polyclonal antibody against mSds3 (Alland et al. 2002), an anti-metLys9H3 (Upstate Biotechnology), anti-AcH3, anti-AcH4 (Upstate Biotechnology), anti-CyclinB1 (Upstate Biotechnology). Immunofluorescence was performed on cells grown in 8-well chamber slides, fixed in 2% paraformaldehyde, permeabilized with 0.1% Triton X-100. Antibodies used are Anti-4XmetLys9H3 [a gift from T. Jenuwein (IMP, The Vienna Biocenter, Vienna, Austria)], anti-AcLys12H4, anti-AcH4, anti-MetH3, anti-AcLys9H3 (Upstate Biotechnology), anti-HP1α (Chemicon), anti-MonoMetH3K4, anti-DiMetH3K4, and anti-TriMetH3K4 (Abcam). Loading was assessed using anti-tubulin (Sigma). Images for Figure 3 were acquired using a Zeiss Axiovert 200M inverted microscope and a CoolSnap HQ CCD camera (Photometrics) driven by the Slidebook 4.0 b 2.5 software (intelligent Imaging Innovations). Pictures were acquired with 1 × 1 binning and a 0.1 mm Z-step. Deconvolution was performed by the nearest neighbors method with 20 iterations.
Metaphase analysis
Metaphases were prepared from primary MEFs 8 d after Crerecombinase infection. Briefly, cells were treated for 1-5 h with colchemid, harvested by trypsinization, and fixed in methanol/acetic acid after incubation for 20 min in 0.075 M KCl. Metaphases were dropped on slides, dried overnight, and stained with DAPI before microscope examination. For centromere and telomere fluorescent in situ hybridization, a Cy3-labeled mouse pan-centromeric probe was used according to the manufacturer's instructions (StarFISH), and a FITC-labeled PNA-FISH telomeric probe (Applied Biosystems) was used when mentioned, using the same conditions. Spectral karyotyping was done using the SkyPaint Kit for mouse samples (Applied Spectral Imaging) according to the manufacturer's protocols. Images were acquired using a Nikon Eclipse E6000 microscope equipped with the SD300 Spectracube and Spectral Imaging acquisition software.
Acknowledgments
We thank David Pellman and Nicole Schreiber-Agus for critical reading of the manuscript and helpful discussions. We also thank Genevieve Almouzni, Beth Sullivan, Anton Chestukhin, Larisa Livotchi, Michael Mourez, and Isabelle Marie for fruitful discussions, as well as the members of the DePinho laboratory. We thank Thomas Jenuwein for the generous gift of the Anti-4XmetLys9H3 antibody, James DeCaprio and Matthew Meyerson for the gift of reagents. We thank Richard Maser for his help with metaphase spreading and FISH staining, and Isabelle Sagot for assistance with deconvolution microscopy. G.D was supported by a fellowship of the Human Frontier Science Program Organization. R.A.D. is an American Cancer Society Research Professor. This work was supported by National Institute of Health grant RO1CA86379 and by grants from the American Cancer Society.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Supplemental material is available at http://www.genesdev.org.
Article and publication are at http://www.genesdev.org/cgi/doi/10.1101/gad.1109403.
References
- Alland L., David, G., Shen-Li, H., Potes, J., Muhle, R., Lee, H.C., Hou Jr., H., Chen, K., and DePinho, R.A. 2002. Identification of mammalian Sds3 as an integral component of the Sin3/histone deacetylase corepressor complex. Mol. Cell. Biol. 22: 2743-2750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayer D.E., Lawrence, Q.A., and Eisenman, R.N. 1995. Mad-Max transcriptional repression is mediated by ternary complex formation with mammalian homologs of yeast repressor Sin3. Cell 80: 767-776. [DOI] [PubMed] [Google Scholar]
- Bannister A.J., Zegerman, P., Partridge, J.F., Miska, E.A., Thomas, J.O., Allshire, R.C., and Kouzarides, T. 2001. Selective recognition of methylated lysine 9 on histone H3 by the HP1 chromo domain. Nature 410: 120-124. [DOI] [PubMed] [Google Scholar]
- Brown D.C., Grace, E., Sumner, A.T., Edmunds, A.T., and Ellis, P.M. 1995. ICF syndrome (immunodeficiency, centromeric instability and facial anomalies): Investigation of heterochromatin abnormalities and review of clinical outcome. Hum. Genet. 96: 411-416. [DOI] [PubMed] [Google Scholar]
- Dobie K.W., Hari, K.L., Maggert, K.A., and Karpen, G.H. 1999. Centromere proteins and chromosome inheritance: A complex affair. Curr. Opin. Genet. Dev. 9: 206-217. [DOI] [PubMed] [Google Scholar]
- Dorland S., Deegenaars, M.L., and Stillman, D.J. 2000. Roles for the Saccharomyces cerevisiae SDS3, CBK1 and HYM1 genes in transcriptional repression by SIN3. Genetics 154: 573-586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekwall K., Olsson, T., Turner, B.M., Cranston, G., and Allshire, R.C. 1997. Transient inhibition of histone deacetylation alters the structural and functional imprint at fission yeast centromeres. Cell 91: 1021-1032. [DOI] [PubMed] [Google Scholar]
- Fischle W., Wang, Y., and Allis, C.D. 2003. Histone and chromatin cross-talk. Curr. Opin. Cell. Biol. 15: 172-183. [DOI] [PubMed] [Google Scholar]
- Jenuwein T. and Allis, C.D. 2001. Translating the histone code. Science 293: 1074-1080. [DOI] [PubMed] [Google Scholar]
- Jeppesen P., Mitchell, A., Turner, B., and Perry, P. 1992. Antibodies to defined histone epitopes reveal variations in chromatin conformation and underacetylation of centric heterochromatin in human metaphase chromosomes. Chromosoma 101: 322-332. [DOI] [PubMed] [Google Scholar]
- Jones P.L., Veenstra, G.J., Wade, P.A., Vermaak, D., Kass, S.U., Landsberger, N., Strouboulis, J., and Wolffe, A.P. 1998. Methylated DNA and MeCP2 recruit histone deacetylase to repress transcription. Nat. Genet. 19: 187-191. [DOI] [PubMed] [Google Scholar]
- Knoepfler P.S. and Eisenman, R.N. 1999. Sin meets NuRD and other tails of repression. Cell 99: 447-450. [DOI] [PubMed] [Google Scholar]
- Lachner M., O'Carroll, D., Rea, S., Mechtler, K., and Jenuwein, T. 2001. Methylation of histone H3 lysine 9 creates a binding site for HP1 proteins. Nature 410: 116-120. [DOI] [PubMed] [Google Scholar]
- Lagger G., O'Carroll, D., Rembold, M., Khier, H., Tischler, J., Weitzer, G., Schuettengruber, B., Hauser, C., Brunmeir, R., Jenuwein, T., et al. 2002. Essential function of histone deacetylase 1 in proliferation control and CDK inhibitor repression. EMBO J. 21: 2672-2681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechner T., Carrozza, M.J., Yu, Y., Grant, P.A., Eberharter, A., Vannier, D., Brosch, G., Stillman, D.J., Shore, D., and Workman, J.L. 2000. Sds3 (suppressor of defective silencing 3) is an integral component of the yeast Sin3.Rpd3 histone deacetylase complex and is required for histone deacetylase activity. J. Biol. Chem. 275: 40961-40966. [DOI] [PubMed] [Google Scholar]
- Maison C., Bailly, D., Peters, A.H., Quivy, J.P., Roche, D., Taddei, A., Lachner, M., Jenuwein, T., and Almouzni, G. 2002. Higher-order structure in pericentric heterochromatin involves a distinct pattern of histone modification and an RNA component. Nat. Genet. 30: 329-334. [DOI] [PubMed] [Google Scholar]
- Nakayama J., Rice, J.C., Strahl, B.D., Allis, C.D., and Grewal, S.I. 2001. Role of histone H3 lysine 9 methylation in epigenetic control of heterochromatin assembly. Science 292: 110-113. [DOI] [PubMed] [Google Scholar]
- Nan X., Tate, P., Li, E., and Bird, A. 1996. DNA methylation specifies chromosomal localization of MeCP2. Mol. Cell. Biol. 16: 414-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nan X., Ng, H.H., Johnson, C.A., Laherty, C.D., Turner, B.M., Eisenman, R.N., and Bird, A. 1998. Transcriptional repression by the methyl-CpG-binding protein MeCP2 involves a histone deacetylase complex. Nature 393: 386-389. [DOI] [PubMed] [Google Scholar]
- O'Neill L.P. and Turner, B.M. 1995. Histone H4 acetylation distinguishes coding regions of the human genome from heterochromatin in a differentiation-dependent but transcription-independent manner. EMBO J. 14: 3946-3957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters A.H., O'Carroll, D., Scherthan, H., Mechtler, K., Sauer, S., Schofer, C., Weipoltshammer, K., Pagani, M., Lachner, M., Kohlmaier, A., et al. 2001. Loss of the Suv39h histone methyltransferases impairs mammalian heterochromatin and genome stability. Cell 107: 323-337. [DOI] [PubMed] [Google Scholar]
- Pines J. and Hunter, T. 1989. Isolation of a human cyclin cDNA: Evidence for cyclin mRNA and protein regulation in the cell cycle and for interaction with p34cdc2. Cell 58: 833-846. [DOI] [PubMed] [Google Scholar]
- Rountree M.R., Bachman, K.E., and Baylin, S.B. 2000. DNMT1 binds HDAC2 and a new co-repressor, DMAP1, to form a complex at replication foci. Nat. Genet. 25: 269-277. [DOI] [PubMed] [Google Scholar]
- Schreiber-Agus N., Chin, L., Chen, K., Torres, R., Rao, G., Guida, P., Skoultchi, A.I., and DePinho, R.A. 1995. An amino-terminal domain of Mxi1 mediates anti-Myc oncogenic activity and interacts with a homolog of the yeast transcriptional repressor SIN3. Cell 80: 777-786. [DOI] [PubMed] [Google Scholar]
- Sharp J.A. and Kaufman, P.D. 2003. Chromatin proteins are determinants of centromere function. Curr. Top. Microbiol. Immunol. 274: 23-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstein R.A., Richardson, W., Levin, H., Allshire, R., and Ekwall, K. 2003. A new role for the transcriptional corepressor SIN3; regulation of centromeres. Curr. Biol. 13: 68-72. [DOI] [PubMed] [Google Scholar]
- Sobel R.E., Cook, R.G., Perry, C.A., Annunziato, A.T., and Allis, C.D. 1995. Conservation of deposition-related acetylation sites in newly synthesized histones H3 and H4. Proc. Natl. Acad. Sci. 92: 1237-1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan B.A., Blower, M.D., and Karpen, G.H. 2001. Determining centromere identity: Cyclical stories and forking paths. Nat. Rev. Genet. 2: 584-596. [DOI] [PubMed] [Google Scholar]
- Sullivan K.F. 2001. A solid foundation: Functional specialization of centromeric chromatin. Curr. Opin. Genet. Dev. 11: 182-188. [DOI] [PubMed] [Google Scholar]
- Taddei A., Roche, D., Sibarita, J.B., Turner, B.M., and Almouzni, G. 1999. Duplication and maintenance of heterochromatin domains. J. Cell Biol. 147: 1153-1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taddei A., Maison, C., Roche, D., and Almouzni, G. 2001. Reversible disruption of pericentric heterochromatin and centromere function by inhibiting deacetylases. Nat. Cell Biol. 3: 114-120. [DOI] [PubMed] [Google Scholar]
- Tuck-Muller C.M., Narayan, A., Tsien, F., Smeets, D.F., Sawyer, J., Fiala, E.S., Sohn, O.S., and Ehrlich, M. 2000. DNA hypomethylation and unusual chromosome instability in cell lines from ICF syndrome patients. Cytogenet, Cell Genet. 89: 121-128. [DOI] [PubMed] [Google Scholar]
- Turner B.M. 2000. Histone acetylation and an epigenetic code. BioEssays 22: 836-845. [DOI] [PubMed] [Google Scholar]
- ____. 2002. Cellular memory and the histone code. Cell 111: 285-291. [DOI] [PubMed] [Google Scholar]
- Van Hooser A.A., Mancini, M.A., Allis, C.D., Sullivan, K.F., and Brinkley, B.R. 1999. The mammalian centromere: Structural domains and the attenuation of chromatin modeling. FASEB J. 13: S216-S220. [DOI] [PubMed] [Google Scholar]
- Verreault A., Kaufman, P.D., Kobayashi, R., and Stillman, B. 1996. Nucleosome assembly by a complex of CAF-1 and acetylated histones H3/H4. Cell 87: 95-104. [DOI] [PubMed] [Google Scholar]
- Wysocka J., Myers, M.P., Laherty, C.D., Eisenman, R.N., and Herr, W. 2003. Human Sin3 deacetylase and trithorax-related Set1/Ash2 histone H3-K4 methyltransferase are tethered together selectively by the cell-proliferation factor HCF-1. Genes & Dev. 17: 896-911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu G.L., Bestor, T.H., Bourc'his, D., Hsieh, C.L., Tommerup, N., Bugge, M., Hulten, M., Qu, X., Russo, J.J., and Viegas-Pequignot, E. 1999. Chromosome instability and immunodeficiency syndrome caused by mutations in a DNA methyltransferase gene. Nature 402: 187-191. [DOI] [PubMed] [Google Scholar]
- Yang L., Mei, Q., Zielinska-Kwiatkowska, A., Matsui, Y., Blackburn, M.L., Benedetti, D., Krumm, A.A., Taborsky Jr., G.J., and Chansky, H.A. 2003. An ERG (ets-related gene)-associated histone methyltransferase interacts with histone deacetylases 1/2 and transcription co-repressors mSin3A/B. Biochem. J. 369: 651-657. [DOI] [PMC free article] [PubMed] [Google Scholar]