Abstract
The assembly of clathrin-coated vesicles on Golgi membranes is initiated by the GTP-binding protein ADP ribosylation factor (ARF), which generates high-affinity membrane-binding sites for the heterotetrameric AP-1 adaptor complex. Once bound, the AP-1 recruits clathrin triskelia, which polymerize to form the coat. We have found that ARF⋅GTP also recruits AP-1 and clathrin onto protein-free liposomes. The efficiency of this process is modulated by the composition of the liposomes, with phosphatidylserine being the most stimulatory phospholipid. There is also a requirement for cytosolic factor(s) other than ARF. Thin-section electron microscopy shows the presence of clathrin-coated buds and vesicles that resemble those formed in vivo. These results indicate that AP-1-containing clathrin-coated vesicles can form in the absence of integral membrane proteins. Thus, ARF⋅GTP, appropriate lipids, and cytosolic factor(s) are the minimal components necessary for AP-1 clathrin-coat assembly.
Keywords: AP-1 adaptor complex
The transport of newly synthesized acid hydrolases from the trans-Golgi network (TGN) to the endosomal/lysosomal system is mediated by clathrin-coated vesicles (CCV; ref. 1). As these vesicles bud from the TGN, they are replaced by new clathrin-coated pits that are generated by the recruitment of cytosolic adaptors and clathrin onto the Golgi membrane. The initial step in this process is the exchange of GTP for GDP on the small GTP-binding protein ADP ribosylation factor (ARF) and the concomitant membrane attachment of ARF. The membrane-bound ARF⋅GTP promotes binding of the Golgi-specific adaptor protein complex AP-1 (2, 3). This heterotetrameric coat protein complex, in turn, recruits cytosolic clathrin triskelia, which assemble into a polyhedral lattice over the membrane-bound AP-1. The AP-1 also functions to concentrate selectively the mannose 6-phosphate receptors (MPRs) with their bound acid hydrolases into the forming CCV.
A central question that remains unresolved is whether proteins other than ARF are required for AP-1 recruitment onto the TGN. One proposal is that the MPRs, together with ARF·GTP, form membrane-docking sites for AP-1, thereby linking coat assembly with cargo selection (4, 5). However, we have found that the MPRs are not essential for the initial binding of AP-1 to the TGN (6). Alternately, we have suggested that ARF⋅GTP activates a docking apparatus that facilitates the recruitment of AP-1 onto the TGN surface (3, 7). Once bound to the TGN, the AP-1 would then interact with trafficking signals in the cytoplasmic tails of the MPRs for sorting. The putative docking molecules, however, have not been identified yet, and it is not known whether they are integral membrane components of the TGN or cytosolic factors that are recruited onto the TGN along with ARF.
Recently, several groups have reported the assembly of coatomer protein I (COPI)- and II (COPII)-coated vesicles, and AP-2-containing CCVs on protein-free artificial liposomes (8–10). These reports established that integral membrane proteins are not essential for the recruitment of these coats, although ARF1 and the small GTP-binding protein Sar1p were required for COPI- and COPII-vesicle formation, respectively. These findings prompted us to evaluate whether AP-1 could be recruited onto liposomes. We find that AP-1 can assemble onto liposomes in an ARF-dependent manner. The efficiency of this process depends on the lipid composition of the liposomes. Further, there is a requirement for a cytosolic component other than ARF, which may represent the proposed docking molecule(s) on Golgi.
MATERIALS AND METHODS
Materials.
l-α-phosphatidylcholine (PC) from soybeans containing either 20% PC (Sigma P5638) or 40% PC (Sigma P3644), phosphatidylinositol (PI) 4-phosphate (PI4P), PI 4,5-bisphosphate (PIP2), phosphatidic acid (PA), the dioleoyl forms of pure PC (DOPC), and phosphatidylethanolamine (DOPE), brefeldin A (BFA), and other common reagents were purchased from Sigma. PI 3,4,5-triphosphate (PIP3) was from Matreya (Pleasant Gap, PA). Phosphatidylserine (PS) and PI were from Avanti Polar Lipids. Recombinant myristoylated ARF1 (11), rat liver Golgi-enriched membranes, bovine adrenal cytosol, rat liver cytosol (3, 7), and rat liver CCVs (12, 13) were made as described. Coat proteins were released from the CCVs with 0.5 M Tris (pH 7) according to the method of Keen et al. (14). The soluble coat fraction was separated from the residual CCV membranes by centrifugation at 240,000 × gmax for 30 min, diluted 20-fold into assay buffer (25 mM Hepes-KOH, pH 7.0/125 mM KAc/2.5 mM Mg(Ac)2/1 mM DTT), and clarified by recentrifugation as above. The coat fraction was stored at 30 μg/ml protein. Bovine adrenal AP-1-depleted cytosol was prepared by immunoaffinity absorption of AP-1 on an anti-γ-adaptin antibody column (7). The antibodies used for Western blotting have been described (3, 7).
Preparation of Liposomes and ARF-Free Golgi Membranes.
Soybean lipids (4 mg) were dissolved in 1 ml of chloroform in glass tubes or in 15-ml Falcon plastic tubes. The chloroform was removed with a stream of nitrogen, and the thin film of lipids was hydrated with 1 ml of assay buffer. The sample was vortexed to release the lipids from the tube, followed by sonication to near translucence. The final liposomes remained in suspension after overnight storage at 4°C. Chemically defined liposomes were prepared the same way from pure lipid components. ARF-depleted Golgi membranes were prepared by incubating Golgi membranes in assay buffer for 15 min at 37°C, followed by centrifugation at 16,000 × g to recover the membranes.
Analysis of Lipid Composition.
Lipids were separated by two-dimensional chromatography on Silica Gel 60 plates (20 × 20 cm; 250-μm thickness; EM Separations Technology, Gibbstown, NJ) as described (15). The solvent systems were chloroform/methanol/18% ammonia (65:35:5, vol/vol) in the first direction and chloroform/acetone/methanol/acetic acid/water (10:4:2:2:1, vol/vol) in the second direction. The lipids were visualized with iodine vapor.
Coat-Recruitment Assays and Immunoblotting.
One- and two-stage coat-recruitment assays were performed essentially as described (3, 7), by using either Golgi membranes (50 μg/ml protein) or liposomes (200 μg/ml). After incubations, the Golgi membranes and liposomes were pelleted at 16,000 × g for 15 min, and the proteins were dissolved by boiling each pellet in 50 μl of 1× SDS sample buffer for 5 min. Half of each sample was then separated by SDS/7–15% PAGE and transferred subsequently to nitrocellulose membranes. Different portions of the blots were probed with antibodies against specific coat proteins, and the labeled bands were visualized by enhanced chemiluminescence (Amersham Pharmacia).
Electron Microscopy.
Golgi membranes and liposomes were incubated at 37°C with ARF and cytosol, supplemented with 15 μg/ml CCV coat fraction for 30 min, followed by centrifugation at 16,000 × g. Membrane pellets were fixed with 1% glutaraldehyde in 0.1 M sodium cacodylate buffer (pH 7.0) for 1 h on ice, postfixed with 1% osmium tetroxide, and contrasted with tannic acid according to the method of Orci et al. (16). Membranes were then embedded in Epon and thin sectioned. Thin sections were contrasted further with uranyl acetate and lead citrate, followed by analysis in a Zeiss 902 electron microscope.
RESULTS
Nucleotide- and Temperature-Dependent Recruitment of Coat Proteins onto Liposomes.
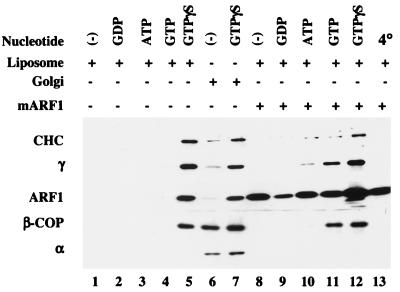
We initially assayed the ability of liposomes made from a commercial soybean lipid preparation containing 20% PC to recruit AP-1 and other types of coats. Incubation of the liposomes with bovine adrenal cytosol and GTPγS resulted in strong recruitment of AP-1, comparable to that obtained with Golgi membranes (Fig. 1, compare lanes 5 and 7). AP-1 binding did not occur at 4°C, in the absence of nucleotides, or when GDP, ATP, or GTP was used instead of GTPγS (Fig. 1, lanes 1–4 and 13). When the cytosol was supplemented with 4 μM myristoylated ARF1, AP-1 binding was evident in the presence of both GTP and GTPγS (Fig. 1, lanes 11 and 12). Similar results have been reported for AP-1 recruitment onto Golgi membranes (7).
Figure 1.
Nucleotide- and temperature-dependent coat recruitment onto liposomes and Golgi-enriched membranes. Liposomes prepared from soybean 20% PC material (200 μg/ml) and Golgi-enriched membranes (50 μg/ml) were incubated with 5 mg/ml gel-filtered bovine adrenal cytosol in the presence or absence of various nucleotides at 1 mM, except for GTPγS, which was at 0.1 mM. Myristoylated ARF1 (mARF1; 4 μM) was added as indicated. Coat proteins were detected by probing with mAb TD.1 for the clathrin heavy chain (CHC; ref. 17), mAb 100/3 for the γ-subunit of AP-1 (γ; ref. 18), mAb 100/2 for the α-subunit of AP-2 (α; ref. 18), mAb M3A5 for β-COP (19), and mAb 1D9 for ARF1 (20). The high ARF background signals in samples with supplemented ARF are caused by nonspecific binding of ARF to the tubes.
In addition to AP-1, COPI was recruited onto the liposomes in a GTPγS-dependent manner (Fig. 1, lanes 5 and 12), confirming the report of Spang et al. (8). However, AP-2 recruitment was not detected. The small amount of AP-2 bound to the Golgi-enriched membranes in the presence of GTPγS (Fig. 1, lanes 6 and 7) probably represents AP-2 binding to endosomes that contaminate the Golgi preparation (22, 23). Because AP-2 did not bind to the liposomes, the clathrin present on these membranes (Fig. 1, lanes 5 and 12) most likely was recruited by the bound AP-1 and possibly AP-3 (data not shown).
Recruitment of AP-1 onto Liposomes Is ARF-Dependent and Facilitated by Cytosolic ARF Guanine Nucleotide-Exchange Factor (GEF).
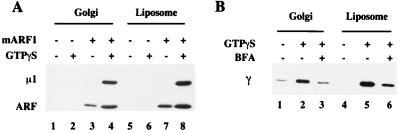
To determine whether ARF is required for AP-1 binding to liposomes, an AP-1-enriched cytosolic fraction devoid of ARF was incubated with either liposomes or ARF-depleted Golgi membranes in the absence or presence of exogenous ARF and GTPγS. The translocation of AP-1 onto the membranes was then determined. As shown in Fig. 2A, AP-1 was recruited onto the liposomes and the Golgi membranes only when ARF and GTPγS were present. This result indicates that AP-1 binding to the liposomes is an ARF-dependent process, just as with Golgi membranes (2, 3).
Figure 2.
AP-1 recruitment onto liposomes requires ARF and is BFA sensitive. (A) Liposomes prepared from soybean 20% PC material (200 μg/ml) and ARF-depleted Golgi membranes (50 μg/ml) were incubated with an AP-1-enriched pool of rat liver cytosol devoid of ARF in the absence or presence of 4 μM ARF and 100 μM GTPγS. The recruitment of the μ1 subunit of AP-1 and ARF was detected by immunoblotting with RY/1 (21) and 1D9, respectively. (B) Liposomes and Golgi-enriched membranes were incubated as in Fig. 1 in the absence or presence of 100 μg/ml BFA and 100 μM GTPγS as indicated. AP-1 binding was detected with mAb 100/3.
The translocation of AP-1 onto the liposomes was inhibited strongly by BFA (Fig. 2B). This fungal metabolite is known to inhibit the Golgi-associated ARF GEF, as well as some cytosolic ARF GEFs (24, 25). Because the liposomes are protein-free, the inhibition of AP-1 recruitment by BFA implicates a cytosolic GEF in the recruitment of ARF onto the liposomes.
Lipid Composition Modulates ARF and AP-1 Binding.
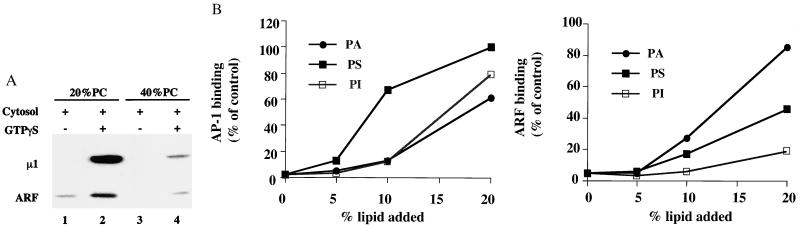
In our initial effort to explore the lipid requirements for AP-1 recruitment, we compared ARF and AP-1 binding to liposomes made from two preparations of soybean PC, one containing 20% PC and the other 40% PC. As shown in Fig. 3A, liposomes made from the 40% PC preparation were unable to recruit ARF and AP-1 efficiently, indicating that the purification process had removed or destroyed the specific lipid(s) required for recruitment of this coat material. A comparison of the phospholipid composition of the two preparations by thin-layer chromatography indicated that the 40% PC material contained significantly less PS and PA, as well as lower levels of phosphatidylinositides (data not shown). The addition of 10% PS to liposomes made from the 40% PC preparation enhanced ARF binding to a small extent but increased AP-1 recruitment 7-fold (Fig. 3B). In contrast, 10% PA and PI had little effect on AP-1 recruitment, even though the PA facilitated ARF binding. When the lipids were added to 20% concentrations, PS restored AP-1 binding to the level obtained with the 20% PC soybean preparation, and the PA and PI also enhanced AP-1 binding, although not to the level seen with the PS. These results indicate that the PS in the 20% PC soybean liposomes most likely accounts for the efficient recruitment of AP-1, with PA and PI having a minor role.
Figure 3.
Lipid-dependent AP-1 recruitment. (A) Liposomes made from soybean lipid fractions containing 20% and 40% PC were incubated with 5 mg/ml gel-filtered rat liver cytosol in the presence or absence of 100 μM GTPγS. The binding of AP-1 and ARF was detected as in Fig. 2A. (B) Liposomes were prepared from 40% PC soybean material supplemented with 5–20% of PA, PS, or PI and incubated as in A. The binding of AP-1 and ARF was determined by immunoblotting and densitometry and was plotted as percentage of binding relative to the 20% PC liposomes.
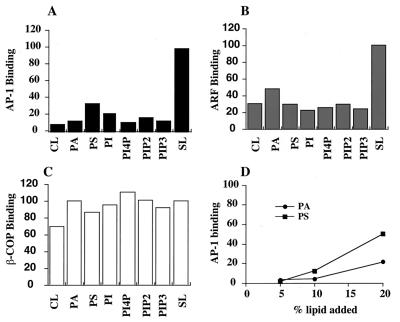
We next measured ARF and AP-1 recruitment onto chemically defined liposomes by using the soybean 20% PC liposomes as the standard for comparative purposes. The simplest form of liposome, consisting of DOPC/DOPE/cholesterol (50:40:10, wt/wt), recruited AP-1 and ARF very poorly (10% and 30% as well as the soybean liposomes, respectively; Fig. 4 A and B). Supplementation with 10% PS enhanced AP-1 binding about 3-fold but had little effect on ARF binding. Liposomes containing 20% PS bound AP-1 even better (Fig. 4D). The addition of 10% PA, PI, or several PI polyphosphates had only small effects on AP-1 binding, and 20% PA was less stimulatory than 20% PS (Fig. 4 A and D). These results indicate that it is not just the negative charge on the acidic lipids that is responsible for the enhanced AP-1 binding. It is also noteworthy that all of the liposomes bound similar amounts of COPI (Fig. 4C), consistent with the report of Spang et al. (8). Thus AP-1 recruitment is affected to a much greater extent by the lipid composition of the liposome than is COPI recruitment.
Figure 4.
(A–C) Coat binding to chemically defined liposomes. Liposomes were made from the combinations of phospholipids described below. Control liposomes (CL) consisted of DOPC/DOPE/cholesterol (50:40:10, wt/wt). The liposomes designated as PA, PS and PI contained 10% PA, PS or PI, respectively, added to CL. The liposomes designated as PI4P and PIP2 contained 5% of each lipid added to CL. The liposomes designated as PIP3 contained 2.5% PIP3 added to CL. The amount of DOPE in these liposomes was adjusted accordingly. SL designates liposome made from soybean 20% PC. The various liposomes were incubated as in Fig. 3. The binding of μ1, β-COP, and ARF was quantitated by immunoblotting and densitometry. Nonspecific binding in the absence of GTPγS was subtracted from the corresponding sample incubated with GTPγS. (D) The CL were supplemented with 5–20% PA and PS, and the binding of AP-1 was determined as above. The values are plotted as the percentage of binding of each protein relative to the 20% PC soybean liposomes.
AP-1 Binding Is Enhanced by Cytosolic Factor(s) Recruited by ARF⋅GTP.
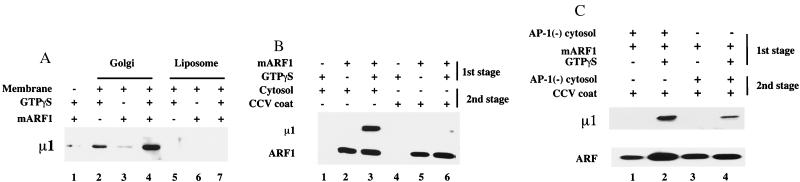
The experiments showed that ARF⋅GTP plus liposomes with a suitable lipid composition are necessary for efficient AP-1 recruitment. We next examined whether these minimal components are also sufficient for AP-1 binding. To pursue this issue, Golgi membranes and soybean-derived liposomes were incubated with a soluble coat fraction derived from rat liver CCVs in the presence or absence of recombinant ARF1 and GTPγS. As shown in Fig. 5A, AP-1 bound to the Golgi membranes in an ARF- and GTPγS-dependent manner, whereas no binding to the liposomes was observed.
Figure 5.
AP-1 binding to liposomes requires cytosolic factor(s) recruited by ARF⋅GTPγS. (A) ARF-depleted Golgi membranes or liposomes prepared from soybean 20% PC material were incubated with 15 μg/ml soluble rat liver CCV coat fraction with or without ARF1 and GTPγS as noted. AP-1 binding was detected with the RY/1 antibody to μ1. The low level of AP-1 binding to the Golgi membranes in the absence of ARF1 may reflect incomplete depletion of ARF during the preincubation (see Materials and Methods). (A) Liposomes were primed with ARF⋅GTPγS in a first-stage incubation with 4 μM ARF1 and 100 μM GTPγS for 30 min at 37°C as noted. After recovery of the liposomes, either 5 mg/ml rat liver cytosol or 15 μg/ml soluble CCV coat fraction was mixed with the primed liposomes on ice for 15 min as indicated. The binding of AP-1 and ARF was determined by immunoblotting. (C) Liposomes were incubated with 4 μM ARF1 and 100 μM GTPγS, with or without 5 mg/ml AP-1-depleted bovine adrenal cytosol for 15 min at 37°C as noted. The liposomes were recovered and mixed with 15 μg/ml soluble CCV coat fraction with or without AP-1-depleted bovine adrenal cytosol on ice for 15 min. The binding of AP-1 and ARF was determined as above.
One explanation for this result is that the Golgi membranes, having an active ARF GEF activity, recruit more ARF⋅GTP than the liposomes that bind only ARF⋅GTP that has undergone spontaneous nucleotide exchange (26). To avoid this problem, a two-stage assay was used. In the first stage, liposomes were incubated with ARF and GTPγS at 37°C for 30 min to load the liposomes with equal amounts of ARF⋅GTPγS. The ARF⋅GTPγS-primed liposomes were then recovered by centrifugation and reincubated in a second stage with either cytosol or the CCV coat fraction for 15 min on ice. Under these conditions, the ARF⋅GTPγS-primed liposomes recruited AP-1 from the cytosol but not from the CCV coat fraction (Fig. 5B). Thus, although the ARF⋅GTP exchange that occurs spontaneously on the liposome is sufficient to allow recruitment of AP-1 from cytosol, the failure to observe an equivalent recruitment of AP-1 from the coat fraction suggests that this material is lacking factor(s) present in the cytosol necessary for AP-1 binding to the liposomes.
Additional evidence in support of this conclusion was obtained by performing two complementary two-stage experiments. In the first experiment, liposomes and AP-1-depleted cytosol were incubated together with ARF and GTPγS in the first stage to allow recruitment of ARF⋅GTPγS and the putative cytosolic factor(s) onto the liposomes. The primed liposomes were then recovered and incubated on ice with the CCV coat fraction, and AP-1 binding was determined. As shown in Fig. 5C, the liposomes primed in this way were capable of binding AP-1 from the CCV coat fraction (lane 2), indicating that the putative cytosolic factor(s) had been recruited from the AP-1-depleted cytosol during the first stage incubation.
In the second experiment, the liposomes were loaded initially with ARF⋅GTPγS in a first-stage incubation. In the second stage, the CCV coat fraction was added together with AP-1-depleted cytosol to supply the putative cytosolic factor(s) presumed to be missing in the coat fraction. Under these conditions, AP-1 was recruited onto the liposomes (Fig. 5C, lane 4). The lower amount of AP-1 recruited in this experiment compared with the previous one probably reflects the difference in liposome-bound ARF⋅GTP, because the ARF⋅GTP exchange activity in the cytosol (lane 2) is much higher than the spontaneous exchange activity of the liposomes (lane 4). Together, these results support the conclusion that AP-1 binding is initiated by the synergistic action of ARF⋅GTP, specific lipid(s), and cytosolic factor(s).
CCV Assembly on Liposomes.
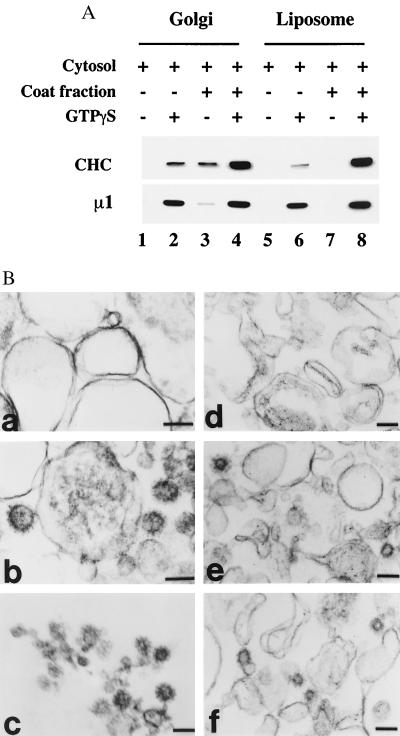
When the liposome and Golgi membranes that were recovered from the standard recruitment assays were analyzed by thin-section electron microscopy, clathrin-coated buds and CCVs were rarely encountered. However, we found that supplementation of the reaction mixtures with 15 μg/ml soluble CCV coat fraction increased clathrin binding to both membrane fractions by ≈20-fold (Fig. 6A). As in the standard assays, the recruitment of AP-1 and clathrin depended on ARF⋅GTPγS. Examination of the liposome and Golgi membranes incubated under these conditions identified abundant clathrin-coated buds and CCVs (Fig. 6B). These structures were very similar in both of two types of membranes, having an average diameter of 60–80 nm. Some of the CCVs in the Golgi membranes seemed to be tethered to the membrane by a thin neck. It is unlikely that these clathrin-coated buds and CCVs have been induced by AP-2, because this adaptor is not recruited onto the liposomes in our assays (Fig. 1 and data not shown). We cannot exclude completely the possibility that these structures are formed, at least in part, by AP-3, because both AP-1 and AP-3 are recruited efficiently under these conditions (data not shown).
Figure 6.
Electron-microscopic analysis of clathrin-coat assembly on Golgi membranes and liposomes. (A) Golgi membranes and soybean 20% PC liposomes were incubated with 5 mg/ml bovine adrenal cytosol supplemented with 15 μg/ml soluble CCV coat fraction, 4 μM mARF1, and 100 μM GTPγS as indicated. The recruitment of clathrin (CHC) and AP-1 (μ1) was determined by immunoblotting. (B) Membrane pellets recovered from Golgi membranes and liposomes that had been incubated as above were fixed and processed for electron microscopy as described in Materials and Methods. Golgi membranes incubated with cytosol and coat fraction in the absence (a) and presence (b) of GTPγS. Liposomes incubated with cytosol and coat fraction in the absence (d) or presence (e and f) of GTPγS. (c) Purified CCVs from rat liver. Clathrin-coat assembly occurred only in the presence of GTPγS (b, e, and f). (Bar = 100 nm.)
DISCUSSION
The data presented in this study establish that AP-1 and clathrin can be recruited onto protein-free liposomes with an efficiency that is comparable to that obtained with Golgi-enriched membranes. In both instances the AP-1 recruitment depends on ARF⋅GTP. However, the translocation of AP-1 onto the liposomes also required cytosolic factor(s) other than ARF and is influenced significantly by the lipid composition of the liposomes.
The importance of the lipid composition of the liposomes for AP-1 recruitment was detected initially in assays with two preparations of soybean PC as lipid sources. Liposomes made from the 20% PC preparation bound AP-1 much better than did the liposomes made from the 40% PC material. The latter material was found to be greatly depleted of PS and PA, and the addition of 10% PS restored most of the AP-1 binding. PA and PI were much less effective in stimulating AP-1 recruitment. Part of the PS effect likely is caused by enhanced ARF⋅GTP binding, but the 2-fold increase in ARF⋅GTP binding cannot account for the 7-fold enhancement of AP-1 recruitment that resulted from the 10% PS supplementation. The soybean liposomes also bound AP-1 much better than a fully synthetic liposome containing DOPC/DOPE/cholesterol. The addition of PS to this synthetic liposome also enhanced AP-1 binding, although not to the extent obtained with the soybean liposomes. Supplementation with the other acidic phospholipids increased AP-1 binding to a lesser extent. In contrast to these results, all of the liposomes bound COPI and AP-3 about equally (Fig. 4C and data not shown). Similar findings have been reported by others for COPI binding to liposomes (8). On the other hand, COPII binding requires acidic phospholipids, particularly PI4P and PIP2, although higher concentrations of PA also work (9). Interestingly, DOPC/DOPE combinations that include PS have a limited capacity to recruit COPII (9). The binding of AP-2 to liposomes has been reported to be independent of lipid composition; however, because this binding did not correlate with clathrin-coated pit formation, the authors suggested that it may be unrelated to coat assembly (10). Under the conditions of our assays, no AP-2 binding to liposomes was detected.
These differences in lipid requirements for binding of the various coat proteins may contribute to the spatial selection for coat assembly. Spang et al. (8) have suggested that regional differences in lipid head group composition, particularly an enrichment of PS in later compartments of the secretory pathway (27), may facilitate the localization of different coats to particular organelles. Our finding that AP-1 recruitment requires a specific lipid composition is consistent with this suggestion.
Previously, we suggested that ARF⋅GTP binding to the TGN serves to activate a docking apparatus that facilitates the recruitment of AP-1 onto the TGN surface (3, 7). In our model, the membrane-bound AP-1 then recruits clathrin, which subsequently polymerizes to form a clathrin-coated bud with a high local density of membrane-apposed adaptor molecules. The concentration of adaptors would serve to entrap the sorted transmembrane proteins, like the MPRs, into the assembling or preformed clathrin-coated pit. This model predicts that the movement of the sorted membrane proteins into the coat lags behind the initial recruitment of AP-1. Others have proposed that it is the MPRs, together with ARF⋅GTP, that form the major membrane-docking sites for AP-1 on the TGN (28). If the ARF⋅GTP-dependent recruitment of AP-1 onto protein-free liposomes accurately reflects the process that occurs on the TGN, then it is very unlikely that integral membrane proteins, such as the MPRs, are essential components of the initial docking apparatus. This conclusion is consistent with our finding that AP-1 binds equally well to Golgi membranes obtained from either normal or MPR-negative fibroblasts (6). However, our experiments do show the need for cytosolic factor(s) other than ARF and ARF GEF for AP-1 recruitment onto liposomes to occur. Thus, binding of ARF⋅GTP to liposomes is not sufficient for recruitment of AP-1 from a soluble CCV coat fraction. Factor(s) in AP-1-depleted cytosol also must be present (Fig. 5). Because the Golgi-membrane preparations bind AP-1 directly from the CCV coat material, we assume that the factor(s) must already be present on the Golgi membranes as a tightly associated peripheral protein. Similar factor(s) may exist on immature secretory granules that bind AP-1 in an ARF⋅GTP-dependent fashion. Tooze and colleagues (29) have reported that Tris stripping of immature secretory granules removes a peripheral protein required for AP-1 recruitment. These results are consistent with our hypothesis that putative docking molecules exist on the TGN for the specific recruitment of AP-1 and clathrin-coat assembly. Obviously, it will be important to identify and characterize the cytosolic factor(s) and to understand its/their role in AP-1 recruitment. The liposome system should serve as a convenient assay for the purification of the factor(s).
The thin-section electron-microscopic analysis of liposomes incubated with cytosol supplemented with a CCV coat fraction plus ARF⋅GTPγS indicated that the coat-assembly process proceeds to the formation of clathrin-coated buds and CCVs. It is important to note that vesicle formation occurred to a much greater extent when the cytosol was supplemented with the soluble coat fraction. This result indicates that the concentration of clathrin, and perhaps AP-1 as well, in the cytosol is limiting under the standard assay conditions. Previously, we reported an analogous finding that ARF was limiting for AP-1 recruitment onto Golgi membranes when GTP served as the nucleotide rather than GTPγS (7).
Although these studies establish that cargo molecules such as the MPRs are not essential for AP-1 recruitment and CCV formation, the findings do not exclude important roles for these molecules in regulating the coat-assembly process. For instance, the cargo molecules could alter the rate of ARF⋅GTP hydrolysis, as does the KDEL receptor ERD2, which regulates the translocation of ARF GAP onto membranes, thereby modulating COPI coat recruitment (30). If the MPRs have a similar function, the ARF⋅GTP-activated high-affinity AP-1 docking sites might remain active until cargo molecules recruit ARF GAP to the site, allowing GTP hydrolysis to occur. In a previous study, we presented evidence that is consistent with this scheme (6). It also has been reported that binding of the cytoplasmic domain of a transmembrane protein of COPI vesicles to coatamer induces a conformational change that favors polymerization (31). In a similar manner, binding of cargo molecules to the μ1 subunit of AP-1 could induce a conformational change that facilitates the coat-polymerization process. Considering the complexity of coat recruitment and assembly, it seems likely that cargo molecules will have regulatory roles in this process (32, 33).
Finally, our data, together with those of Spang et al. (8), establish that the three coat proteins known to require ARF⋅GTP for membrane binding—AP-1, AP-3, and COPI—can be recruited onto protein-free liposomes in an ARF⋅GTP-dependent manner. This system should prove useful for characterizing the various requirements for the recruitment of these different coats.
Acknowledgments
We thank Linton Traub for supplying several antibodies; Philip Majerus and Marina Ermolaeva for help with the lipid analysis; Lorrain LaRose for electron-microscopic analysis; and Rosalind Kornfeld and Linton Traub for valuable comments concerning the manuscript. This research was supported by National Institutes of Health Grant CA 08759 and by National Institutes of Health Training Grant HL 0708823.
ABBREVIATIONS
- ARF
ADP ribosylation factor
- TGN
trans-Golgi network
- COP
coatomer protein
- BFA
brefeldin A
- CCV
clathrin-coated vesicle
- PC
phosphatidylcholine
- PS
phosphatidylserine
- PA
phosphatidic acid
- PI
phosphatidylinositol
- PI4P
PI 4-phosphate
- PIP2
PI 4,5-bisphosphate
- PIP3
PI 3,4,5-triphosphate
- GEF
guanine nucleotide-exchange factor
- MPRs
mannose 6-phosphate receptors
- DOPC
dioleoyl form of pure PC
- DOPE
dioleoyl form of phosphatidylethanolamine
References
- 1.Kornfeld S, Mellman I. Annu Rev Cell Biol. 1989;5:483–525. doi: 10.1146/annurev.cb.05.110189.002411. [DOI] [PubMed] [Google Scholar]
- 2.Stamnes M A, Rothman J E. Cell. 1993;73:999–1005. doi: 10.1016/0092-8674(93)90277-w. [DOI] [PubMed] [Google Scholar]
- 3.Traub L M, Ostrom J A, Kornfeld S. J Cell Biol. 1993;123:561–573. doi: 10.1083/jcb.123.3.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Le Borgne R, Griffiths G, Hoflack B. J Biol Chem. 1996;271:2162–2170. doi: 10.1074/jbc.271.4.2162. [DOI] [PubMed] [Google Scholar]
- 5.Le Borgne R, Hoflack B. J Cell Biol. 1997;137:335–345. doi: 10.1083/jcb.137.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhu Y X, Traub L M, Kornfeld S. Mol Biol Cell. 1999;10:537–549. doi: 10.1091/mbc.10.3.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhu Y X, Traub L M, Kornfeld S. Mol Biol Cell. 1998;9:1323–1337. doi: 10.1091/mbc.9.6.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spang A, Matsuoka K, Hamamoto S, Schekman R, Orci L. Proc Natl Acad Sci USA. 1998;95:11199–11204. doi: 10.1073/pnas.95.19.11199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matsuoka K, Orci L, Amherdt M, Bednarek S Y, Hamamoto S, Schekman R, Yeung T. Cell. 1998;93:263–275. doi: 10.1016/s0092-8674(00)81577-9. [DOI] [PubMed] [Google Scholar]
- 10.Takei K, Haucke V, Slepnev V, Farsad K, Salazar M, Chen H, De Camilli P. Cell. 1998;94:131–141. doi: 10.1016/s0092-8674(00)81228-3. [DOI] [PubMed] [Google Scholar]
- 11.Liang J O, Kornfeld S. J Biol Chem. 1997;272:4141–4148. doi: 10.1074/jbc.272.7.4141. [DOI] [PubMed] [Google Scholar]
- 12.Campbell C, Squicciarini J, Shia M, Pilch P F, Fine R E. Biochemistry. 1984;23:4420–4426. doi: 10.1021/bi00314a028. [DOI] [PubMed] [Google Scholar]
- 13.Kedersha N L, Rome L H. J Cell Biol. 1986;103:699–709. doi: 10.1083/jcb.103.3.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Keen J H, Willingham M C, Pastan I H. Cell. 1979;16:303–312. doi: 10.1016/0092-8674(79)90007-2. [DOI] [PubMed] [Google Scholar]
- 15.Kates M. Techniques of Lipidology: Isolation, Analysis and Identification of Lipids. 2nd Ed. New York: Elsevier; 1986. p. 311. [Google Scholar]
- 16.Orci L, Glick B S, Rothman J E. Cell. 1986;46:171–184. doi: 10.1016/0092-8674(86)90734-8. [DOI] [PubMed] [Google Scholar]
- 17.Nathke I S, Heuser J, Lupas A, Stock J, Turck G W, Brodsky F M. Cell. 1992;68:899–910. doi: 10.1016/0092-8674(92)90033-9. [DOI] [PubMed] [Google Scholar]
- 18.Ahle S, Mann A, Eichelsbacher U, Ungewickell E. EMBO J. 1988;7:919–929. doi: 10.1002/j.1460-2075.1988.tb02897.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Allan V J, Kreis T E. J Cell Biol. 1986;103:2229–2239. doi: 10.1083/jcb.103.6.2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Houle M G T, Kahn R A, Naccache P H, Bourgoin S. J Biol Chem. 1995;270:22795–22800. doi: 10.1074/jbc.270.39.22795. [DOI] [PubMed] [Google Scholar]
- 21.Traub L M, Kornfeld S, Ungewickell E. J Biol Chem. 1995;270:4933–4942. doi: 10.1074/jbc.270.9.4933. [DOI] [PubMed] [Google Scholar]
- 22.Seaman M N, Ball C L, Robinson M S. J Cell Biol. 1993;123:1093–1105. doi: 10.1083/jcb.123.5.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang L H, Rothberg K G, Anderson R G. J Cell Biol. 1993;123:1107–1117. doi: 10.1083/jcb.123.5.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Randazzo P A, Yang Y C, Rulka C, Kahn R A. J Biol Chem. 1993;268:9555–9563. [PubMed] [Google Scholar]
- 25.Sata M, Donaldson J G, Moss J, Vaughan M. Proc Natl Acad Sci USA. 1998;95:4204–4208. doi: 10.1073/pnas.95.8.4204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Terui T, Kahn R A, Randazzo P A. J Biol Chem. 1994;269:28130–28135. [PubMed] [Google Scholar]
- 27.Zinser E, Daum G. Yeast. 1995;11:493–536. doi: 10.1002/yea.320110602. [DOI] [PubMed] [Google Scholar]
- 28.Le Borgne R, Hoflack B. Curr Opin Cell Biol. 1998;10:499–503. doi: 10.1016/s0955-0674(98)80065-3. [DOI] [PubMed] [Google Scholar]
- 29.Dittie A S, Hajibagher N, Tooze S A. J Cell Biol. 1996;132:523–536. doi: 10.1083/jcb.132.4.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aoe T, Cukierman E, Lee A, Cassel D, Hsu V W. EMBO J. 1997;16:7305–7316. doi: 10.1093/emboj/16.24.7305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reinhard C, Harter C, Bremser M, Brugger B, Sohn K, Helms J B, Wieland F. Proc Natl Acad Sci USA. 1999;96:1224–1228. doi: 10.1073/pnas.96.4.1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aridor M, Bannykh S I, Rowe T, Balch W E. J Biol Chem. 1999;274:4389–4399. doi: 10.1074/jbc.274.7.4389. [DOI] [PubMed] [Google Scholar]
- 33.Bremser M, Nickel W, Schweikert M, Ravazzola M, Amherdt M, Hughes C A, Sollner T H, Rothman J E, Wieland F T. Cell. 1999;96:495–506. doi: 10.1016/s0092-8674(00)80654-6. [DOI] [PubMed] [Google Scholar]