Abstract
Fish contain essential long chain polyunsaturated fatty acids (PUFAs), particularly docosahexaenoic acid (DHA), an omega-3 (or n-3) PUFA, but are also the main source of exposure to methylmercury (MeHg), a potent developmental neurotoxicant. Since n-3 PUFAs support neural development and function, benefits deriving from a diet rich in n-3s have been hypothesized to protect against deleterious effects of gestational MeHg exposure. To determine whether protection occurs at the behavioral level, female Long-Evans rats were exposed, in utero, to 0, 0.5, or 5 ppm of Hg as MeHg via drinking water, approximating exposures of 0, 40, and 400 μg Hg/kg/day and producing 0, 0.29, and 5.50 ppm of total Hg in the brains of siblings at birth. They also received pre- and postnatal exposure to one of two diets, both based on the AIN-93 semipurified formulation. A “fish-oil” diet was high in, and a “coconut-oil” diet was devoid of, DHA. Diets were approximately equal in α-linolenic acid and n-6 PUFAs. As adults, the rats were first assessed with a spatial discrimination reversal (SDR) procedure and later with a visual (nonspatial) discrimination reversal (VDR) procedure. MeHg increased the number of errors to criterion for both SDR and VDR during the first reversal, but effects were smaller or nonexistent on the original discrimination and on later reversals. No such MeHg-related deficits were seen when the rats were retested on SDR after two years of age. These results are consistent with previous reports and hypotheses that gestational MeHg exposure produces perseverative responding. No interactions between Diet and MeHg were found, suggesting that n-3 PUFAs do not guard against these behavioral effects. Brain Hg concentrations did not differ between the diets, either. In geriatric rats, failures to respond were less common and response latencies were shorter for rats fed the fish oil diet, suggesting that exposure to a diet rich in n-3s may lessen the impact of age-related declines in response initiation.
Keywords: Methylmercury, Fish oil, Development, Operant behavior, Discrimination reversal, PUFA, Aging
1. Introduction
Developmental exposure to methylmercury (MeHg) at doses that result in low micromolar concentrations of mercury in whole brain (Newland and Reile, 1999; Newland et al., 2006b) produce long-lasting behavioral effects that are manifested in adulthood and become exacerbated in aging (Kinjo et al., 1993; Newland and Rasmussen, 2000; Newland et al., 2004; Rice, 1996). Included among these effects are impaired sensory function in nonhuman primates (Rice, 1996; Rice and Gilbert, 1982, 1990), retarded behavior in transition in rodents and nonhuman primates (Newland et al., 2004; Newland et al., 1994; Paletz et al., 2006), deficits in complex, high-rate operant behavior (Newland and Rasmussen, 2000), disrupted performance on timing in fixed-interval schedules of reinforcement (Rice, 1992), delayed object permanence (Burbacher et al., 1988; Gunderson et al., 1988b), and impaired facial recognition (Gunderson et al., 1988a). Performance on tasks that tap memory function, however, are relatively spared after developmental MeHg exposure (Elsner et al., 1988; Gilbert et al., 1993; Goldey et al., 1994; Newland and Paletz, 2000). This behavioral toxicity is consistent with damage to the cerebral cortex, a region that is especially susceptible to low-level developmental MeHg exposure. Even at very low, submicromolar, concentrations, MeHg disrupts cell signaling, calcium currents, and induces dysmorphology in in vitro models of cortical development (Barone et al., 1998; Parran et al., 2003; Shafer et al., 2002).
Consumption of certain types of fish, notably long-lived predators, is the primary route of MeHg exposure. Marine fish also contain docosahexaenoic acid (DHA), an essential omega-3 polyunsaturated fatty acid (n-3 PUFA) that supports brain development and visual and motor function (Egeland and Middaugh, 1997; Reisbick and Neuringer, 1997). Dietary deficiencies in n-3 PUFAs reduce brain weight (Wainwright et al., 1992), impair vision (Okaniwa et al., 1996; Reisbick et al., 1997; Yamamoto et al., 1987; Yamamoto et al., 1988), and produce motor deficits (Greiner et al., 1999). There is mixed evidence about whether n-3 PUFAs affect such cognitive functions as learning, behavior change, or memory (Becker and Kyle, 2001; Wainwright, 1997; Wainwright et al., 1999). Nevertheless, it is reasonable to hypothesize that benefits derived from consumption of n-3 PUFAs may protect against or otherwise counter some of MeHg’s effects (Davidson et al., 1998; Egeland and Middaugh, 1997; Grandjean et al., 1997; Mahaffey, 1998).
In studies involving a choice between two sources of reinforcement, the acquisition of choice but not its steady-state expression was especially sensitive to gestational MeHg. In a common choice procedure, an animal is presented with a left and right lever to press, and the reinforcement rate on one lever is scheduled to occur independently of that on the other lever. Under these conditions, animals allocate responses to each lever in accordance with the relative ratio of the two reinforcement rates (Davison and McCarthy, 1988). When the ratio changes, allocation of behavior adjusts to the new conditions, but gestational MeHg exposure retards this adjustment (Newland et al., 2004; Newland et al., 1994). In these studies, MeHg exposure may have strengthened the impact of reinforcers delivered prior to the change of conditions to such an extent that the reinforced behavior was resistant to change. This outcome is consistent with accounts of response perseveration in that allocation of behavior under the previous arrangements would persist despite the change in the structure of the environment. Response perseveration has also been observed in a study of the acquisition of fixed-ratio responding in gestationally-exposed rats (Paletz et al., 2006).
The present study was designed to determine the effects of gestational MeHg and gestational plus lifetime exposure to n-3 PUFAs using discrimination reversal procedures, which permit an examination of perseverative responding on a lever that no longer produces any reinforcers. The first phase comprised a spatial discrimination reversal task in which up to seven reversals were evaluated. In the second phase, the same animals performed a more difficult visual discrimination reversal. Finally, the rats were reevaluated on the spatial task, 9 months after their behavior was initially assessed and when most were over two years of age. Female Long Evans rats, used as breeders, began their diets 5 weeks, and MeHg exposure 2 weeks, before breeding commenced. MeHg exposure of the offspring ended at weaning. Female offspring were used in the present study so as to facilitate comparisons with chronic exposure to the female breeders, which were studied in other experiments (Day et al., 2005).
2. Materials and Methods
2.1. Subjects
The subjects were 45 female Long-Evans rats (F1 generation) bred in the laboratory and weaned on postnatal day (PND) 21. Each was selected from a different litter so that litter served as the statistical unit of analysis. While in utero, they received concomitant exposure to one of two diets and one of three doses of methylmercury (MeHg) via maternal diet and drinking water, respectively, forming a 2 (Diet) × 3 (MeHg) factorial design (detailed below). Table 1 shows the number of subjects selected from each of the 6 exposure conditions. At weaning, the subjects were injected subcutaneously with an electronic identification chip (Biomedic Data Systems, Seaford, DE) and provided with free access to tap water. When rats reached 250 grams, daily food allotment was adjusted to maintain that body weight. Subjects were housed in standard cages, two per cage but separated by a transparent divider that ran diagonally in the cage so they could be fed and watered individually. Home cages were located in environmentally-controlled rooms with a 12:12 light-dark cycle in effect (lights on at 7:00 am).
Table 1.
Exposure, age when tested, and number of subjects per age per cell group
| Procedure (Phase) | MeHg Dose | Diet | |||
|---|---|---|---|---|---|
| Coconut Oil (CO)
|
Fish Oil (FO)
|
||||
| Agea | nb | Agea | nb | ||
| Spatial Discrimination Reversal (SDR-Adult) | 0.0 ppm | 15 | 4c | ||
| 18 | 3 | 18 | 7 | ||
| 0.5 ppm | 18 | 8 | 18 | 7e | |
| 5.0 ppm | 15 | 5d | |||
| 18 | 3e | 18 | 8f | ||
| Visual Discrimination Reversal (VDR) | 0.0 ppm | 17 | 3c | ||
| 20 | 2 | 20 | 2 | ||
| 0.5 ppm | 20 | 5 | 20 | 4 | |
| 5.0 ppm | 17 | 3d | |||
| 20 | 2 | 20 | 2e | ||
| Spatial Discrimination Reversal (SDR-Geriatric) | 0.0 ppm | 24 | 4c | ||
| 27 | 2 | 27 | 5 | ||
| 0.5 ppm | 27 | 4 | 27 | 4 | |
| 5.0 ppm | 24 | 5d | |||
| 27 | 3e | 27 | 3e | ||
Age, in months, when the phase began.
Number of subjects included in statistical analyses.
Subjects from a second breeding cohort used to control for age differences within the CO, 5-ppm exposure group.
Subjects from a second breeding cohort were included to make up for failed breeding attempts in the CO, 5-ppm exposure group.
One of these subjects did not receive previous exposure to the FI schedule and drug challenges.
Two of these subjects did not receive previous exposure to the FI schedule and drug challenges.
Pups were evaluated for developmental milestones including surface righting, elevation of head, gait, eye opening, onset of walking, startle reflex, and negative geotaxis, with no observed effects. One cohort was 15 months at the beginning of the present experiment and the other was 18 months. Table 1 shows the number of rats of each age within each exposure condition. For the 5 months prior to the present investigation, 41 of the subjects pressed the right lever under a fixed-interval (FI) schedule of reinforcement and received acute exposures to cocaine, imipramine, clomipramine, and desipramine. The 4 rats not exposed to the FI schedule and drug challenges were included here to maintain adequate sample sizes. It took longer for two of these rats to acquire the two-response sequence (see Behavioral methods), but after training they were indistinguishable from FI rats from respective exposure groups. In post-hoc tests, data analyses (see Data analysis and statistical methods) were conducted without these rats but the conclusions did not change. Table 1 indicates the exposure groups to which these 4 rats belonged.
All procedures were approved by the Auburn University Institutional Animal Care and Use Committee. The colony was housed in an AAALAC accredited facility under conditions meeting PHS guidelines. Rat health was monitored daily by the research staff and personnel from the Department of Laboratory Animal Health at Auburn University.
2.2. Breeding
At 10 weeks of age, 72 female and 36 male Long-Evans rats (Harlan, Indianapolis, IN) were bred (F0 generation). The F0 females were maintained on one of two customized diets and exposed to one of three concentrations of MeHg (see Exposures). The F0 males were maintained on rat chow and never received MeHg exposure. Breeding cages contained tap water and the maternal diet. Each male was paired with a single female during every other dark cycle, and these pairings did not change throughout breeding. In cases in which a male was bred with two females, the females were always members of different exposure groups. Births before 5:00 pm were assigned to PND 0 for that day. All births after 5:00 pm were assigned to PND 0 for the subsequent day. Litters were culled to 8 pups and at least 3 females.
Because of pregnancy and breeding failures in the coconut oil diet, 5 ppm MeHg group (see Exposures), an additional breeding cycle was required for this group and their coconut oil controls (for further details, see Paletz et al., 2006). Consequently, there was a 3-month age differential among some of the F1 offspring (see Table 1).
2.3. Exposures
2.3.1. Diets
Both F0 and F1 females were fed a customized diet based on the AIN-93 semi-purified formulation (supplied initially by Dyets, Inc., Bethlehem, PA, and later by Research Diets, Inc., New Brunswick, NJ). F0 females began receiving the diet 5 weeks before breeding, so F1 offspring received exposure to the same diet from conception to death (for an illustrated timeline, see Day et al., 2005; Paletz et al., 2006). The base fat mixture of the diet consisted of 42.8% palm oil, 9.2% safflower oil, and 15.0% soybean oil. For 23 rats, the fat mixture contained 33% EPAX (Pronova Biocare, Lysaker, Norway) fish oil (FO diet), making it rich in the n-3 polyunsaturated fatty acids (PUFAs), eicosapentaenoic acid (20:5n-3, EPA) and docosahexaenoic acid (22:6n-3, DHA). The fat mixture for the other 22 rats consisted of 33% coconut oil (CO diet), an oil containing no EPA or DHA. The fat mixtures contained 1.0 – 1.6% α-linolenic acid (18:3n-3, ALA), a biosynthetic precursor to EPA and DHA, and they also contained similar concentrations of n-6 PUFAs, about 18% and 22% of total fats for CO and FO diets, respectively. Overall, the ratios of n-6 to n-3 PUFAs were 2.1:1 and 16.5:1 for fish and coconut oil diets respectively (for a more detailed breakdown, see Paletz et al., 2006). During pregnancy and offspring development, a “growth” diet containing 7% fat from the mixture was used. At all other times a “mature” diet containing 4% fat from the mixture was used. Selenium content of the diet was adjusted to be 0.40 ppm during breeding and 0.18 ppm at all other times.
During the course of the experiment, 15 subjects died. Necropsies revealed that 11 rats died of natural causes as they aged beyond 18 months and 4 died of complications due to urolithiasis (Table 2), which was provoked by the inadvertent incorporation of racemic, rather than levo, bitartaric acid to stabilize choline in diets shipped to many sites in the U.S. and Canada (Newland et al., 2005). The presence of urolithiasis (kidney and bladder stones) in these rats is described elsewhere (Klurfeld, 2002; Newland et al., 2005). Briefly, urolithiasis was distributed throughout the colony across all MeHg groups and did not interact with MeHg with respect to survival among the F1 generation, those reported on here. Since urolithiasis affected only 4 subjects and urolithiasis does not produce any known signs or symptoms resembling those of MeHg exposure (Brown et al., 1977; Mandel, 1996; Smith, 1990), the possibility that results were biased by renal disease seems low. Shortly after detection of initial cases of urolithiasis, when the rats were about 3 – 6 months of age, choline bitartrate was replaced with 1.1 g/kg of choline chloride, equimolar for choline.
Table 2.
Exposure and number of deaths before and after completing the third reversal
| Procedural Phase | MeHg Dose | Diet | |||
|---|---|---|---|---|---|
| Coconut Oil (CO)
|
Fish Oil (FO)
|
||||
| Early Deathsa | Later Deathsb | Early Deathsa | Later Deathsb | ||
| SDR-A (18 – 20 months)c | 0.0 ppm | -- | -- | -- | -- |
| 0.5 ppm | -- | -- | -- | 2 (1) | |
| 5.0 ppm | -- | -- | -- | 1 | |
| VDR (20 – 26 months)c | 0.0 ppm | 1 | -- | 2 | -- |
| 0.5 ppm | -- | 1 | 1 (1) | -- | |
| 5.0 ppm | -- | -- | 3 (1) | -- | |
| SDR-G (27 – 29 months)c | 0.0 ppm | -- | -- | -- | -- |
| 0.5 ppm | 2 | -- | -- | 1 (1) | |
| 5.0 ppm | -- | -- | 1d | -- | |
Number of deaths before completion of the third reversal (R3), with number resulting from urolithiasis listed in parentheses. These subjects were dropped from statistical analyses. Hyphens indicate that no deaths occurred.
Number of deaths after completion of the third reversal (R3), with number resulting from urolithiasis listed in parentheses. These subjects were included in statistical analyses. Hyphens indicate that no deaths occurred.
Age range of the older F1 rats (i.e., from the initial breeding cycle) during the entire phase; F1 rats from the second breeding cycle, and, consequently, 3 months younger, did not die during the experiment.
One rat died during the 1-month period between the end of the VDR phase and the beginning of the SDR-G phase.
2.3.2. Methylmercury
Two weeks before breeding and 3 weeks after beginning the diets, the F0 females began consuming water containing 0, 0.5, or 5 ppm of mercury (Hg) as methylmercuric chloride (Alfa Aesar, Ward Hill, MA), producing exposures of roughly 0, 40 and 400 μg/kg/day, respectively, based on average daily consumption, with some elevation during pregnancy resulting from increased water consumption (Newland and Reile, 1999). Sodium carbonate (< 5 nanomolar) was used to buffer the MeHg and added to all 3 water mixtures (cf. Stern et al., 2001). On PND 16, when the pups were capable of reaching the water spout, MeHg water was removed from the cage and replaced with the 0 ppm water. The F1 offspring received plain tap water after weaning on PND 21. Therefore, the subjects never received direct exposure to MeHg. As there is apparently no MeHg exposure via breast milk (Newland and Reile, 1999; Stern et al., 2001), this can be considered a gestational exposure.
An approximately equal number of male and female pups from each exposure group was euthanized on PND 1 and their brains collected for analysis of Hg content by atomic absorption spectrophotometry, as done in Newland and Reile, 1999. Total Hg contents in the 0.5- and 5-ppm exposure groups were 0.29 and 5.50 ppm, respectively, regardless of diet group. No Hg was detected in the brains of control rats.
2.4. Testing apparatus
Behavioral procedures were conducted in 16 commercial operant chambers (Med-Associates, Model ENV-008) containing one rear-mounted lever and two front, retractable levers (each calibrated to 0.20 N), a pellet dispenser situated between the two front (left and right) levers and filled with 20 mg sucrose pellets (Research Diets, Inc., New Brunswick, NJ), sonalert tones (2900 and 4500 Hz, nominally; calibrated to 70 dbC), a house lamp (28 V 100 ma), and an LED (light emitting diode) above each lever. Each chamber was surrounded by a sound-attenuating cabinet, with built-in ventilating fan that circulated air into the experimental environment and provided masking white noise. Programs for experimental procedures and data collection were written using MED-PC IV (Med-Associates, St. Albans, VT). Session events were recorded with 0.01” resolution.
2.5. Behavioral methods
An autoshaping procedure was used to establish lever pressing (Bushnell, 1988; Reed et al., 2006). Reinforcement consisted of the delivery of one sucrose pellet paired with a brief, 4500 Hz tone. Body weights did not differ among any of the exposure groups. Sessions for each of 3 squads of subjects were conducted daily at different, but consecutive, times; assignment of subjects to squads and chambers was distributed across exposure groups. Each session lasted 60 trials. Fans, lights, tones, levers, and pellet dispensers were tested before and after sessions for each squad of rats to ensure that equipment was functioning properly. Electronic identification chips used to track subjects were scanned prior to each session to confirm identity.
2.5.1. Spatial discrimination reversal
The spatial discrimination reversal (SDR) procedure was as follows. Each trial began with the onset of an alternating tone stimulus (2900 Hz; 0.3-sec on, 0.6-sec off). A single rear lever press turned off the tone stimulus and extended the two front levers. A response on one of these two levers (e.g., left) produced reinforcement while a response on the other did not. Regardless of position, this response retracted both levers, ending the trial. Trials also ended if a lever press did not occur within 15 seconds after trial onset (i.e., a rear lever press) or within 15 seconds after lever extension (i.e., a front lever press).
For the “original discrimination” (OD) sessions, only left-lever presses were followed by reinforcement (OD-left), and this continued until the accuracy criterion was met: 3 consecutive sessions in which at least 51 out of 60 trials (i.e., ≥85% of trials) ended in reinforcement. Then, only right-lever presses were reinforced (first reversal or R1-right). After reaching the same performance criterion on R1-right, the contingency reversed back to the left lever (second reversal, or R2-left), and so on, until a total of 7 discrimination reversals were arranged (OD-left through R7-right). The initial SDR phase (SDR-A) began when the rats were adults, at 15 – 18 months of age, and it lasted about 45 sessions. A second SDR phase (SDR-G) was presented when the subjects were geriatric, beginning at 24 – 27 months old (see Table 1). Sessions for the SDR-A phase were conducted daily while those during the SDR-G phase were conducted 2 – 3 times per week.
2.5.2. Visual discrimination reversal
The visual discrimination reversal (VDR) procedure was implemented after all surviving subjects had completed all reversals of the SDR-A phase. This procedure was similar to that of the SDR except that the LED above one lever was lit during each trial. During the original discrimination, a front-lever press was reinforced when made on the lever beneath the lit LED (OD-light). The side on which the LED was lit during each trial was randomly determined following each reinforced response. If a trial did not end in reinforcement, a correction trial was presented with the position of the lit LED unchanged until a response was reinforced or until the session ended, whichever came first. This correction procedure was necessary for the VDR because otherwise, unlike with the SDR, a rat could collect half of all reinforcers by responding exclusively on either side. Performance on correction trials did not contribute to the accuracy criterion. After criterion, lever presses were reinforced only when they occurred on the side beneath the unlit LED (R1-dark), and so on. The VDR phase lasted 6 months because some rats required a large number of sessions to complete R1-dark. Sessions were conducted daily for the first month and 1 – 2 times per day, 2 – 3 times per week thereafter.
2.6. Data analysis and statistical methods
Litter served as the statistical unit of analysis for all analyses. The experiments were structured as a 2 (Diet) × 3 (MeHg) full factorial design with repeated measures across reversals. There were 7 or 8 subjects, each representing a unique litter, assigned to each cell (see Table 1) at the beginning of the study. The following dependent variables were used:
Sessions to criterion – Sessions required to reach accuracy criterion for each reversal.
Reinforcers to criterion – Reinforced (correct) trials required to reach accuracy criterion for each reversal.
Commission errors – Trials ending with a non-reinforced, front-lever press for each reversal.
Omission errors – Trials without a rear-lever or a front-lever press for each reversal.
Commission Error Run Lengths – The number of commission errors divided by the number of “runs” of commission errors in the reversal. Runs constituted sequences of commission errors, irrespective of omission errors, leading up to the next reinforced response or the end of the session, whichever occurred first. For example, the sequence R, O, E, E, E, R, E, O, E, R, E, R, O, R… (where R = reinforced responses, O = omission errors, and E = commission errors) includes 3 runs of commission errors with lengths of 3, 2, and 1, respectively. Note that run lengths have a minimum of 1 and a maximum of 60.
Rear-lever latency – Time between the onset of the trial (alternating tone) and the rear-lever press (which extends the “choice” levers on the front panel). Trials without a rear-lever press were excluded.
Choice latency – Time between a rear-lever press and a front-lever press. Trials without a front-lever press were excluded.
ITI responses – Average number of responses (per trial) during intertrial intervals.
Repeated-measures analysis of variance (RMANOVA) tests were performed using SPSS® 13 (SPSS Science, Chicago, IL). MeHg (0, 0.5, and 5 ppm) and Diet (CO, FO) served as the two between-subjects factors. Reversal condition (OD, R1,…, Rn) served as the within-subjects factor and included only completed reversals (i.e., those for which accuracy criterion was met). The degrees of freedom for within-subjects effects were adjusted using the Huynh-Feldt correction for sphericity. Customized, univariate contrasts were performed whenever an interaction was detected to characterize more fully the Reversal(s) that contributed to the interaction. Significant contrasts were followed by further pairwise comparisons to determine which MeHg groups were different; such comparisons were not necessary for Diet because it involved only two groups. Significant main effects were followed by post-hoc Tukey tests (HSD). To ensure that the data set complied with the assumptions required by ANOVAs, logarithmic transforms were applied where the data were positively skewed or where variability increased with the mean value. This included only the commission error run length data.
For the SDR-A (adult) phase, 3 reversals (OD-left through R3-right) were included in statistical analyses because all rats completed these, and subsequent reversals contributed no additional information. For comparative purposes, analyses of the VDR and SDR-G (gerontological) phases also included the first 3 reversals, although not all rats completed these.
3. Results
3.1. Spatial discrimination reversal
There were no effects of either MeHg or Diet, alone or in combination, on the rate with which the rats acquired the rear-to-front-lever response chain. All 45 rats completed the first 3 reversals (OD-left through R3-right) of the SDR-A phase, and 42 of these rats completed all 7 reversals (OD-left through R7-right). Three rats died before completing the SDR-A phase, and their deaths were due to reasons unrelated to exposure; one of these rats had small uroliths detected post mortem but had shown no clinical signs of illness (see Table 2).
Repeated measures ANOVAs were conducted across OD-left through R3-right of the SDR-A phase. Reversal, itself, had a within-subjects effect on all dependent measures listed above (all Ps<0.02); i.e., behavior changed significantly across reversals.
3.1.1. Interactions between Diet and MeHg
It has been speculated that a fish diet may protect against exposure to MeHg. However, no statistical interactions between Diet and MeHg were found for any measure (all Ps>0.1).
3.1.2. MeHg effects
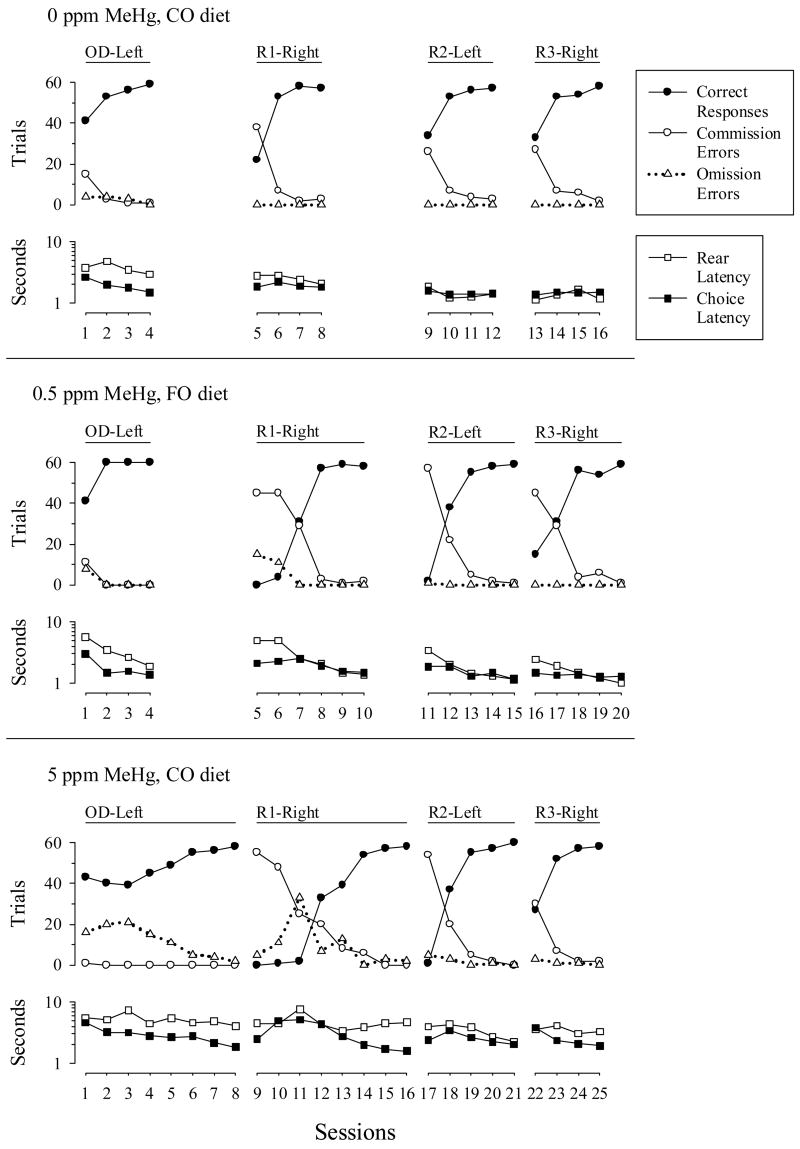
Although no interactions between Diet and MeHg were observed, there were effects of MeHg alone. Fig. 1 illustrates performances observed among rats in each of the MeHg groups. The number of commission errors was lowest during OD-left for the 3 rats shown, but for the 0.5 ppm (center) and 5 ppm (bottom) rats, these errors were highest and correct responses (i.e., reinforced responses) lowest during the first session of each reversal. The 5 ppm rats failed to respond on many trials (omission errors) at the beginning of OD-left and R1-right. Overall, rats had the most difficulty with the task during R1-right. The 0-, 0.5- and 5-ppm rats required 4, 6, and 8 sessions, respectively, to complete R1-right. Recall that 3 sessions with at least 85% accuracy were required before the next reversal commenced. Both rear-lever and choice latencies were slightly longer for the 5 ppm rat across sessions.
Fig. 1.
Illustrative rats showing spatial discrimination reversals (SDR-A) through R3-right. Correct, reinforced trials (closed circles), errors of commission (open circles), errors of omission (open triangles), rear-lever latency (open squares), and choice latency (closed squares) across sessions. A rat gestationally exposed to 0 ppm methylmercury and coconut oil diet (top), 0.5 ppm methylmercury and fish oil diet (center), and 5 ppm methymercury and coconut oil diet (bottom) are shown. The ordinates show trials and seconds (note logarithmical scaling). Connected lines and axes, separated by spaces, indicate each reversal condition. Experimental conditions are aligned vertically. Rate of progression through the reversal conditions varied across rats and depended on the expediency of reaching criterion within each condition.
Fig. 2 shows group data for each MeHg group through R7-right. There was a significant MeHg-Reversal interaction effect on commission errors [F(6,117)=3.1, P=0.009] (Fig 2, top), and contrasts revealed that R1-right contributed to this result [F(2,42)=6.1, P=0.005] while other reversals, including OD-left, did not. As seen in Fig. 2, the 0.5 ppm and 5 ppm groups each committed more errors than the 0 ppm group during R1-right (both Ps<0.006). The two exposed groups, statistically indistinguishable from each other (P=0.8), made about 60% more errors than controls during R1-right.
Fig. 2.
Performance measures on spatial discrimination reversals (SDR-A). Errors of commission (top), average commission error run lengths (center), and average choice latency (bottom) for each methylmercury group. Data are shown for all rats and all reversal conditions. Note logarithmic scaling and presentation of geometric means for commission error run lengths. Error bars represent ± 1 SEM. (^) p<0.01 for the 5 ppm group (n=16) compared to the 0 ppm group (n=14); ($) p<0.01 for the 0.5 ppm group (n=15) compared to the 0 ppm group; (&) p<0.05 for the 5 ppm group compared to the 0 ppm group.
As with commission errors, there was a MeHg-Reversal interaction effect on commission error run lengths [F(6,117)=2.4. P=0.029] (Fig. 2, center), with R1-right contributing to this interaction [F(2,42)=3.2, P=0.049]. However, differences during R1-right were only between the 0- and 5-ppm groups (P=0.015); the 0- and 0.5-ppm groups were indistinguishable (P=0.2) as were the 0.5- and 5-ppm groups (P=0.3). Fig. 2 (center) shows that during R1-right, the 5 ppm rats averaged about 5 errors per run, while the 0 ppm group averaged about 3 errors per run (geometric averages).
Choice latency decreased the most between OD-left and R1-right, especially for the 0.5 ppm group (Fig. 2, bottom), but there was no MeHg-Reversal interaction effect on this or any other variable (Ps>0.2) except for ITI responses for which there was a marginal effect (P=0.07).
There was a significant, between-subjects (i.e., overall, irrespective of Reversal) main effect of MeHg on commission errors [F(2,39)=3.4, P=0.045], but this was due to the large MeHg effect at R1-right, already discussed, which skewed means across the first 3 reversals; inclusion of OD-left through R7-right in an exploratory RMANOVA showed how this effect is diminished (i.e., P=0.09). Only a marginal main effect of MeHg was detected on sessions to criterion (P=0.06), but otherwise there were no between-subjects effects of MeHg on any other measure (Ps>0.1).
3.1.3. Diet effects
For all measures, there were no main (Diet) or interaction (Diet-Reversal) effects during the SDR-A phase (all Ps>0.1). Therefore, diet did not play a role when the rats were 1.5 years of age.
3.1.4. Effects after aging
At 2 years, when the rats returned to performing the SDR (SDR-G), 33 of the original 45 rats were still alive. Of these 33, three failed to complete the first 3 reversals before becoming ill or dying. All remaining 30 rats completed at least R3-right, and five completed R6-left, the highest condition completed in this phase. Consequently, sample sizes for some of the cell groups were too small to support analyses of interactions between Diet and MeHg (see Table 1). Tests of MeHg (n ≥ 8) and Diet (n ≥ 12), including interactions with Reversal, as well as main effects of these three variables, could be analyzed for OD-left through R3-right, however. There was a main effect of Reversal on all dependent measures assessed (Ps<0.008) with the exception of rear-lever latency, for which there was a marginal effect (P=0.07), and omission errors (P=0.6).
3.1.4.1. MeHg effects
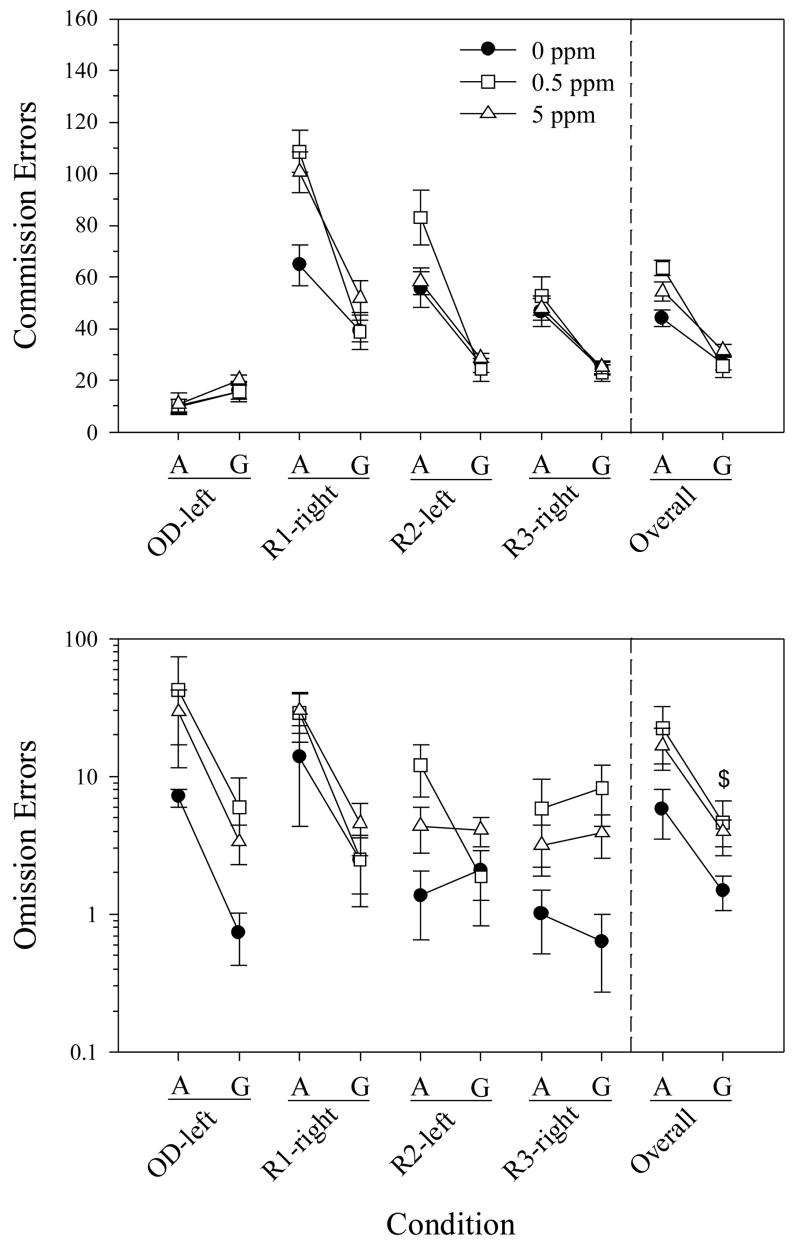
Fig. 3 (top) shows how commission errors changed from about 1.5 years of age (SDR-A) to 2 years of age (SDR-G) for OD-left through R3-right. The MeHg-related difference observed in R1-right when the rats were younger was no longer apparent when they were old. That is, after the animals had aged, there was no longer an interaction between MeHg and Reversal for either commission errors or commission error run lengths [both Fs(6,72)<1.0, Ps>0.4]. Also, there was no longer an overall main effect of MeHg on commission errors [Fs(2,24)=0.9, P=0.4]. On the other hand, there was a significant main effect of MeHg on omission errors [F(2,24)=3.5, P=0.047] (Fig. 3, bottom, far right points) and a marginal main effect on commission error run lengths (P=0.06) not previously observed; differences were between the 0 ppm and 0.5 ppm, but not the 5 ppm, groups for both of these measures. The values represented in Fig. 3 are slightly different from those in Fig. 2 (e.g., R2-left in tops of Figs. 2 and 3), because rats that died between the SDR-A phase and R3-left of the SDR-G phase are not included in Fig. 3. Overall, however, omitting those rats did not substantially change the values, especially at R1-right of the SDR-A phase where the effect was detected.
Fig. 3.
Spatial discrimination reversal in old rats (SDR-G) compared to their performance when younger (SDR-A). Shown are commission errors (top) and omission errors (bottom) when adult (A) and when “geriatric” (G) for each methylmercury group. Solid lines, separated by spaces, connect pairs of identical reversal conditions. Data within each pair are shown for only those rats that completed R3-right in both the adult and geriatric stages of life. Error bars represent ± 1 SEM. Note logarithmic scaling. ($) p<0.05 for the 0.5 ppm group (n=8) compared to the 0 ppm group (n=11); for the 5 ppm group, n=11.
With the exception of the effects noted above, and a marginal MeHg-Reversal interaction for omission errors (P=0.07), MeHg did not impact any other measure in the SDR-G phase (Ps>0.1).
3.1.4.2. Diet effects
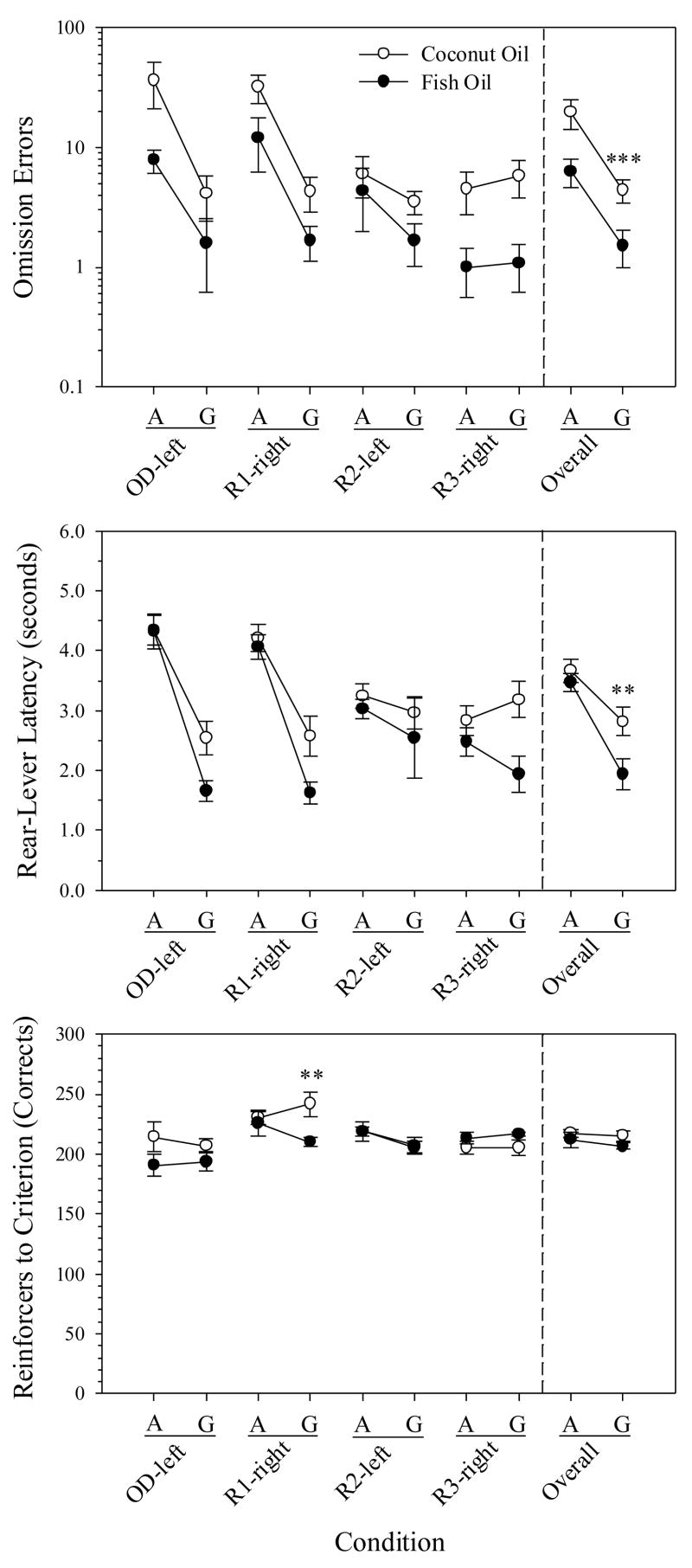
Unlike during the SDR-A phase, the CO rats had more omission errors (Fig. 4, top) and longer rear-lever latencies (Fig. 4, center), overall and irrespective of Reversal, than the FO rats [both Fs(1,24)>7.7, Ps<0.02]. There was also a marginal effect of Diet on ITI responses (P=0.05) and sessions to criterion (P=0.07). No main effects of Diet were found for remaining measures (Ps>0.1), although there was a Diet-Reversal interaction effect on reinforcers to criterion [F(3,72)=3.1, P=0.03] (Fig. 4, bottom). This interaction was due entirely to the first reversal; contrasts revealed that the FO rats required fewer reinforcers than the CO rats to complete R1-right (P=0.02), but the groups were indistinguishable on OD-left, R2-left, or R3-right (Ps>0.1). There was no Diet-Reversal interaction for any other measure (Ps>0.1).
Fig. 4.
Spatial discrimination reversal in old rats (SDR-G) compared to their performance when younger (SDR-A). Shown are omission errors (top), rear-lever latency (center), and reinforced trials, or corrects (bottom) when adult (A) and when “geriatric” (G) for each diet group. Plots are structured as in Fig. 3. Error bars represent ± 1 SEM. Note logarithmic scaling. (***) p<0.01 and (**) p<0.05 for the coconut oil group (n=18) compared to the fish oil group (n=12).
To examine the possibility that omissions in the old rats were due to slowed trial initiations (rear-lever latencies), a post-hoc analysis of ITI responses was undertaken. If the rat reached the rear lever too late, then the rear-lever press would have been recorded as an ITI response. Following trials with an omission error, the CO rats, relative to the FO rats, had a higher frequency of ITIs with a single response (23.0% for CO vs. 15.4% for FO), a lower frequency of ITIs with zero responses (66.7% for CO vs. 73.1% for FO), and a similar frequency of ITIs with two or more responses (10.3% for CO vs. 11.5% for FO). Therefore, the marginally higher number of ITI responses among CO rats are plausibly attributable to single responses, not response bursts, during the ITI.
3.2. Visual discrimination reversal
A total of 42 rats were alive at the beginning of the VDR phase when the animals were 17 – 20 months old, and 8 more from the initial breeding cycle died by the end of this phase, about 6 months later (see Table 2). Of the surviving rats, 12 failed to complete R3-dark because they never reached criterion; the VDR was more difficult than the SDR, requiring about three times as many sessions to complete the third reversal.
Due to the reduced sample size, some of the cell groups became too small to support analyses of interactions between Diet and MeHg (see Table 1). However, interactions between each of these individual factors and Reversal (OD-light through R3-dark), as well as their main effects, were appropriate because sample sizes were adequate for each level of MeHg (n ≥ 7) and Diet (n ≥ 8). Therefore, results of interactions between Diet and MeHg are not reported here. There was a main effect of Reversal alone on all measures (Ps<0.05) except for rear-lever latency (P=0.2).
3.2.1. MeHg effects
Overall, the 0- and 0.5-ppm rats completed more reversals than the 5 ppm rats, with R10-light, R8-light, and R4-light as the highest reversals completed by a rat in these groups, respectively.
There was an interaction between MeHg and Reversal for commission errors [F(6,51)=3.5, P=0.006] (Fig. 5, top), and contrasts indicated that differences among groups occurred only in R1-dark [F(2,20)=4.7, P=0.022]. Specifically, the 5 ppm rats produced more errors during R1-right than either the 0 ppm rats (P=0.039) or the 0.5 ppm rats (P=0.008); the 0- and 0.5-ppm groups were indistinguishable (P=0.5). Errors of commission were the lowest and highest during OD-light and R1-dark, respectively. With the exception of a marginal MeHg-Reversal interaction for commission error run lengths (Fig. 5, center, P=0.09) to which R1-dark did not contribute, there were no MeHg-Reversal interactions for other measures (Ps>0.2) including choice latency (Fig. 5, bottom, P=0.9). Despite the large number of errors committed during the VDR relative to the SDR-A phase (compare Figs. 2 and 5, top), the average run-length of commission errors was generally lower in the SDR-A phase (compare Figs. 2 and 5, center), especially during R1 and, to a lesser extent, R2 and R3. There were no overall main effects of MeHg for any measure, including commission errors (all Ps>0.1), so all MeHg effects involved an interaction with reversal. Irrespective of group, choice latencies (Fig. 5, bottom) were shorter during OD-light and R1-dark of the VDR phase than during these conditions of the SDR-A phase (see Fig. 2, bottom), but group averages were similar during R2 and R3 across both phases.
Fig. 5.
Performance measures on visual discrimination reversals (VDR). Errors of commission (top), average commission error run lengths (center), and average choice latency (bottom) across reversal conditions through R3-dark for each methylmercury group. Data are shown for all rats that completed R3-dark. Note logarithmic scaling and presentation of geometric means for commission error run lengths. Error bars represent ± 1 SEM. (^) p<0.05 for the 5 ppm group (n=7) compared to the 0 ppm group (n=7); (#) p<0.01 for the 5 ppm compared to the 0.5 ppm group (n=9).
3.2.2. Diet effects
A marginal Diet-Reversal interaction was found for commission errors (P=0.08), but no interaction was found for any of the other measures (Ps>0.2) during the VDR phase (data not shown). Similarly, there was no main effect of Diet on any measure (all Ps>0.4).
4. Discussion
The present experiment began when the rats were 15 – 18 months of age. Developmental exposure to methylmercury (MeHg) significantly impaired the performance of a discrimination reversal during adulthood, an effect that was seen even at the lower (0.5 ppm) exposure level and that was most prominent when the first spatial and visual discrimination reversals were encountered. Assessment of interactions between lifelong Diet (fish oil vs. coconut oil) and gestational MeHg was statistically feasible for the SDR-A phase, but there was no interaction; potential interactions could not be examined in the VDR and SDR-G phases due to attrition. In a previous study (Paletz et al., 2006), no interactions were found between the n-3 polyunsaturated fatty acids (PUFAs) and gestational MeHg when rats were assessed on a series of increasing fixed-ratio (FR) schedules of reinforcement.
In an ongoing longitudinal study of developmental Hg exposure among Seychelles Islands children (Davidson, et al., 2006), no correlation has been found between mercury (Hg) exposure and measures of IQ and achievement. The Seychellois diet largely consists of fish, which contain, among other nutrients, essential n-3 PUFAs. In light of findings from the Seychelles cohort, it has been speculated that a fish diet may afford protection against Hg exposure (Davidson et al., 1998; Egeland and Middaugh, 1997; Grandjean et al., 1997; Mahaffey, 1998), and inorganic Hg, the form retained in the central nervous system following MeHg exposure, binds readily to PUFAs (Nakada and Imura, 1983). However, the results of the previous (Paletz et al., 2006) and present studies with rats receiving concomitant exposure to gestational MeHg and a diet rich in n-3 PUFAs suggest that such a diet does not protect against MeHg’s effect on behavioral plasticity. It should be noted that the procedures used here and in the previous study are different from IQ and other achievement measures; these assessment measures do not explicitly assess behavior change.
MeHg alone (i.e., regardless of diet) retarded the acquisition of the first reversal (R1) during the initial SDR (SDR-A) and VDR phases. The sensitivity of the first reversal has been noted often with this procedure (Bushnell and Bowman, 1979; Gilbert and Rice, 1987; Rice, 1985; Schantz and Bowman, 1989; Widholm et al., 2001). For the SDR-A phase, both 0.5- and 5-ppm rats were significantly impaired in this capacity, but for the VDR, only the 5 ppm rats were affected. Such differences were not apparent after R1, suggesting that the effect may have been specific to novel changes in behavior-environment contingencies. In other words, exposure compromised the ability to shift behavior from left to right (SDR) or from light to dark (VDR) the first time this reversal was encountered, but as experience with the reversing contingencies accrued, developmental MeHg exposure no longer affected the commission of errors. In addition, benefits of prior experience with the discrimination reversals were present when the animals were quite old.
The results of the SDR-A phase replicate those of an investigation involving prenatal exposure to the same doses of MeHg tested here, approximately 0, 40, and 400 μg/kg/day (Reed et al., 2006). In that study, the number of errors during R1-right and R3-right was larger for both exposure groups than for controls, although the result was only statistically significant for the high-dose group. Also in that study, choice latencies during R1-right and R2-left for the high-dose group were shorter than for controls, unlike in the present study. It is unclear why choice latencies were not affected in the present investigation, although one or more methodological differences between the studies may account, in part, for this outcome. Compared to rats in the previous study, the rats in the present experiment (a) were 3 – 6 months older when first tested, (b) had previous experience in the operant chamber, (c) experienced several drug challenges, and (d) were exposed to a different pair of diets (selenium levels were manipulated in Reed et al., 2006).
The results obtained for the VDR phase stand in contrast to those of a previous study with infant and juvenile monkeys (Rice, 1992). In that study, 12 monkeys received gestational and postnatal MeHg exposure to 0 – 50 μg/kg/day, a dose range comparable to that tested here given the difference between rats and primates with respect to MeHg’s blood-binding properties (see Magos, 1987; Newland and Reile, 1999). Despite other signs of neurotoxicity among the high-dose monkeys, there was no effect of MeHg on errors to criterion at either age, even during R1. Procedural differences may have contributed to the discrepancy between those and the present results, but the studies were, by replication standards, relatively comparable. It is also possible that discrepancies were due to species- or age-related differences. Note that the rats tested here were more mature than the monkeys. Support for the importance of maturity at the time of testing comes from another experiment in which 2 month-old rats with gestational exposure to doses of MeHg similar to those used here were tested using the VDR procedure (Schreiner et al., 1986). No effect on accuracy was reported, although the authors did not include a direct measure of errors to criterion in their analysis. Interestingly, they did report that ITI responses were significantly increased for the group that received the high dose of ~ 400 μg/kg/day.
4.1. MeHg and behavioral transitions
The present results suggest that developmental exposure to MeHg impairs behavior in a changing environment (i.e., in transition) rather than discrimination or memory per se, a conclusion that is consistent with that of other studies involving developmental exposure and that failed to find effects on the latter (Buelke-Sam et al., 1985; Gilbert et al., 1993; Schreiner et al., 1986). The effects during R1 observed here and elsewhere (Reed et al., 2006) are also consistent with the results of studies that tested the acquisition of choice using concurrent schedules of reinforcement (Newland et al., 2004; Newland et al., 1994). In those studies, exposed subjects resembled controls during steady-state performance. The relative number of responses on a lever (i.e., choice of that lever) approximately matched the relative number of reinforcers delivered for responding on that lever for both the exposed and control animals. When the reinforcement rates available from the levers changed, however, exposed monkeys’ (Newland et al., 1994) and rats’ (Newland et al., 2004) behavior transitioned more slowly. The SDR procedure maintains an important feature of the concurrent schedule procedure in that reinforcement density changes with respect to spatial location at the onset of the transition. For the SDR, reinforcement density always changes from 0% to 100% on one side and from 100% to 0% on the other. For concurrent schedule transitions, the shifts in reinforcement density are more subtle, e.g., from 50% to 80% on one side and from 50% to 20% on the other, but the directionality of shifts and the establishment of experience with one set of reinforcement contingencies before reinforcement rates change is similar.
MeHg-induced effects on behavioral transitions are not entirely specific to procedures that arrange contingencies on two levers. When the response requirement on a single lever becomes more demanding under rapidly increasing fixed-ratio schedules of reinforcement, rats exposed during gestation produced more responding, i.e., “better” performance, than controls (Paletz et al., 2006; Reed et al., in press). This apparently paradoxical effect was interpreted as diminished sensitivity to a change in the source of reinforcement or increased efficacy of reinforcement prior to the change. Either mechanism could result in behavior that appears perseverative, as reflected in the retarded transitions in the present study and in an earlier one (Reed et al., 2006).
The commission error run lengths, defined as the average number of errors committed before a correct, reinforced response, was used to characterize the tendency for behavior to persist immediately following an initial disconfirming (i.e., non-reinforced) response. These run lengths were higher for both MeHg-exposed groups during R1-right of the SDR, but the result was only significant for the 5 ppm rats (see Fig. 2, center). For the VDR, run lengths were not different between groups during R1-dark (see Fig. 5, center), possibly due to the difficulty of the visual task. Therefore, MeHg increased perseveration in at least the spatial task. This relative increase in perseverative responding is consistent with outcomes stemming from damage to the cerebral cortex, the region most heavily targeted by gestational MeHg exposure (Barone et al., 1998; Berlin et al., 1975; Burbacher et al., 1990; O’Kusky, 1983; Rice, 1983). Lesions within the cortex lead to increases in perseveration on discrimination reversal procedures (Chudasama and Robbins, 2003; Ridley et al., 1993), and they also increase responding on large ratio reinforcement schedules (Kheramin et al., 2005), consistent with MeHg effects reported in Paletz et al., 2006 (see Newland et al., 2006a). Cortical regions also appear to mediate behavioral choice (Schultz et al., 2000; Tremblay and Schultz, 1999) in situations like the SDR (and VDR) and concurrent schedules. Taken together, it seems plausible that effects observed here were produced, in part, by cortical damage due to MeHg exposure.
4.2. Comparing SDR and VDR
It is interesting that the results of the SDR-A and VDR phases were similar because the stimuli involved do not invoke identical sensory systems. MeHg has been shown to affect higher-order visual function in monkeys (Rice and Gilbert, 1982, 1990). In these monkey studies, only visual acuity for detecting shapes and fine details of stimuli was impaired. The procedure used here probably did not tap this aspect of vision, as detecting the presence of a light over a lever requires little attention to detail. The exceptional difficulty of the VDR as compared with the SDR has been reported by others (Murray et al., 1995; Widholm et al., 2003). These reports and the generally poor vision of rats (Prusky et al., 2002) suggests that rats’ behavior comes under the control of visual stimuli with difficulty. Because MeHg effects surfaced during R1, and the primary effect for both the SDR and VDR was on the same measure (errors of commission), it seems likely that MeHg affected some mechanism common to both tasks.
These tasks bear some commonalities with others that tap cortical function, such as the Stroop and Wisconsin Card-Sorting tasks (Kolb and Wishaw, 2003). All three require that behavior come under the control of a novel stimulus dimension after a baseline on a different discrimination has been established.
4.3. Effects of n-3 PUFAs
Diet effects appeared only after the rats were old. During the SDR-G phase, when over 2 years of age, the rats consuming a fish oil (FO) diet initiated a trial faster than those on the coconut oil (CO) diet and they showed fewer omission errors, in which a trial was missed altogether. Taken together, these outcomes suggest that exposure to the CO diet delays response initiation and may cause motor slowing. Such an interpretation is consistent with the pattern of ITI responses seen in the CO group and with post-hoc analyses that showed that the number of ITI responses may actually have been higher among CO rats because they occasionally responded too late.
After aging, the number of reinforcers required to complete R1-right was higher for the CO group than for the FO group. Since omission errors across all conditions were also significantly greater for the CO rats, it is possible that failures to respond prevented the CO rats from reaching the accuracy criterion of at least 51 of a possible 60 (i.e., 85%) reinforcers across each of 3 consecutive sessions. Therefore, the effect on reinforcers to criterion may not necessarily represent a lack of sensitivity to reinforcement among the CO rats (i.e., because they required more reinforcers), but rather may be consistent with the notion that consuming a lifelong diet rich in essential n-3 PUFAs can afford protection against age-related slowing. Epidemiological studies have identified a relationship between n-3 PUFAs and cognitive function during middle age (Kalmijn et al., 2004) and an inverse relationship between fish consumption and cognitive decline in individuals 65 and over (Kalmijn et al., 1997; Morris et al., 2005); an association between the n-3 PUFA, docosahexaenoic acid (DHA) and reduced risk of dementia has also been reported (Schaefer et al., 2006). Since cognitive function depends, in part, on timely response initiation, the present results may provide empirical support for these findings.
4.4. Concluding Remarks
Gestational exposure to MeHg impaired behavior as it transitioned from the original discrimination to the first reversal of the SDR-A and VDR arrangements. The low error rate seen in the adult rats performing the spatial task after the first reversal (SDR-A) and later when the rats were old and more experienced (SDR-G) suggests that MeHg’s effects can be overcome with experience on a specific learning task, a sort of behavior therapy or environmental enrichment, and that this improved performance extends well into aging.
These exposures, which result in about 40 and 400 μg/kg/day of Hg exposure, produced an effect in the SDR-A phase. The lower dose is about 2.5 orders of magnitude higher than the current RfD of 0.1 μg/kg/day for methylmercury in the U.S. (National Research Council, 2000) and produced about 0.29 ppm of Hg in the neonatal brain. While it is not yet clear how best to accommodate species differences when evaluating chronic exposure, it is worth noting that rat blood binds approximately ten times as much Hg as many other species, including human and nonhuman primates (Magos, 1987). As a result, with chronic gestational exposure, the brain:blood ratio for Hg is 0.13 for rats consuming chow (Newland and Reile, 1999) and between 0.10 and 0.25 for rats consuming high and low selenium diets, respectively (Newland et al., 2006b), as compared to slightly higher than 1.0 for human and nonhuman primates (Magos, 1987). A diet rich in fish oil did not ameliorate the effects of developmental MeHg exposure, but it did produce some effects of its own during aging.
Acknowledgments
This work was supported by ES10865 from NIEHS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barone S, Jr, Haykal-Coates N, Parran DK, Tilson HA. Gestational exposure to methylmercury alters the developmental pattern of trk-like immunoreactivity in the rat brain and results in cortical dysmorphology. Brain Res Dev Brain Res. 1998;109:13–31. doi: 10.1016/s0165-3806(98)00038-8. [DOI] [PubMed] [Google Scholar]
- Becker CC, Kyle DJ. The importance of DHA in optimal cognitive function in rodents. In: Mostofsky D, Yehuda S, Salem N Jr, editors. Fatty Acids: Physiological and Behavioral Functions. Totowa, NJ: Humana Press Inc; 2001. pp. 357–75. [Google Scholar]
- Berlin M, Grant CA, Hellberg J, Hellstrom J, Schultz A. Neurotoxicity of methylmercury in squirrel monkeys. Cerebral cortical pathology, interference with scotopic vision, and changes in operant behavior. Arch Environ Health. 1975;30:340–8. doi: 10.1080/00039896.1975.10666717. [DOI] [PubMed] [Google Scholar]
- Brown NO, Parks JL, Green RW. Canine urolithiasis: Retrospective analysis of 438 cases. J Am Assoc Lab Anim Sci. 1977;110:414–18. [PubMed] [Google Scholar]
- Buelke-Sam J, Kimmel CA, Adams J, Nelson CJ, Vorhees CV, Wright DC, St Omer V, Korol BA, Butcher RE, Geyer MA. Collaborative Behavioral Teratology Study: Results. Neurobehav Toxicol Teratol. 1985;7:591–624. [PubMed] [Google Scholar]
- Burbacher TM, Grant KS, Mottet NK. Retarded object permanence development in methylmercury exposed Macaca fascicularis infants. Dev Psychol. 1988;22:771–76. [Google Scholar]
- Burbacher TM, Rodier PM, Weiss B. Methylmercury developmental neurotoxicity: A comparison of effects in humans and animals. Neurotoxicol Teratol. 1990;12:191–202. doi: 10.1016/0892-0362(90)90091-p. [DOI] [PubMed] [Google Scholar]
- Bushnell PJ. Behavioral effects of acute p-xylene inhalation in rats: Autoshaping, motor activity, and reversal learning. Neurotoxicol Teratol. 1988;10:569–77. doi: 10.1016/0892-0362(88)90094-3. [DOI] [PubMed] [Google Scholar]
- Bushnell PJ, Bowman RE. Persistence of impaired reversal learning in young monkeys exposed to low levels of dietary lead. J Toxicol Environ Health. 1979;5:1015–23. doi: 10.1080/15287397909529810. [DOI] [PubMed] [Google Scholar]
- Chudasama Y, Robbins TW. Dissociable contributions of the orbitofrontal and infralimbic cortex to pavlovian autoshaping and discrimination reversal learning: Further evidence for the functional heterogeneity of the rodent frontal cortex. J Neurosci. 2003;23:8771–80. doi: 10.1523/JNEUROSCI.23-25-08771.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson PW, Myers GJ, Cox C, Axtell C, Shamlaye C, Sloane-Reeves J, Cernichiari E, Needham L, Choi A, Wang Y, Berlin M, Clarkson TW. Effects of prenatal and postnatal methylmercury exposure from fish consumption on neurodevelopment: Outcomes at 66 months of age in the Seychelles Child Development Study. JAMA. 1998;280:701–7. doi: 10.1001/jama.280.8.701. [DOI] [PubMed] [Google Scholar]
- Davison M, McCarthy D. The Matching Law: A Research Review. Hillsdale, NJ: Erlbaum; 1988. [Google Scholar]
- Day JJ, Reed MN, Newland MC. Neuromotor deficits and mercury concentrations in rats exposed to methyl mercury and fish oil. Neurotoxicol Teratol. 2005;27:629–41. doi: 10.1016/j.ntt.2005.03.011. [DOI] [PubMed] [Google Scholar]
- Egeland GM, Middaugh JP. Balancing fish consumption benefits with mercury exposure. Science. 1997;278:1904–05. doi: 10.1126/science.278.5345.1904. [DOI] [PubMed] [Google Scholar]
- Elsner J, Hodel B, Suter KE, Oelke D, Ulbrich B, Schreiner G, Cuomo V, Cagiano R, Rosengren LE, Karlsson JE, Haglid KG. Detection limits of different approaches in behavioral teratology, and correlation of effects with neurochemical parameters. Neurotoxicol Teratol. 1988;10:155–67. doi: 10.1016/0892-0362(88)90080-3. [DOI] [PubMed] [Google Scholar]
- Gilbert SG, Rice DC. Low-level lifetime lead exposure produces behavioral toxicity (spatial discrimination reversal) in adult monkeys. Toxicol Appl Pharmacol. 1987;91:484–90. doi: 10.1016/0041-008x(87)90070-6. [DOI] [PubMed] [Google Scholar]
- Gilbert SG, Burbacher TM, Rice DC. Effects of in utero methylmercury exposure on a spatial delayed alternation task in monkeys. Toxicol Appl Pharmacol. 1993;123:130–6. doi: 10.1006/taap.1993.1229. [DOI] [PubMed] [Google Scholar]
- Goldey ES, O’Callaghan JP, Stanton ME, Barone S, Jr, Crofton KM. Developmental neurotoxicity: Evaluation of testing procedures with methylazoxymethanol and methylmercury. Fundam Appl Toxicol. 1994;23:447–64. doi: 10.1006/faat.1994.1127. [DOI] [PubMed] [Google Scholar]
- Grandjean P, Weihe P, White RF, Debes F, Araki S, Yokoyama K, Murata K, Sorensen N, Dahl R, Jorgensen PJ. Cognitive deficit in 7-year-old children with prenatal exposure to methylmercury. Neurotoxicol Teratol. 1997;19:417–28. doi: 10.1016/s0892-0362(97)00097-4. [DOI] [PubMed] [Google Scholar]
- Greiner RS, Moriguchi T, Hutton A, Slotnick BM, Salem N. Rats with low levels of brain docosahexaenoic acid show impaired performance in olfactory-based and spatial learning tasks. Lipids. 1999;34:S239–S43. doi: 10.1007/BF02562305. [DOI] [PubMed] [Google Scholar]
- Gunderson VM, Grant-Webster KS, Burbacher TM, Mottet NK. Visual recognition memory deficits in methylmercury-exposed Macaca fascicularis infants. Neurotoxicol Teratol. 1988a;10:373–9. doi: 10.1016/0892-0362(88)90041-4. [DOI] [PubMed] [Google Scholar]
- Gunderson VM, Grant-Webster KS, Burbacher TM, Mottet NK. Retarded object permanence development in methylmercury exposed Macaca fascicularis infants. Dev Psychol. 1988b Nov;22 doi: 10.1016/0892-0362(88)90041-4. 1986. [DOI] [PubMed] [Google Scholar]
- Kalmijn S, Fesken EJ, Launer LJ, Kromhout D. Polyunsaturated fatty acids, antioxidants, and cognitive function in very old men. Am J Epidemiol. 1997;145:33–41. doi: 10.1093/oxfordjournals.aje.a009029. [DOI] [PubMed] [Google Scholar]
- Kalmijn S, van Boxtel MP, Ocke M, Verschuren WM, Kromhout D, Launer LJ. Dietary intake of fatty acids and fish in relation to cognitive performance at middle age. Neurology. 2004;62:275–80. doi: 10.1212/01.wnl.0000103860.75218.a5. [DOI] [PubMed] [Google Scholar]
- Kheramin S, Body S, Herrera FM, Bradshaw CM, Szabadi E, Deakin JF, Anderson IM. The effect of orbital prefrontal cortex lesions on performance on a progressive ratio schedule: Implications for models of inter-termporal choice. Behav Brain Res. 2005;156:145–52. doi: 10.1016/j.bbr.2004.05.017. [DOI] [PubMed] [Google Scholar]
- Kinjo Y, Higashi H, Nakano A, Sakamoto M, Sakai R. Profile of subjective complaints and activities of daily living among current patients with Minamata disease after 3 decades. Environ Res. 1993;63:241–51. doi: 10.1006/enrs.1993.1144. [DOI] [PubMed] [Google Scholar]
- Klurfeld DM. Kidney and bladder stones in rodents fed purified diets. J Nutr. 2002;132:3482. doi: 10.1093/jn/132.12.3784. [DOI] [PubMed] [Google Scholar]
- Kolb B, Wishaw IQ. Fundamentals of Human Neuropsychology. 5. New York: Worth Publishers; 2003. [Google Scholar]
- Magos L. The absorption, distribution, and excretion of methyl mercury. In: Eccles CU, Annau Z, editors. The Toxicity of Methyl Mercury. Baltimore: Johns Hopkins; 1987. pp. 24–44. [Google Scholar]
- Mahaffey KR. Methylmercury exposure and neurotoxicity. JAMA. 1998;280:737–38. doi: 10.1001/jama.280.8.737. [DOI] [PubMed] [Google Scholar]
- Mandel N. Mechanism of stone formation. Semin Nephrol. 1996;16:364–74. [PubMed] [Google Scholar]
- Morris MC, Evans DA, Tangney CC, Bienias JL, Wilson RS. Fish consumption and cognitive decline with age in a large community study. Arch Neurol. 2005;62:1849–53. doi: 10.1001/archneur.62.12.noc50161. [DOI] [PubMed] [Google Scholar]
- Murray TK, Ridley RM, Snape MF, Cross AJ. The effect of dizocilpine (MK-801) on spatial and visual discrimination tasks in the rat. Behav Pharmacol. 1995;6:540–49. [PubMed] [Google Scholar]
- Nakada S, Imura N. Susceptibility of lipids to mercurials. Journal of Applied Toxicology. 1983;3:131–4. doi: 10.1002/jat.2550030305. [DOI] [PubMed] [Google Scholar]
- National Research Council. Toxicological Effects of Methylmercury. Washington, D.C: National Academy Press; 2000. [Google Scholar]
- Newland MC, Reile PA. Blood and brain mercury levels after chronic gestational exposure to methylmercury in rats. Toxicol Sci. 1999;50:106–16. doi: 10.1093/toxsci/50.1.106. [DOI] [PubMed] [Google Scholar]
- Newland MC, Rasmussen EB. Aging unmasks adverse effects of gestational exposure to methylmercury in rats. Neurotoxicol Teratol. 2000;22:819–28. doi: 10.1016/s0892-0362(00)00107-0. [DOI] [PubMed] [Google Scholar]
- Newland MC, Paletz EM. Animal studies of methylmercury and PCBs: What do they tell us about expected effects in humans? Neurotoxicology. 2000;21:1003–27. [PubMed] [Google Scholar]
- Newland MC, Reile PA, Langston JL. Gestational exposure to methylmercury retards choice in transition in aging rats. Neurotoxicol Teratol. 2004;26:179–94. doi: 10.1016/j.ntt.2003.12.004. [DOI] [PubMed] [Google Scholar]
- Newland MC, Yezhou S, Logdberg B, Berlin M. Prolonged behavioral effects of in utero exposure to lead or methyl mercury: Reduced sensitivity to changes in reinforcement contingencies during behavioral transitions and in steady state. Toxicol Appl Pharmacol. 1994;126:6–15. doi: 10.1006/taap.1994.1084. [DOI] [PubMed] [Google Scholar]
- Newland MC, Donlin WD, Paletz EM, Banna KM. Developmental Behavioral Toxicitiy of Methylmercury: Consequences, Conditioning, and Cortex. In: Levin ED, Buccafusco JJ, editors. Animal Models of Cognitive Impairment. Boca Raton, FL: CRC Press; 2006a. pp. 101–46. [PubMed] [Google Scholar]
- Newland MC, Reed MN, LeBlanc A, Donlin WD. Brain and blood mercury and selenium after chronic and developmental exposure to methylmercury. Neurotoxicology. 2006b;27:710–20. doi: 10.1016/j.neuro.2006.05.007. [DOI] [PubMed] [Google Scholar]
- Newland MC, Reile PA, Sartin EA, Hart M, Craig-Schmidt MC, Mandel INM. Urolithiasis in rats consuming a dl bitartrate form of choline in a purified diet. Comp Med. 2005;55:354–67. [PubMed] [Google Scholar]
- Okaniwa Y, Yuasa S, Yamamoto N, Watanabe S, Kobayashi T, Okuyama H, Nomura M, Nagata Y. A high linoleate and a high alpha-linolenate diet induced changes in learning behavior of rats. Effects of a shift in diets and reversal of training stimuli. Biol Pharm Bull. 1996;19:536–40. doi: 10.1248/bpb.19.536. [DOI] [PubMed] [Google Scholar]
- O’Kusky J. Methylmercury poisoning of the developing nervous system: Morphological changes in neuronal mitochondria. Acta Neuropathol. 1983;61:116–22. doi: 10.1007/BF00697390. [DOI] [PubMed] [Google Scholar]
- Paletz EM, Craig-Schmidt MC, Newland MC. Gestational exposure to methylmercury and n-3 fatty acids: Effects on high- and low-rate operant behavior in adulthood. Neurotoxicol Teratol. 2006;28:59–73. doi: 10.1016/j.ntt.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Parran DK, Barone S, Jr, Mundy WR. Methylmercury decreases NGF-induced TrkA autophosphorylation and neurite outgrowth in PC12 cells. Brain Res Dev Brain Res. 2003;141:71–81. doi: 10.1016/s0165-3806(02)00644-2. [DOI] [PubMed] [Google Scholar]
- Prusky GT, Harker KT, Douglas RM, Whishaw IQ. Variation in visual acuity within prigmented, and between pigmented and albino rat strains. Behav Brain Res. 2002;136:339–48. doi: 10.1016/s0166-4328(02)00126-2. [DOI] [PubMed] [Google Scholar]
- Reed MN, Paletz EM, Newland MC. Gestational Exposure to Methylmercury and Selenium: Effects on a Spatial Discrimination Procedure in Adulthood. Neurotoxicology. 2006;27:721–32. doi: 10.1016/j.neuro.2006.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed MN, Banna KM, Donlin WD, Newland MC. Effects of gestational exposure to methylmercury and dietary selenium on reinforcement efficacy in adulthood. Neurotoxicol Teratol. doi: 10.1016/j.ntt.2007.10.003. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisbick S, Neuringer M. Omega-3 fatty acid deficiency and behavior. In: Yehuda S, Mostofsky DI, editors. Handbook of Essential Fatty Acid Biology. Totowa, NJ: Humana Press; 1997. pp. 397–426. [Google Scholar]
- Reisbick S, Neuringer M, Gohl E, Wald R, Anderson GJ. Visual attention in infant monkeys: Effects of dietary fatty acids and age. Dev Psychol. 1997;33:387–95. doi: 10.1037//0012-1649.33.3.387. [DOI] [PubMed] [Google Scholar]
- Rice DC. Central nervous system effects of perinatal exposure to lead or methylmercury. In: Clarkson T, Nordberg G, Sager P, editors. Reproductive and Developmental Toxicity of Metals. New York: Plenum Press; 1983. pp. 517–39. [Google Scholar]
- Rice DC. Chronic low-lead exposure from birth produces deficits in discrimination reversal in monkeys. Toxicol Appl Pharmacol. 1985;77:201–10. doi: 10.1016/0041-008x(85)90319-9. [DOI] [PubMed] [Google Scholar]
- Rice DC. Effects of pre- plus postnatal exposure to methylmercury in the monkey on fixed interval and discrimination reversal performance. Neurotoxicology. 1992;13:443–52. [PubMed] [Google Scholar]
- Rice DC. Sensory and cognitive effects of developmental methylmercury exposure in monkeys, and a comparison to effects in rodents. Neurotoxicology. 1996;17:139–54. [PubMed] [Google Scholar]
- Rice DC, Gilbert SG. Early chronic low-level methylmercury poisoning in monkeys impairs spatial vision. Science. 1982;216:759–61. doi: 10.1126/science.7079739. [DOI] [PubMed] [Google Scholar]
- Rice DC, Gilbert SG. Effects of developmental exposure to methyl mercury on spatial and temporal visual function in monkeys. Toxicol Appl Pharmacol. 1990;102:151–63. doi: 10.1016/0041-008x(90)90092-9. [DOI] [PubMed] [Google Scholar]
- Ridley RM, Clark BA, Durnford LJ, Baker HF. Stimulus-bound perseveration after frontal ablations in marmosets. Neuroscience. 1993;52 doi: 10.1016/0306-4522(93)90409-9. [DOI] [PubMed] [Google Scholar]
- Schaefer EJ, Bongard V, Beiser AS, Lamon-Fava S, Robins SJ, Au R, Tucker KL, Kyle DJ, Wilson PW, Wolf PA. Plasma phosphatidylcholine docosahexaenoic acid content and risk of dementia and Alzheimer disease: The Framingham Heart Study. Arch Neurol. 2006;63:1545–50. doi: 10.1001/archneur.63.11.1545. [DOI] [PubMed] [Google Scholar]
- Schantz SL, Bowman RE. Learning in monkeys exposed perinatally to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) Neurotoxicol Teratol. 1989;11:13–9. doi: 10.1016/0892-0362(89)90080-9. [DOI] [PubMed] [Google Scholar]
- Schreiner G, Ulbrich B, Bass R. Testing strategies in behavioral teratology: II. Discrimination learning. Neurobehav Toxicol Teratol. 1986;8:567–72. [PubMed] [Google Scholar]
- Schultz W, Tremblay L, Hollerman JR. Reward processing in primate orbitofrontal cortex and basal ganglia. Cereb Cortex. 2000;10:272–84. doi: 10.1093/cercor/10.3.272. [DOI] [PubMed] [Google Scholar]
- Shafer TJ, Meacham CA, Barone S., Jr Effects of prolonged exposure to nanomolar concentrations of methylmercury on voltage-sensitive sodium and calcium currents in PC12 cells. Brain Res Dev Brain Res. 2002;136:151–64. doi: 10.1016/s0165-3806(02)00360-7. [DOI] [PubMed] [Google Scholar]
- Smith LH. The pathophysiology and medical treatment of urolithiasis. Semin Nephrol. 1990;10:31–52. [PubMed] [Google Scholar]
- Stern S, Cox C, Cernichiari E, Balys M, Weiss B. Perinatal and lifetime exposure to methylmercury in the mouse: Blood and brain concentrations of mercury to 26 months of age. Neurotoxicology. 2001;22:467–77. doi: 10.1016/s0161-813x(01)00047-x. [DOI] [PubMed] [Google Scholar]
- Tremblay L, Schultz W. Relative reward preference in primate orbitofrontal cortex. Nature. 1999;398:704–8. doi: 10.1038/19525. [DOI] [PubMed] [Google Scholar]
- Wainwright P. Essential fatty acids and behavior. In: Yehuda S, Mostofsky DI, editors. Handbook of Essential Fatty Acid Biology. Totowa, NJ: Humana Press; 1997. pp. 299–341. [Google Scholar]
- Wainwright PE, Huang YS, Bulman-Fleming B, Dalby D, Mills DE, Redden P, McCutcheon D. The effects of dietary n-3/n-6 ratio on brain development in the mouse: A dose response study with long-chain n-3 fatty acids. Lipids. 1992;27:98–103. doi: 10.1007/BF02535807. [DOI] [PubMed] [Google Scholar]
- Wainwright PE, Xing HC, Ward GR, Huang YS, Bobik E, Auestad N, Montalto M. Water maze performance is unaffected in artificially reared rats fed diets supplemented with arachidonic acid and docosahexaenoic acid. J Nutr. 1999;129:1079–89. doi: 10.1093/jn/129.5.1079. [DOI] [PubMed] [Google Scholar]
- Widholm JJ, Seo BW, Strupp BJ, Seegal RF, Schantz SL. Effects of perinatal exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin on spatial and visual reversal learning in rats. Neurotoxicol Teratol. 2003;25:459–71. doi: 10.1016/s0892-0362(03)00014-x. [DOI] [PubMed] [Google Scholar]
- Widholm JJ, Clarkson GB, Strupp BJ, Crofton KM, Seegal RF, Schantz SL. Spatial reversal learning in Aroclor 1254-exposed rats: Sex-specific deficits in associative ability and inhibitory control. Toxicol Appl Pharmacol. 2001;174:188–98. doi: 10.1006/taap.2001.9199. [DOI] [PubMed] [Google Scholar]
- Yamamoto N, Saitoh M, Moriuchi A, Nomura M, Okuyama H. Effect of dietary alpha-linolenate/linoleate balance on brain lipid compositions and learning ability of rats. J Lipid Res. 1987;28:144–51. [PubMed] [Google Scholar]
- Yamamoto N, Hashimoto A, Takemoto Y, Okuyama H, Nomura M, Kitajima R, Togashi T, Tamai Y. Effect of the dietary alpha-linolenate/linoleate balance on lipid compositions and learning ability of rats. II. Discrimination process, extinction process, and glycolipid compositions. J Lipid Res. 1988;29:1013–21. [PubMed] [Google Scholar]