Abstract
Background and Aim
Chronic inflammation is a risk factor for colon cancer in patients with ulcerative colitis (UC). The molecular mechanisms linking inflammation and colon carcinogenesis are incompletely understood. We tested the hypothesis that TLR4 is involved in tumorigenesis in the setting of chronic inflammation.
Methods
Tissues from UC patients with cancer were examined for TLR4 expression. Colitis-associated neoplasia was induced using azoxymethane (AOM) injection followed by dextran sodium sulfate (DSS) treatment in TLR4-deficient (TLR4−/−) or wild-type (WT) mice. Inflammation, polyps, and microscopic dysplasia were scored. Cox-2 and PGE2 production were analyzed by real-time PCR, immunohistochemistry, or enzyme immunoassay. EGFR phosphorylation and amphiregulin production were examined by Western blot analysis and ELISA, respectively.
Results
We show that TLR4 is overexpressed in human and murine inflammation-associated colorectal neoplasia. TLR4−/− mice were markedly protected from colon carcinogenesis. Mechanistically, we show that TLR4 is responsible for induction of Cox-2, increased PGE2 production and activation of EGFR signaling in chronic colitis. Amphiregulin, an EGFR ligand, was induced in a TLR4, COX-2-dependent fashion and contributes to activation of EGFR phosphorylation in colonic epithelial cells.
Conclusion
TLR4 signaling is critical for colon carcinogenesis in chronic colitis. TLR4 activation appears to promote the development of colitis-associated cancer by mechanisms including enhanced Cox-2 expression and increased EGFR signaling. Inhibiting TLR4 signaling may be useful in prevention or treatment of colitis-associated cancer.
Introduction
Inflammation is considered a risk factor for many common malignancies including cancers of the lung 1, breast 2, and colon 3. Colon cancer is the third most common cancer and the third leading cause of cancer-related mortality in the United States 4. The link between inflammation and colon cancer offers the possibility of identifying novel ways to prevent cancer. However, the molecular mechanisms whereby chronic inflammation predisposes to cancer remain elusive.
The clearest link between inflammation and colon cancer is seen in patients with inflammatory bowel disease (IBD)5. Colorectal cancer is one of the most serious complications of IBD, accounting for increased mortality in these disorders 6. The severity of inflammation correlates with the risk of colorectal cancer in patients with IBD 7, 8. Consistent with a role of inflammation in colorectal neoplasia, animal models have demonstrated that the multi-functional transcription factor NF-κB is required for colorectal neoplasia 9. Therefore, focusing on the relationship between chronic inflammation and carcinogenesis may provide insights into the pathogenesis of colitis-associated cancer (CAC) and possibly sporadic colorectal cancer.
The colon contains 100 trillion bacteria, and commensal bacteria have been implicated in the development of sporadic colorectal cancer 10. Commensal bacteria may promote colorectal cancer by a variety of mechanisms including generation of reactive oxygen intermediates resulting in chromosomal instability 11. Bacteria are required for eliciting chronic inflammation and colon cancer in animal models of CAC 12, 13. Therefore, we have focused on TLR4 and its role in CAC. TLR4 is normally expressed at low levels in the intestinal mucosa 14–16 while it is up-regulated in patients with IBD 17. In acute colitis, we previously demonstrated that TLR4 is a potent inducer of cyclooxygenase-2 (Cox-2) expression 18. TLR4 may also be important for evasion of tumor surveillance 19. Altogether, these data raise the intriguing possibility that TLR4 promotes colon cancer in the setting of chronic inflammation, and this serves as the focus of the present study.
In the current study, we demonstrate first that TLR4 is over-expressed in human colon cancers arising in chronic ulcerative colitis. Over-expression of TLR4 was then confirmed in an animal model of inflammation-induced colon tumorigenesis wherein healthy, wild-type (WT) mice were given azoxymethane (AOM) to induce colonic neoplasia in the setting of chronic inflammation. Importantly, mice genetically lacking TLR4 were protected against colon tumorigenesis in this animal model of inflammation-induced carcinogenesis. Mechanistically, we show that TLR4 is required for expression of Cox-2 and enhanced PGE2 production in chronic colitis. TLR4-dependent tumorigenesis was also associated with activation of EGFR signaling. These findings are significant because both Cox-2 and EGFR have been linked to the development of colon tumors 20, 21. The current results provide important insights into the previously unrecognized role of TLR4 signaling in CAC and strengthen the rationale for developing chemopreventive therapies that target TLR4.
Materials and Methods
Animal studies
TLR4−/− mice were purchased from Oriental Bio Service, Inc. (Kyoto, Japan). All knockout mice were backcrossed to C57Bl/6J mice at least 8 generations. C57BL/6J mice were obtained from Jackson Laboratory as controls (Jackson Laboratory, Bar Harbor, Maine). Mice were kept in specific-pathogen free (SPF) conditions and fed by free access to a standard diet and water. All experiments were done according to Mount Sinai School of Medicine animal experimental ethics committee guidelines.
Following previously established methods for inducing colonic neoplasia 22, six to ten week old gender-matched mice were injected with 7.4mg/kg of AOM (Sigma, St. Louis, MO) intraperitoneally (i.p.) at the beginning of the experiment (day 0). After 14 days, mice were treated with 3% DSS (MW 36–50 kDa: ICN, Aurora, Ohio) in their drinking water for 7 days. This was followed by 14 days of normal water, another 7 days of 3% DSS treatment, and then normal water for an additional 14 days. During the DSS treatment and recovery phase, body weights, stool consistency, and stool occult blood were monitored, as described previously 23.
Mice were sacrificed on day 56. Colons were removed, opened longitudinally and stained with 1% Alcian Blue (Sigma, St. Louis, MO). High-resolution mucosal pictures were obtained using a Leica MZ APO dissecting microscope (Leica, Bannockburn, IL) with SONY Power HAD DXC-970MD 3CCD Color digital video camera, and Scion Image 1.60 image software (Scion Co., Frederick, MD). After macroscopic assessment, cecum, proximal, and distal parts of the colon were fixed in 10% buffered formalin, embedded in paraffin, sectioned and stained with hematoxylin and eosin. Histological assessment was performed by two independent gastrointestinal pathologists (R.X., H.C.) blinded to the mouse genotype and treatment. Severity of mucosal inflammation was graded using a standard scoring system (Table 1). Dysplastic lesions were determined by previously established criteria 24. To quantify the microscopic extent of dysplasia, paraffin-embedded colons were cut in 5 μm thick serial sections and every 20th section was analyzed for dysplasia. Number, size, and the percentage of the mucosal surface area containing dysplasia were determined under the microscope. The size of the lesions was calculated using a scale micrometer on the microscope.
Table 1.
Scoring system for of chronic mucosal inflammation
| Histological scoring* | ||||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| Crypt damage | Acute inflammatory index (neutrophils/eosinophils) | Edema | Erosions/ulceration | Chronic inflammatory index (mononuclear cell infiltration) | Epithelial regeneration | Distortion/branching |
| intact = 0
basal 1/3 = 1 basal 2/3 = 2 entire loss = 3 Loss of crypt + epi= 4 |
0–4 | 0–4 | 0–4 | normal distribution = 0
intermediate =1 full thickness of lymphoplasmacytosis =2 |
no tissue repair = 3
Surface epithelium not intact =2 Slight epithelial injury = 1 Complete regeneration or normal tissue = 0 |
normal crypt= 0
mild = 1 moderate = 2 severe = 3 |
Cell lines and reagents
The human colon cell line SW480 (1 × 106 cells/well) was maintained in Dulbecco’s modified Eagle’s medium supplemented with 2% heat-inactivated FCS, 2mM L-glutamine, penicillin/streptomycin and was incubated in 6-well plates overnight at 37 °C in a 5% CO2 humidified incubator. The next day, cells were incubated with Ultrapure lipopolysaccharide (LPS, 2μg/ml), Eschericha coli 0111: B4 (Invivogen, San Diego, CA) for varying time periods.
Real Time PCR
Total RNA was isolated using RNA Bee (Tel-Test, Inc., Friendwood, TX) according to the manufacturer’s instructions. A total of 1 μg RNA was used as the template for single strand cDNA synthesis utilizing the Transcriptor First Strand cDNA Synthesis Kit (Roche, Indianapolis, IN) according to the manufacturer’s instructions. Quantitative real-time PCR was performed for Cox-2, and β-actin using TaqMan probes. The primers and probes used in this study can be found on-line in Supplemental material. The cDNA was amplified using TaqMan universal PCR Master Mix (Roche, Indianapolis, IN) on an ABI Prism 7900HT sequence detection system (Applied Biosystems, Foster City, CA), programmed for 95°C for 10 minutes, then 40 cycles of: 95 °C for 15 seconds, 60 °C for 1 minute. The amplification results were analyzed using SDS 2.2.1 software (Applied Biosystems, Foster City, CA) and the gene of interest was normalized to the corresponding β-actin results. Data were expressed as fold induction relative to the lowest gene product amplified.
RNA interference (RNAi)
SW480 cells were plated at a density of 1.5 × 105 cells/well in 12-well plates 24hours before the first transfection. TLR4 small interfering RNA (siRNA) oligonucleotide corresponding to the sequence (GGUAAGGAAUGAGCUAGUAUU) was purchased from Dharmacon (Chicago, IL). Fifty nM of siRNA were transfected twice every 24 hours with X-trim gene siRNA transfection reagent (Roche, Indianapolis, IN) as per the manufacturer’s instructions. Forty-eight hours after the first transfection, cells were stimulated with LPS (2μg/ml) for the indicated period of time. Negative control siRNA (50 nM), which has no significant homology to any known gene sequences from mouse, rat, or human, was used as a control (Ambion, Austin, TX).
Western Blot Analysis
De-identified human colon resections were obtained under the auspices of the Mount Sinai Medical Center Institutional Review Board. Snap frozen tumor and surrounding colonic tissues from six ulcerative colitis patients, and actively inflamed colonic tissues from four ulcerative colitis patients undergoing colectomy were used. Mouse colon samples were taken at the time of sacrifice and frozen at −80°C. Details of tissue preparation and antibodies used for Western blot are included in Supplemental material. The mouse TLR4 band intensity was calculated using NIH image 1.62 by normalizing with the intensity of the corresponding β-actin band.
Enzyme linked immunosorbent assay (ELISA)
For the amphiregulin ELISA, SW480 (1 × 106 cells/well) were plated in 6 well plates. Cells were treated with LPS (2μg/ml) for the indicated periods. For ex vivo colonic tissue cultures, 100 mg of tissue from each part of the colon (not including the polypoid lesions) were cultured for 24 hours in 12 well flat bottom plates in serum free RPMI 1640. Supernatants were harvested for measurement of amphiregulin or TNF-α. ELISA (R&D Systems, Minneapolis, MN) was performed per the manufacturer’s instructions.
Immunofluorescent and immunohistochemical studies
Paraffin-embedded human colectomy specimens of normal colon (n=11) (obtained from resection edge of sporadic colorectal cancers) or colitis-associated dysplasia or cancer (n=15; all patients with ulcerative colitis) were stained with biotinylated mouse monoclonal anti-human TLR4 (1:500, eBioscience, San Diego, CA) overnight at 4°C, followed by streptavidin-FITC (10 μg/ml, eBioscience, San Diego, CA) for 1 hour at room temperature. Details of staining methodology and antibodies used are found in Supplemental material.
Assessment of proliferation
Colonic tissue sections were examined for cell proliferation (bromodeoxyuridine: BrdU labeling) in the polypoid lesions as well as surrounding colonic mucosa. Mice were injected with 120 mg/kg of BrdU (Sigma, St. Louis, MO) i.p., 90 minutes prior to sacrificing, and colonic tissues was stained for BrdU using a BrdU staining kit (Zymed Laboratories Inc, South San Francisco, CA) according to the manufacturer’s instructions. The number of BrdU-positive cells per well-oriented crypt were calculated in every 3 crypts for each colon segment at high magnification under light microscopy.
NF-κB Assay
For measurement of NF-κB activation, nuclear extracts were isolated from snap frozen mouse colonic tissues using a nuclear extraction kit (Active Motif, Carlsbad, CA) according to the manufacturer’s instructions. After measurement of protein concentration by the Bradford method, 1μg of nuclear extract was used to measure the DNA-binding activity of NF-κB p65 using TransAM NF-κB p65 Chemiluminescent kit (Active Motif, Carlsbad, CA) according to the manufacturer’s instructions. Chemiluminescent intensities were calculated as relative light units (RLU) and normalized with the mean RLU from untreated animals.
Measurement of PGE2
Production of PGE2 in the tissue culture supernatant was determined using a monoclonal EIA kit (Cayman, Ann Arbor, MI) according to the manufacturer’s instructions and as described previously 18, 25. Briefly, colonic samples from TLR4−/− and WT mice were washed in cold PBS containing penicillin, streptomycin, and fungizone (100U/ml each). 100 mg tissue fragments from the distal part of the colon closest to the anus were cultured for 24 hours in 12 well flat bottom plates in serum free RPMI 1640. Culture supernatants were harvested for PGE2 measurement.
Statistical analysis
Data were presented as mean (± SD). The significance was analyzed by Student t-test and Fisher’s exact test with Microsoft Excel and StatView software, respectively. P values were considered significant when < 0.05.
Results
TLR4 expression is up-regulated in colitis-associated tumors
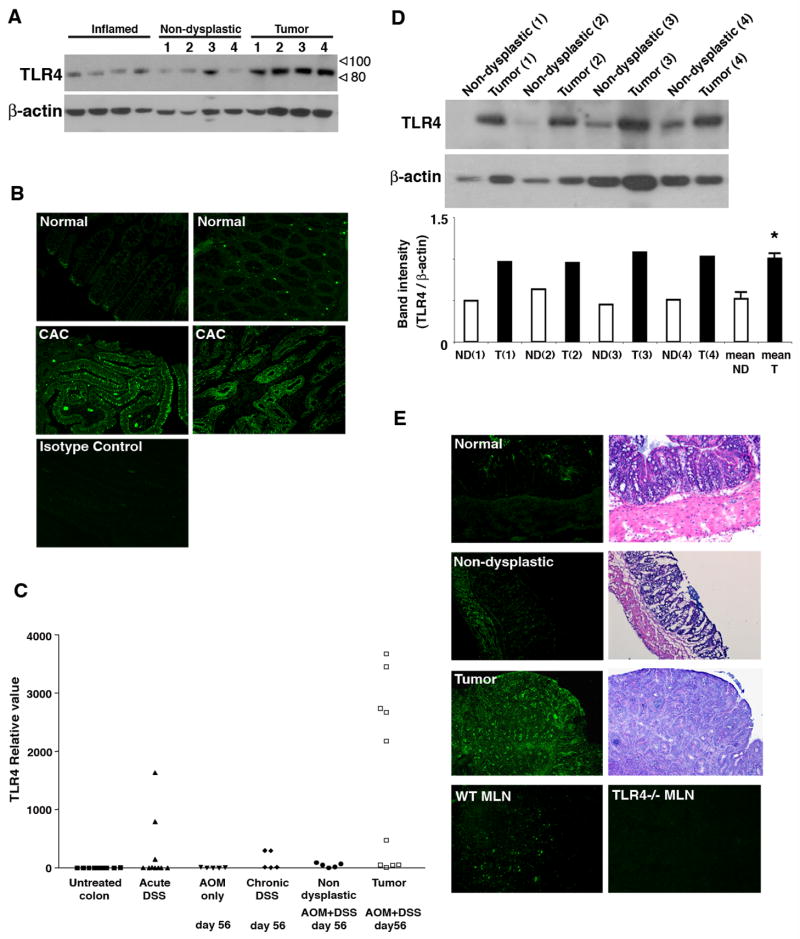
TLR4 expression is known to be low in the normal colon but increased in IBD 16, 26–28. We hypothesized that TLR4 is up-regulated in colitis-associated tumors. Colon tumor specimens from patients with UC with dysplasia or cancer were examined for TLR4 expression by Western blot analysis and immunofluorescent staining (Figure 1A, B). Non-dysplastic colon tissue from the same UC patients was used as a control. In addition, we examined samples from patients with active UC undergoing colectomy to assess the effect of moderate to severe inflammation on expression of TLR4. Non-dysplastic colon had low expression of TLR4 protein whereas samples of protein from the matched tumor specimens demonstrated higher expression of TLR4 (Figure 1A). Colon samples from active UC demonstrated expression of TLR4 comparable to the affected, non-dysplastic colon in UC cancer patients (Figure 1A). Immunostaining showed that, in normal colon, only cells at the base of the crypt and scattered cells in the lamina propria express TLR4 (Figure 1B). Colitis-associated tumors showed predominantly epithelial staining of TLR4 with varying degrees of TLR4 expression in cells of the lamina propria.
Figure 1. TLR4 expression is up-regulated in colitis-associated tumors.
A. Expression of TLR4 in normal mucosa and tumors from patients with UC. Samples of tumor tissue (n=4) and corresponding surrounding non-dysplastic colon (n=4) were examined for TLR4 protein by Western blot analysis. Colonic tissues from patients with active ulcerative colitis were also examined (Inflamed) (n=4). 25 μg/lane of protein was loaded per lane. β-actin staining is used as a loading control.
B. Immunofluorescent staining for TLR4 in human colitis-associated cancer. Normal human colon tissue (2 representative examples of 11 patients) (Top panels) and colon cancer in patients with ulcerative colitis (2 representative examples of 15 patients) (middle panels) were stained for TLR4.
C. TLR4 mRNA expression in the AOM-DSS model. Normal colon (n=10), acute phase after 7 days of DSS (n=10), day 56 after injection of AOM (n=5), day 56 of chronic DSS colitis after two cycles of DSS (n=5), non-dysplastic mucosa in AOM+DSS treated mice at day 56 (n=5), and tumor tissues taken from AOM+DSS treated mice at day 56 were analyzed for TLR4 mRNA expression by real-time PCR. P <0.05 for tumor tissue versus non-dysplastic mucosa, non-dysplastic mucosa versus normal colon, and chronic DSS versus normal mucosa.
D. Western blot analysis for TLR4 in murine colon samples. Immunoblots of tissue lysates (25 μg/lane) prepared from colonic samples of tumor versus corresponding surrounding non-dysplastic mucosa (n=4). Graph shows ratios of TLR4 band intensity normalized with β-actin band (ND= Non-dysplastic mucosa, T= Tumor). Mean ratios of non-dysplastic mucosa and tumor are shown at the end (*P<0.0001).
E. Immunofluorescent staining for TLR4 in the mouse CAC model. Serial sections of tumors or surrounding mucosa are stained with H&E for histology and immunofluorescent staining for TLR4. Mesenteric lymph node (MLN) is used as a positive control; mesenteric lymph node from a TLR4−/− mouse is used as a negative control.
To further examine the role of TLR4 in colitis-associated tumorigenesis, we used a well-established model of CAC 24, 29. In this model, animals receive AOM, a genotoxic agent, followed by two cycles of DSS to induce inflammation. Using the AOM-DSS model, all WT mice developed polypoid or flat tumors. The microscopic features recapitulate the features of human dysplasia and polyps in colitis patients 30. We hypothesized that TLR4 expression would be increased in colorectal neoplasia in this CAC model. To investigate this possibility, we first examined mRNA expression of various TLRs in tumor tissues and surrounding non-dysplastic mucosa by real-time PCR (Table 2). Compared to the untreated colon, all TLRs examined demonstrated increased expression with inflammation. Of the TLRs tested, however, only TLR4 was increased in the tumor tissue compared with the surrounding inflamed mucosa. We then examined TLR4 mRNA expression under various conditions, e.g. untreated, acute DSS, chronic DSS (at day 56), AOM alone, AOM+DSS at day 56 (Figure 1C). Although TLR4 mRNA expression is increased following acute or chronic DSS compared with untreated mice, the highest level of expression was seen in tumors caused by AOM+DSS. Expression of TLR4 protein by Western blot was significantly elevated in tumors that developed in WT mice compared to the surrounding non-dysplastic mucosa (Figure 1D). Immunofluorescent staining for TLR4 also showed an increase in TLR4 expression in polypoid tumors (Figure 1E). Little TLR4 staining was seen in the normal epithelium or in the epithelium in chronic colitis. Muscularis propria stains for TLR4 consistent with a previous report 31. We conclude that colorectal tumors over-express TLR4 and that TLR4 may play a role in tumorigenesis.
Table 2.
Expression of TLRs in the AOM-DSS model.
| TLRs | Normal mucosa (untreated) | Non-dysplastic surrounding mucosa | Tumor | P value* |
|---|---|---|---|---|
| TLR2 | 2.4 ± 2.0 | 4975.0 ± 6835.7 | 206.0 ± 582.9 | 0.207 |
| TLR3 | 359.2 ± 238.1 | 8555.8 ± 14520.5 | 6700.0 ± 17380.6 | 0.197 |
| TLR4 | 2.7 ± 3.0 | 48.1 ± 36.3 | 1538.7 ± 1546.0 | 0.027 |
| TLR5 | 19.7 ± 5.8 | 375.9 ± 706.2 | 318.9 ± 836.2 | 0.453 |
| TLR9 | 100.0 ± 76.9 | 446.8 ± 533.1 | 300.1 ± 410.7 | 0.298 |
Comparing tumor with inflamed surrounding mucosa.
TLR4 promotes development of colorectal tumors in the setting of chronic colitis
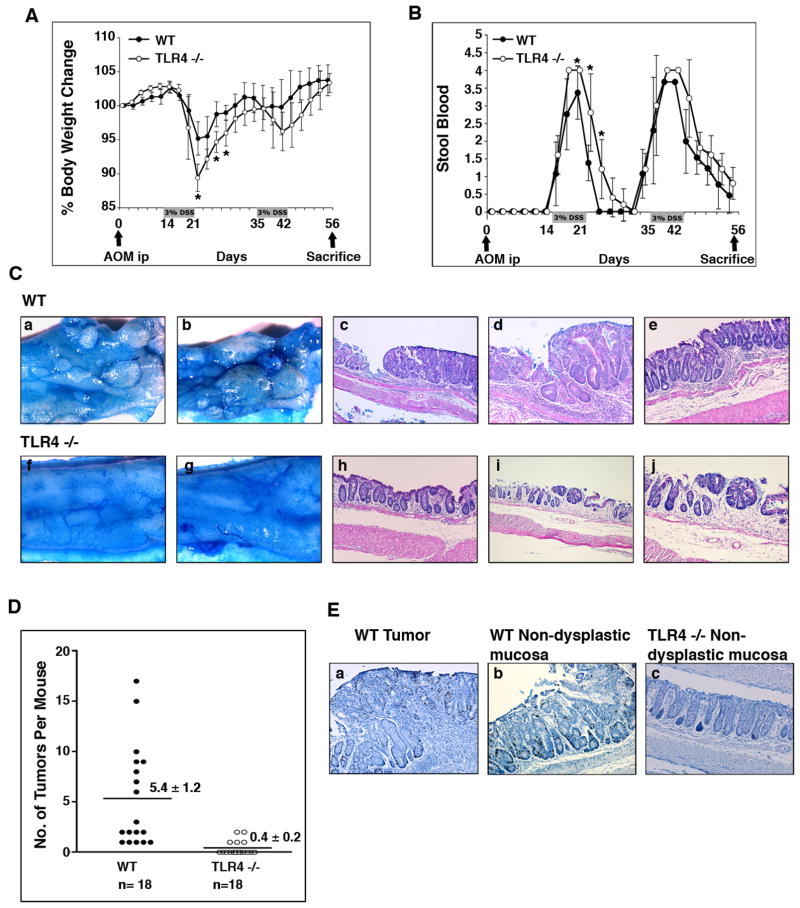
To examine the role of TLR4 in colitis-associated tumorigenesis, we first addressed whether absence of TLR4 altered the susceptibility to developing CAC. TLR4−/− and WT mice were treated with AOM-DSS as described in Materials and Methods and developed weight loss and bleeding during DSS treatment (Figure 2A, B). We found a striking difference between WT and TLR4−/− mice with respect to development of polyps and dysplasia. All WT mice grossly showed multiple polypoid lesions but no such visible lesions were seen in TLR4−/− mice (Figure 2C). When examined microscopically, all WT mice (n=18) had at least one dysplastic lesion and up to 17 lesions per mouse (Figure 2C, D). In contrast, only five of eighteen TLR4−/− mice (27.8%) had one or two dysplastic lesions (Figure 2C, D). A significant increase in the number of dysplastic lesions was observed between WT and TLR4−/− mice (P < 0.001) (Figure 2D, Table 3).
Figure 2. TLR4−/− mice are protected from colonic tumors in the setting of inflammation.
A, B. AOM and DSS were administered as shown. Weight change (A) and stool blood (B) were examined during the study period. The data represent the average (± SD) of four independent experiments with a total of 36 mice (TLR4−/− (n=18), and WT controls (n=18)) (*p<0.05).
C. Tumors in the CAC model. Colons were cut open longitudinally and the mucosal surface was stained with 1% Alcian blue. All WT mice showed multiple polypoid lesions (a, b) but not the TLR4−/− mice (f, g). Microscopically, two examples of WT dysplasias are shown at lower (c) and higher (d) magnifications. Non-dysplastic mucosa from a WT mouse is shown in e. Most TLR4−/− mucosa following AOM-DSS appeared as in panel h. An example of a dysplastic lesion in a TLR4−/− mouse is shown at lower (i) and higher (j) magnifications.
D. Incidence of dysplasia. The number of dysplastic lesions was counted per mouse under the microscope. Data are expressed as mean ± SEM (P < 0.0001).
E. Proliferation in the CAC model. BrDU incorporation was assessed using anti-BrDU Ab staining. Representative pictures are taken from a WT dysplastic lesion (a), and WT non-dysplastic mucosa (b). Mucosa of AOM-DSS treated TLR4−/− mice (c) is also shown.
Table 3.
Incidence and size of polyps
| WT (n=18) | TLR4−/− (n=18) | P value | |
|---|---|---|---|
| Incidence (%) | 100 | 27.8 | 0.027 |
| Tumors/animal (range) | 5.4 ± 1.2 (1 – 17) | 0.4 ± 0.2 (0 – 2) | 0.00016 |
| Tumor size (mm) (range) | 3.6 ± 0.4 (1 – 10) | 1.3 ± 0.3 (0.5 – 1) | 0.02 |
| Percentage of mucosal surface with tumor (range) | 22.6% ± 3.1 (1 – 65) | 4.4% ± 0.6 (1 – 5) | 0.0025 |
The size and severity of dysplasia were also different between WT mice and TLR4−/− mice (Table 3). The few dysplastic lesions found in TLR4−/− mice were small, flat, and low grade. By contrast, 48% of dysplastic lesions (47/97) in WT mice were polypoid. BrdU staining of colon showed that proliferation was greatly increased in dysplasia and the surrounding epithelium of WT mice compared to non-dysplastic mucosa in TLR4−/− mice (Figure 2E). The number of BrdU positive cells was significantly higher in WT dysplastic (33.4 positive cells/100 epithelial cells ± 8.3SD) and non-dysplastic mucosa (20.4 cells ± 6.2SD) compared with the non-dysplastic mucosa in TLR4−/− mice (14.3 cells ± 3.8SD). These results demonstrate that TLR4 is required for initiation and growth of colorectal tumors in a mouse model of CAC.
NF-κB activation is decreased in acute but not chronic DSS colitis in TLR4−/− mice
Having demonstrated the link between TLR4 and colorectal tumors, we turned our attention to the mechanism by which this may occur. The severity of inflammation correlates with the risk of colorectal cancer in patients with IBD 7, 8. Using a scoring system for chronic colitis that examines features seen in human colitis, there was no significant difference between TLR4−/− and WT mice with respect to overall severity of colitis (Figure 3). A common connection between inflammation and carcinogenesis is the activation of signaling pathways that promote cell proliferation and increase cell survival such as the transcription factor NF-κB 9. TNF-α is a pro-inflammatory cytokine that is regulated by TLR-mediated NF-κB activation and has been linked to inflammation and carcinogenesis 32. Therefore, we examined transcriptional activity of NF-κB and TNF-α production in the colonic tissues from the CAC model. There were no significant differences in either NF-κB activation or TNF-α production between WT and TLR4−/− mice in the CAC model (Figure 3).
Figure 3. Comparison of inflammatory activity in TLR4−/− versus WT mice.
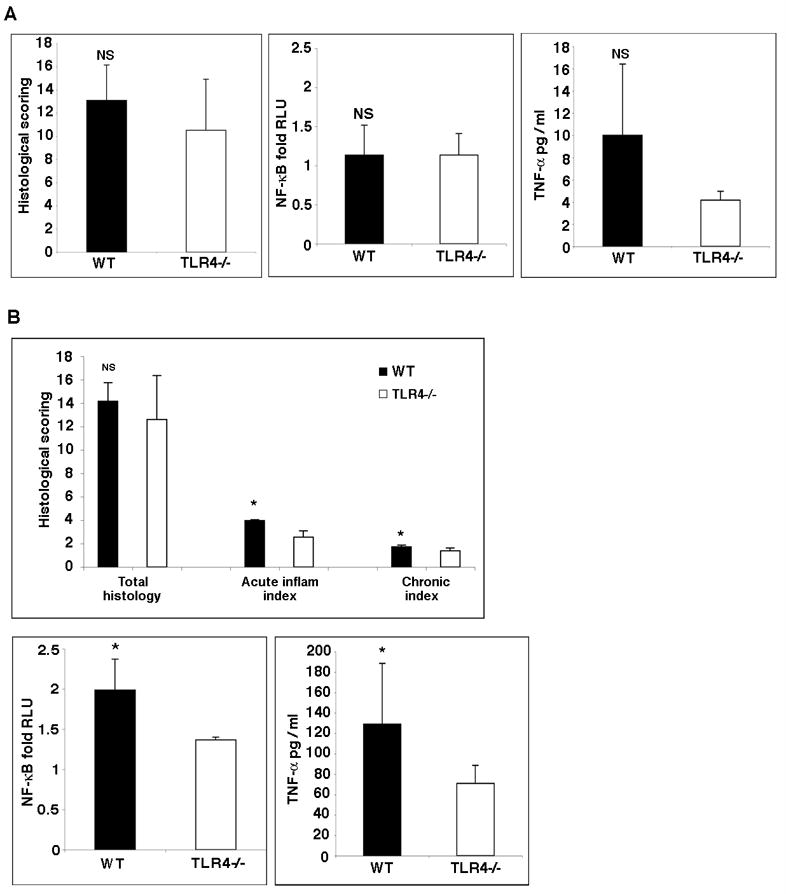
A. Histology scores in WT versus TLR4−/− mice at day 56 following AOM+DSS (left panel). Table 1 demonstrates the criteria used for scoring. The total histology score was similar in WT and TLR4−/− mice. NF-κB activation state (middle panel) and TNF-α secretion (right panel) in WT versus TLR4−/− mice at day 56 following AOM+DSS. There are no significant differences between WT and TLR4−/− mice in NF-κB activation or TNF-α production in the intestine.
B. Histology scores in WT versus TLR4−/− mice at day 7 following DSS. The acute and chronic sub-scores for the indices are shown. NF-κB activation state and TNF-α secretion in WT versus TLR4−/− mice after seven days of DSS treatment. TLR4−/− mice have significantly decreased NF-κB activation and TNF-α secretion.
We reasoned, however, that inflammation at earlier time points might be different between WT and TLR4−/− mice. We have previously shown that following acute DSS treatment, TLR4−/− mice have significantly decreased recruitment of neutrophils 23. Although the total inflammatory scores following acute DSS treatment were similar between WT and TLR4−/− mice, there was a small but significant decrease in the sub-score for acute inflammation in TLR4−/− mice compared with WT mice (Figure 3B). Thus, we examined NF-κB activation and TNF-α production after seven days of DSS treatment. WT mice had significantly higher activity of NF-κB and increased production of TNF-α compared with TLR4−/− mice in the acute phase of colitis (Figure 3B). These results indicate that the decrease in inflammatory mediators such as NF-κB activation and TNF-α production in TLR4−/− mice may contribute to the subsequent protection from colorectal dysplasia. These data also highlight that in spite of expression of many other TLRs during inflammation (Table 2), the absence of TLR4 results in a decreased ability to mount a robust pro-inflammatory response.
Mucosal Cox-2 expression and PGE2 production are decreased in TLR4−/− mice in the CAC model
Cox-2 is critical for development of colorectal neoplasia 20. We hypothesized that decreased expression of Cox-2 in TLR4−/− mice was associated with protection against tumorigenesis. Using real-time PCR, we found that mucosal Cox-2 expression was significantly decreased in TLR4−/− mucosa compared to WT mucosa in the CAC model (Figure 4A). Mucosal Cox-2 expression was found in lamina propria cells and to a lesser extent in IEC by immunofluorescent staining (Figure 4B). We confirmed by double staining that the majority of Cox-2 positive lamina propria cells were also positive for the macrophage marker CD68 (Figure 4C). We also performed double-staining of Cox-2 and a marker of myofibroblasts and found Cox-2-expressing myofibroblasts at the base of the crypts (Figure 4C).
Figure 4. Mucosal Cox-2 expression is decreased in TLR4−/− mice in the CAC model.
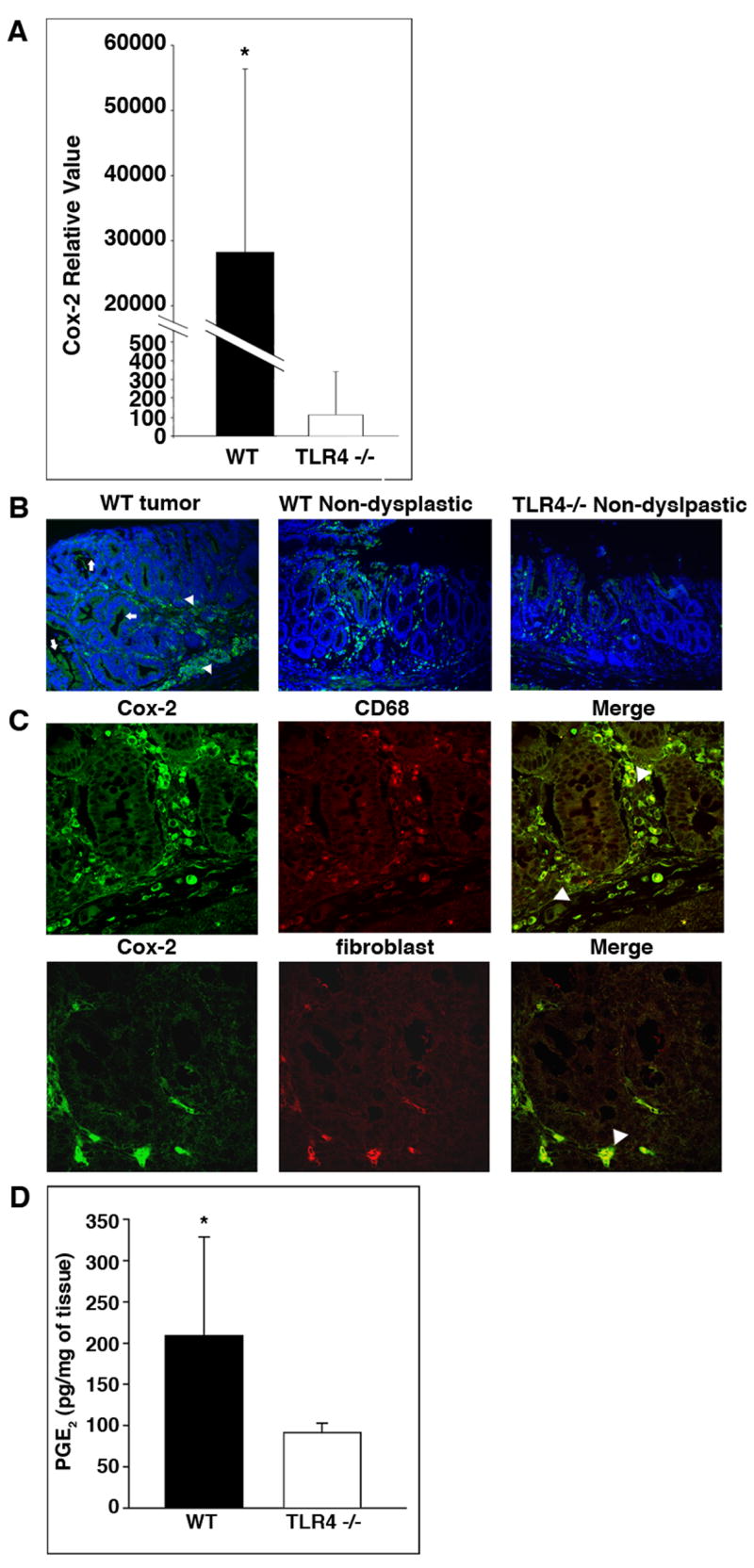
A. TaqMan real-time PCR was used to compare mucosal expression of Cox-2 mRNA in WT and TLR4−/− mice in the CAC model (n=6 each). Data are represented as mean ± SD of relative values of expression (*P < 0.05).
B. Immunofluorescent staining for Cox-2 in the colon. Representative pictures show Cox-2 positive cells (green - FITC) in lamina propria cells (arrowhead) and IEC (arrow) in WT mice and TLR4−/− mice.
C. Double immunostaining for Cox-2 (green - FITC) and the macrophage marker CD68 (red - TRITC) in WT mucosa (top panel) or anti-fibroblast antibodies (red-TRITC) (bottom panel). Arrows indicate double positive cells (right most panels).
D. PGE2 production by colonic tissue from TLR4−/− or WT mice in the CAC model. Colonic tissues from TLR4−/− (n=9) and WT mice (n=10). Data are shown as mean ± SD. (*P < 0.05).
Given that TLR4−/− mice have decreased expression of mucosal Cox-2 compared to WT mice, we investigated whether PGE2 production was also reduced. PGE2 production is important for both the formation and growth of colorectal tumors 33. We found that PGE2 production by colonic tissue from TLR4−/− mice was significantly less than in WT mice (Figure 4D). Given the well-established link between Cox-2 and colon carcinogenesis 20, these data suggest that TLR4 signaling may promote the development of CAC, in part, by inducing Cox-2 expression and PGE2 production.
Phosphorylation of EGFR is decreased in TLR4−/− mucosa
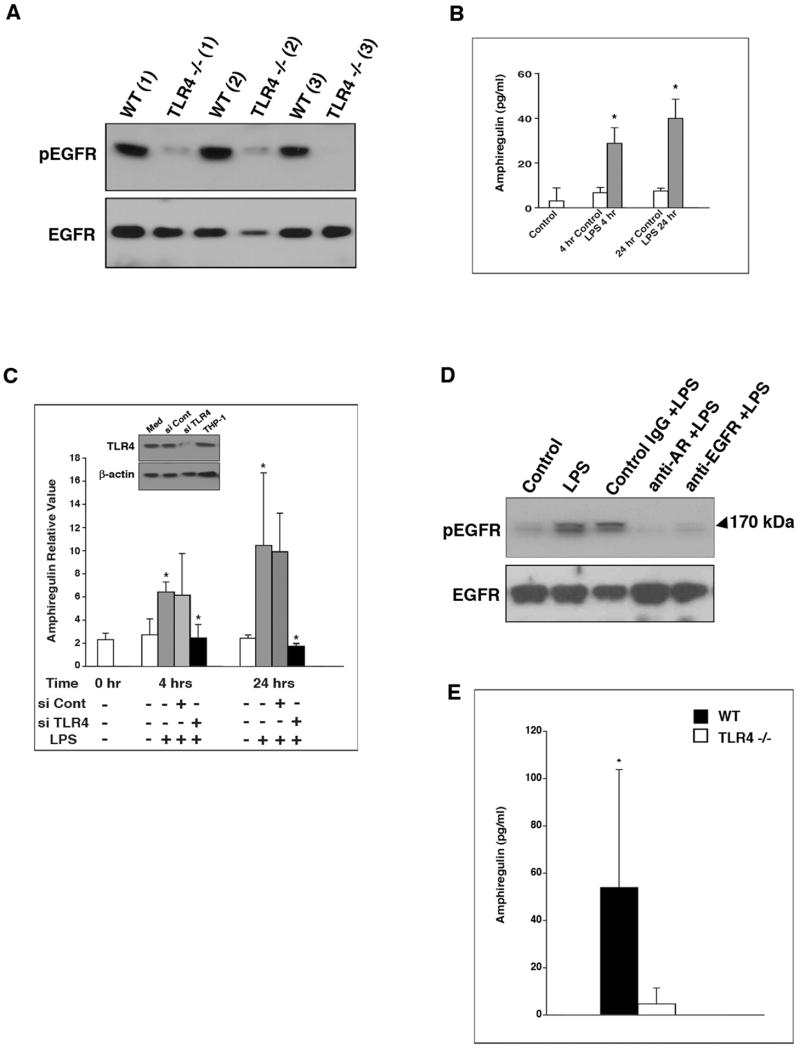
Several studies have suggested that PGE2 stimulates colorectal carcinogenesis in part by activation of EGFR signaling 34, 35. We reasoned that TLR4−/− mice might be protected against colorectal carcinogenesis in chronic colitis because of decreased EGFR activation. Consistent with this hypothesis, levels of phosphorylated EGFR were significantly lower in colonic mucosa from TLR4−/− mice compared with WT mice (Figure 5A).
Figure 5. TLR4 signal regulates EGFR tyrosine phosphorylation and amphiregulin expression in colonocytes.
A. Western blot analysis of phosphorylated EGFR and EGFR in the colon. Results from three representative samples obtained from WT or TLR4−/− mice are shown. 25μg/lane of protein was loaded per lane. The membrane was sequentially probed for phospho-EGFR and EGFR. Beta-actin was used to demonstrate equal protein loading.
B. LPS induces the release of amphiregulin protein. SW480 cells were treated with vehicle (control) or LPS (2 μg/ml) for the indicated periods. Amphiregulin concentration in supernatants was measured by ELISA. Data are expressed as mean ± SD of relative values of expression in three individual experiments with triplicate samples (*P < 0.05).
C. LPS-mediated induction of amphiregulin mRNA is TLR4-dependent. SW480 cells were transiently transfected with control siRNA or siRNA against TLR4 and then stimulated with LPS (2μg/ml) for the indicated periods of time. Untransfected control samples were not LPS treated. Levels of amphiregulin mRNA were determined by real time PCR. The data are represented as mean ± SD of relative values of expression in three individual experiments with triplicate samples (*P < 0.05). Inset: Western blot shows TLR4 expression with medium alone, control siRNA, and TLR4 siRNA in SW480 cells. The last bar is THP-1 cells as a positive control.
D. LPS-mediated activation of EGFR is ligand-dependent. SW480 cells were pre-treated with antibodies to the ligand-binding site of EGFR, anti-amphiregulin antibody or control IgG for two hours. Subsequently, cells were stimulated with LPS (2μg/ml) for 30 minutes. Blots of whole cell lysates (25 μg/lane) were sequentially probed for phospho-EGFR and EGFR. The data are one representative experiment of three with similar results. β-actin was used as an internal control for protein loading.
E. TLR4 deficiency is associated with reduced colonic mucosal production of amphiregulin. The production of amphiregulin was measured in colonic mucosa from WT (n=10) and TLR4−/− (n=8) mice. Data are expressed as mean ± SD (*P < 0.05).
Additional studies were carried out to elucidate the mechanism by which activation of TLR4 stimulates EGFR tyrosine kinase activity. Amphiregulin, an EGFR ligand, is believed to play a role in colon carcinogenesis 36 and can induce COX-2 37. Hence, experiments were carried out to determine whether amphiregulin plays a role in TLR4-mediated activation of EGFR. Treatment with LPS caused a several-fold increase in production of amphiregulin in SW480 cells (Figure 5B). In contrast to nonspecific siRNA, LPS-mediated induction of amphiregulin was abrogated by siRNA to TLR4 (Figure 5C). We next addressed whether ligand binding was involved in LPS-mediated stimulation of EGFR tyrosine kinase activity. We found that antibody blockade of the EGFR ligand-binding site as well as a neutralizing antibody to amphiregulin abrogated LPS-mediated activation of EGFR (Figure 5D).
Given the compelling evidence that amphiregulin links the TLR4 pathway with activation of EGFR in human colonocytes, we hypothesized that TLR4−/− mice have reduced levels of amphiregulin in the colon. Mucosal amphiregulin release measured by ELISA was also significantly reduced in TLR4−/− mice compared with WT mice in the CAC model (Figure 5E). Collectively, these results strongly suggest that the reduction in EGFR tyrosine kinase activity in colonic mucosa from TLR4−/− mice (Figure 5A) is likely to be explained at least, in part, by reduced production of amphiregulin.
Discussion
The present study establishes a molecular link between TLR4 and colon cancer in the setting of chronic inflammation (Figure 6). We demonstrate for the first time that TLR4 is highly expressed in colon cancers from patients with long-standing ulcerative colitis and in colonic tumors in a murine model of CAC. Our studies show a critical role for TLR4 in development of colitis-associated dysplasia since, in the absence of TLR4, colonic polyps do not occur. The ligand for TLR4, LPS, is abundant in the colonic lumen raising the intriguing possibility that colonic bacteria induce growth of colonic tumors through activation of TLR4. Chronic infection or chronic inflammation resulting in TLR4 activation may occur in other common malignancies, such as Helicobacter pylori and gastric cancer 38. In addition, hyaluronan, a non-bacterial TLR4 ligand present in chronically inflamed tissues, may activate TLR4 and contribute to carcinogenesis 39. Our results highlight the possibility of interfering with the host-microbial innate immune response to prevent or treat cancer.
Figure 6. Model of TLR4-mediated colon carcinogenesis.
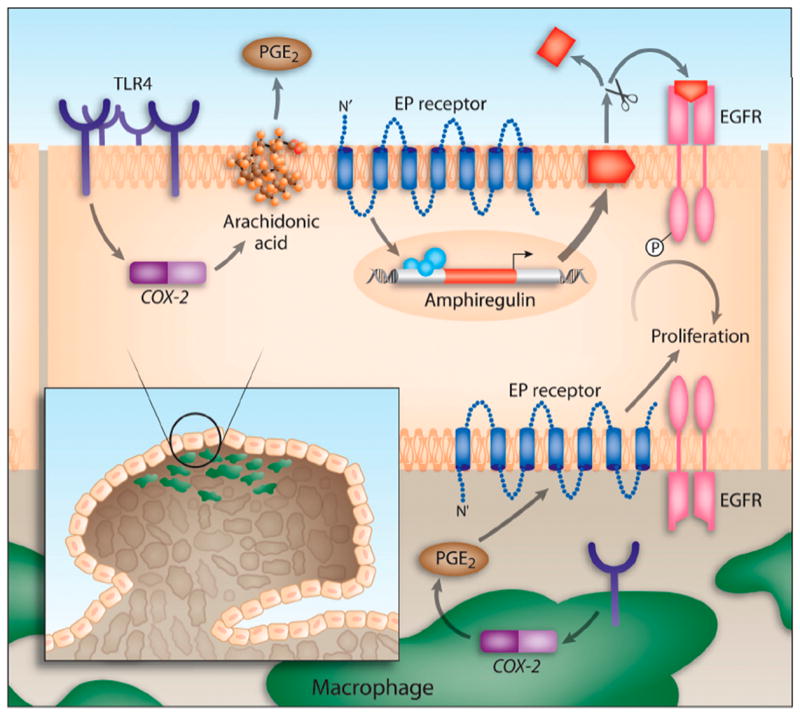
TLR4 expression is increased in chronic intestinal inflammation. TLR4 signaling in response to LPS induces Cox-2 expression and PGE2 production. PGE2 through its receptors (EP) can act in a paracrine or autocrine fashion on colonocytes to stimulate the expression and release of amphiregulin, an EGFR ligand. EGFR signaling is associated with increased proliferation of colonocytes. Likewise, TLR4 expression in tumor-associated macrophages may also respond to LPS by inducing Cox-2 and PGE2, which may then act on the epithelium to stimulate proliferation of colonocytes.
NF-κB activation in both colonocytes and macrophages has been shown to be important in development of CAC and supports the connection between inflammation and colon cancer 9. In Greten et al.’s study examining the contribution of epithelial versus macrophage-dependent activation of NF-κB on colonic tumorigenesis, epithelial NF-κB activation was necessary for high tumor numbers whereas macrophage-dependent NF-κB activation contributed to tumor size. NF-κB can be activated by many upstream stimuli including TLRs. Our studies suggest that TLR4 may be a principal receptor, upstream of NF-κB, promoting the development of colonic tumors especially during the acute inflammatory stage. In our model, TLR4 expression is found in both epithelial cells as well as lamina propria mononuclear cells in neoplastic areas. In vitro, TLR4 can directly induce proliferation of IEC through EGFR activation 18. In vivo, there are likely to be multiple pathways leading to dysregulated epithelial proliferation in response to TLR4.
The intestinal epithelium is in intimate contact with the underlying lamina propria containing macrophages and subepithelial myofibroblasts; these cells have been shown to express TLR4 15, 16. Tumor-associated macrophages play a clear role in supporting tumor growth in a variety of systems 40. Some of the effects on the intestinal epithelium may be mediated by TLR4-dependent signals derived from the stroma. We show that Cox-2 expression and PGE2 levels are markedly different between WT versus TLR4−/− colons. Immunostaining demonstrates expression of Cox-2 predominantly in tumor-associated macrophages and myofibroblasts, but we know from our previous work that IEC also express Cox-2 in a TLR4-dependent way following acute injury 18. The finding of increased PGE2 production in our model is consistent with previous evidence of increased levels of PGE2 and deregulated expression of enzymes involved in the synthesis and catabolism of PGs in inflamed human colonic mucosa and colon cancers 41, 42. Recent work by Brown et al. demonstrates that mesenchymal stromal cells express Cox-2 and migrate to colonic epithelial progenitors during injury in a MyD88-dependent fashion 43. Without the migration of PGE2-producing stromal cells, epithelial proliferation is impaired. In addition, recent work demonstrates that bacteria can produce reactive oxygen intermediates that induce Cox-2 expression in macrophages leading, in turn, to chromosomal instability 11. These data highlight the interplay of bacteria and host cells in promoting colorectal cancer.
The reciprocal interaction between the epithelium and the stroma is complex. PGE2 can stimulate the release of EGFR ligands, such as amphiregulin 36. Thus, bioactive lipids produced by the stroma can amplify IEC proliferation through induction of trophic growth factors. At least in vitro, we show that amphiregulin is the principal contributor to EGFR activation. We have not shown, however, that the decrease in amphiregulin in TLR4−/− mice is the reason that EGFR activation is decreased. Likewise, EGFR signaling has been shown previously to increase Cox-2 expression and PGE2 secretion resulting in a positive feedback loop that contributes to deregulated cell proliferation44. More work is necessary to understand whether a positive feedback loop between EGFR and Cox-2 is at play in our model. In summary, several well-established mediators of human colon carcinogenesis, namely Cox-2, PGE2, and the EGFR, are modulated by TLR4 signaling.
For our studies, we have chosen the AOM-DSS model of CAC 24. AOM is a colonic genotoxic carcinogen that is extensively used for the investigation of colorectal carcinogenesis in rodents. Although by itself, AOM does not cause dysplasia or cancer in C57/BL6 mice, in combination with repeated cycles of DSS, it increases the incidence of dysplastic lesions 29. In particular, this model has been used to interrogate the inflammation-cancer link in the colon 9. Although no single animal model suitably reproduces all the features of human CAC, increased Cox-2 expression and enhanced PGE2 levels characterize this model and are reminiscent of human CAC 45.
Another question raised by our work is whether the effect of TLR4 is unique or whether other TLRs can contribute to development of malignancy. The colonic microbiota is diverse and should activate many TLRs. It is possible that other TLRs have the potential to induce dysplasia but that their contribution is weaker than TLR4. The prediction would be that the absence of all TLR signaling would also prevent colon cancer. We have evaluated whether CAC could be induced in MyD88−/− mice and all 15 mice succumbed from colitis following the first cycle of DSS (data not shown). MyD88−/− mice are fragile and may die as a result of sepsis, hemorrhage, or both, at least in part because MyD88 and TLR signaling are necessary for repair of the intestinal epithelium. It also suggests that targeting MyD88 as a therapeutic or chemopreventive intervention is likely to be impractical.
In summary, our results point to a critical role of TLR4 in development of colon cancer. Based on this study, we postulate that targeted inhibition of TLR4 may be effective in preventing development of colon cancer in IBD. TLR4 inhibitors are currently being evaluated in clinical trials of sepsis 46. One of the issues with the use of TLR4 antagonists is that our previous work suggests that during acute injury, TLR4 signaling is beneficial. Perhaps the value of TLR4 antagonists would be in patients with quiescent colitis with dysplasia or at high risk for dysplasia. Ideally, TLR4 signaling would be dampened but not eliminated. Cox-2 inhibitors are effective in reducing the risk of adenomatous polyps but unfortunately long-term use is associated with increased cardiovascular complications 47, 48. Therefore, new approaches to preventing colon carcinogenesis are required. Our studies will permit rational therapies to be developed that combine blockade of TLR4 and other signaling pathways, such as EGFR signaling, in the prevention or treatment of CAC.
Supplementary Material
Acknowledgments
Supported by NIH grants AI052266 (MTA), DK069594 (MTA), CA111469 (KS), Career Development Award from CCFA (MF), Uehara Memorial Foundation Research Fellowship (MF), and the New York Crohn’s Foundation (AJD).
Abbreviations
- TLR
toll-like receptor
- WT
wild-type
- DSS
dextran sodium sulfate
- PAMP
pathogen associated molecular pattern
- LPS
lipopolysaccharide
- Cox
cyclooxygenase
- EGFR
epidermal growth factor receptor
- PG
prostaglandin
- IEC
intestinal epithelial cells
Footnotes
The authors have no conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sin DD, Man SF, McWilliams A, Lam S. Progression of airway dysplasia and C-reactive protein in smokers at high risk of lung cancer. Am J Respir Crit Care Med. 2006;173:535–9. doi: 10.1164/rccm.200508-1305OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rao VP, Poutahidis T, Ge Z, Nambiar PR, Boussahmain C, Wang YY, Horwitz BH, Fox JG, Erdman SE. Innate immune inflammatory response against enteric bacteria Helicobacter hepaticus induces mammary adenocarcinoma in mice. Cancer Res. 2006;66:7395–400. doi: 10.1158/0008-5472.CAN-06-0558. [DOI] [PubMed] [Google Scholar]
- 3.Gunter MJ, Stolzenberg-Solomon R, Cross AJ, Leitzmann MF, Weinstein S, Wood RJ, Virtamo J, Taylor PR, Albanes D, Sinha R. A prospective study of serum C-reactive protein and colorectal cancer risk in men. Cancer Res. 2006;66:2483–7. doi: 10.1158/0008-5472.CAN-05-3631. [DOI] [PubMed] [Google Scholar]
- 4.Surveillance, Epidemiology, and End Results (SEER) Program and the National Center for Health Statistics. http://seer.cancer.gov/
- 5.Itzkowitz SH, Yio X. Inflammation and cancer IV. Colorectal cancer in inflammatory bowel disease: the role of inflammation. Am J Physiol Gastrointest Liver Physiol. 2004;287:G7–17. doi: 10.1152/ajpgi.00079.2004. [DOI] [PubMed] [Google Scholar]
- 6.Eaden JA, Abrams KR, Mayberry JF. The risk of colorectal cancer in ulcerative colitis: a meta-analysis. Gut. 2001;48:526–35. doi: 10.1136/gut.48.4.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rutter M, Saunders B, Wilkinson K, Rumbles S, Schofield G, Kamm M, Williams C, Price A, Talbot I, Forbes A. Severity of inflammation is a risk factor for colorectal neoplasia in ulcerative colitis. Gastroenterology. 2004;126:451–9. doi: 10.1053/j.gastro.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 8.Gupta RB, Harpaz N, Itzkowitz S, Hossain S, Matula S, Kornbluth A, Bodian C, Ullman T. Histologic Inflammation Is a Risk Factor for Progression to Colorectal neoplasia in Ulcerative Colitis: a Cohort Study. Gastroenterology. 2007 doi: 10.1053/j.gastro.2007.08.001. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Greten FR, Eckmann L, Greten TF, Park JM, Li ZW, Egan LJ, Kagnoff MF, Karin M. IKKbeta links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell. 2004;118:285–96. doi: 10.1016/j.cell.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 10.Hope ME, Hold GL, Kain R, El-Omar EM. Sporadic colorectal cancer--role of the commensal microbiota. FEMS Microbiol Lett. 2005;244:1–7. doi: 10.1016/j.femsle.2005.01.029. [DOI] [PubMed] [Google Scholar]
- 11.Wang X, Huycke MM. Extracellular superoxide production by Enterococcus faecalis promotes chromosomal instability in mammalian cells. Gastroenterology. 2007;132:551–61. doi: 10.1053/j.gastro.2006.11.040. [DOI] [PubMed] [Google Scholar]
- 12.Sellon RK, Tonkonogy S, Schultz M, Dieleman LA, Grenther W, Balish E, Rennick DM, Sartor RB. Resident enteric bacteria are necessary for development of spontaneous colitis and immune system activation in interleukin-10-deficient mice. Infect Immun. 1998;66:5224–31. doi: 10.1128/iai.66.11.5224-5231.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kado S, Uchida K, Funabashi H, Iwata S, Nagata Y, Ando M, Onoue M, Matsuoka Y, Ohwaki M, Morotomi M. Intestinal microflora are necessary for development of spontaneous adenocarcinoma of the large intestine in T-cell receptor beta chain and p53 double-knockout mice. Cancer Res. 2001;61:2395–8. [PubMed] [Google Scholar]
- 14.Abreu MT, Vora P, Faure E, Thomas LS, Arnold ET, Arditi M. Decreased expression of Toll-like receptor-4 and MD-2 correlates with intestinal epithelial cell protection against dysregulated proinflammatory gene expression in response to bacterial lipopolysaccharide. J Immunol. 2001;167:1609–16. doi: 10.4049/jimmunol.167.3.1609. [DOI] [PubMed] [Google Scholar]
- 15.Otte JM, Cario E, Podolsky DK. Mechanisms of cross hyporesponsiveness to Toll-like receptor bacterial ligands in intestinal epithelial cells. Gastroenterology. 2004;126:1054–70. doi: 10.1053/j.gastro.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 16.Hausmann M, Kiessling S, Mestermann S, Webb G, Spottl T, Andus T, Scholmerich J, Herfarth H, Ray K, Falk W, Rogler G. Toll-like receptors 2 and 4 are up-regulated during intestinal inflammation. Gastroenterology. 2002;122:1987–2000. doi: 10.1053/gast.2002.33662. [DOI] [PubMed] [Google Scholar]
- 17.Cario E, Gerken G, Podolsky DK. Toll-like receptor 2 enhances ZO-1-associated intestinal epithelial barrier integrity via protein kinase C. Gastroenterology. 2004;127:224–38. doi: 10.1053/j.gastro.2004.04.015. [DOI] [PubMed] [Google Scholar]
- 18.Fukata M, Chen A, Klepper A, Krishnareddy S, Vamadevan AS, Thomas LS, Xu R, Inoue H, Arditi M, Dannenberg AJ, Abreu MT. Cox-2 Is Regulated by Toll-Like Receptor-4 (TLR4) Signaling: Role in Proliferation and Apoptosis in the Intestine. Gastroenterology. 2006;131:862–77. doi: 10.1053/j.gastro.2006.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang B, Zhao J, Li H, He KL, Chen Y, Chen SH, Mayer L, Unkeless JC, Xiong H. Toll-like receptors on tumor cells facilitate evasion of immune surveillance. Cancer Res. 2005;65:5009–14. doi: 10.1158/0008-5472.CAN-05-0784. [DOI] [PubMed] [Google Scholar]
- 20.Wang D, Mann JR, DuBois RN. The role of prostaglandins and other eicosanoids in the gastrointestinal tract. Gastroenterology. 2005;128:1445–61. doi: 10.1053/j.gastro.2004.09.080. [DOI] [PubMed] [Google Scholar]
- 21.Dannenberg AJ, Lippman SM, Mann JR, Subbaramaiah K, DuBois RN. Cyclooxygenase-2 and epidermal growth factor receptor: pharmacologic targets for chemoprevention. J Clin Oncol. 2005;23:254–66. doi: 10.1200/JCO.2005.09.112. [DOI] [PubMed] [Google Scholar]
- 22.Okayasu I, Ohkusa T, Kajiura K, Kanno J, Sakamoto S. Promotion of colorectal neoplasia in experimental murine ulcerative colitis. Gut. 1996;39:87–92. doi: 10.1136/gut.39.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fukata M, Michelsen KS, Eri R, Thomas LS, Hu B, Lukasek K, Nast CC, Lechago J, Xu R, Naiki Y, Soliman A, Arditi M, Abreu MT. Toll-like receptor-4 is required for intestinal response to epithelial injury and limiting bacterial translocation in a murine model of acute colitis. Am J Physiol Gastrointest Liver Physiol. 2005;288:G1055–65. doi: 10.1152/ajpgi.00328.2004. [DOI] [PubMed] [Google Scholar]
- 24.Cooper HS, Murthy S, Kido K, Yoshitake H, Flanigan A. Dysplasia and cancer in the dextran sulfate sodium mouse colitis model. Relevance to colitis-associated neoplasia in the human: a study of histopathology, B-catenin and p53 expression and the role of inflammation. Carcinogenesis. 2000;21:757–68. doi: 10.1093/carcin/21.4.757. [DOI] [PubMed] [Google Scholar]
- 25.Morteau O, Morham SG, Sellon R, Dieleman LA, Langenbach R, Smithies O, Sartor RB. Impaired mucosal defense to acute colonic injury in mice lacking cyclooxygenase-1 or cyclooxygenase-2. J Clin Invest. 2000;105:469–78. doi: 10.1172/JCI6899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abreu MT, Vora P, Faure E, Thomas LS, Arnold ET, Arditi M. Decreased expression of Toll-like receptor-4 and MD-2 correlates with intestinal epithelial cell protection against dysregulated proinflammatory gene expression in response to bacterial lipopolysaccharide. Journal of Immunology. 2001;167:1609–16. doi: 10.4049/jimmunol.167.3.1609. [DOI] [PubMed] [Google Scholar]
- 27.Abreu MT, Arnold ET, Thomas LS, Gonsky R, Zhou Y, Hu B, Arditi M. TLR4 and MD-2 expression is regulated by immune-mediated signals in human intestinal epithelial cells. Journal of Biological Chemistry. 2002;277:20431–7. doi: 10.1074/jbc.M110333200. [DOI] [PubMed] [Google Scholar]
- 28.Cario E, Podolsky DK. Differential alteration in intestinal epithelial cell expression of toll-like receptor 3 (TLR3) and TLR4 in inflammatory bowel disease. Infection & Immunity. 2000;68:7010–7. doi: 10.1128/iai.68.12.7010-7017.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Suzuki R, Kohno H, Sugie S, Nakagama H, Tanaka T. Strain differences in the susceptibility to azoxymethane and dextran sodium sulfate-induced colon carcinogenesis in mice. Carcinogenesis. 2006;27:162–9. doi: 10.1093/carcin/bgi205. [DOI] [PubMed] [Google Scholar]
- 30.Itzkowitz SH, Harpaz N. Diagnosis and management of dysplasia in patients with inflammatory bowel diseases. Gastroenterology. 2004;126:1634–48. doi: 10.1053/j.gastro.2004.03.025. [DOI] [PubMed] [Google Scholar]
- 31.Rumio C, Besusso D, Arnaboldi F, Palazzo M, Selleri S, Gariboldi S, Akira S, Uematsu S, Bignami P, Ceriani V, Menard S, Balsari A. Activation of smooth muscle and myenteric plexus cells of jejunum via Toll-like receptor 4. J Cell Physiol. 2006;208:47–54. doi: 10.1002/jcp.20632. [DOI] [PubMed] [Google Scholar]
- 32.Suganuma M, Okabe S, Marino MW, Sakai A, Sueoka E, Fujiki H. Essential role of tumor necrosis factor alpha (TNF-alpha) in tumor promotion as revealed by TNF-alpha-deficient mice. Cancer Res. 1999;59:4516–8. [PubMed] [Google Scholar]
- 33.Castellone MD, Teramoto H, Williams BO, Druey KM, Gutkind JS. Prostaglandin E2 promotes colon cancer cell growth through a Gs-axin-beta-catenin signaling axis. Science. 2005;310:1504–10. doi: 10.1126/science.1116221. [DOI] [PubMed] [Google Scholar]
- 34.Pai R, Soreghan B, Szabo IL, Pavelka M, Baatar D, Tarnawski AS. Prostaglandin E2 transactivates EGF receptor: a novel mechanism for promoting colon cancer growth and gastrointestinal hypertrophy. Nat Med. 2002;8:289–93. doi: 10.1038/nm0302-289. [DOI] [PubMed] [Google Scholar]
- 35.Buchanan FG, Wang D, Bargiacchi F, DuBois RN. Prostaglandin E2 regulates cell migration via the intracellular activation of the epidermal growth factor receptor. J Biol Chem. 2003;278:35451–7. doi: 10.1074/jbc.M302474200. [DOI] [PubMed] [Google Scholar]
- 36.Shao J, Lee SB, Guo H, Evers BM, Sheng H. Prostaglandin E2 stimulates the growth of colon cancer cells via induction of amphiregulin. Cancer Res. 2003;63:5218–23. [PubMed] [Google Scholar]
- 37.Moraitis D, Du B, De Lorenzo MS, Boyle JO, Weksler BB, Cohen EG, Carew JF, Altorki NK, Kopelovich L, Subbaramaiah K, Dannenberg AJ. Levels of cyclooxygenase-2 are increased in the oral mucosa of smokers: evidence for the role of epidermal growth factor receptor and its ligands. Cancer Res. 2005;65:664–70. [PubMed] [Google Scholar]
- 38.Kawahara T, Teshima S, Oka A, Sugiyama T, Kishi K, Rokutan K. Type I Helicobacter pylori Lipopolysaccharide Stimulates Toll-Like Receptor 4 and Activates Mitogen Oxidase 1 in Gastric Pit Cells. Infection & Immunity. 2001;69:4382–9. doi: 10.1128/IAI.69.7.4382-4389.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Termeer C, Benedix F, Sleeman J, Fieber C, Voith U, Ahrens T, Miyake K, Freudenberg M, Galanos C, Simon JC. Oligosaccharides of Hyaluronan activate dendritic cells via toll-like receptor 4. Journal of Experimental Medicine. 2002;195:99–111. doi: 10.1084/jem.20001858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.de Visser KE, Eichten A, Coussens LM. Paradoxical roles of the immune system during cancer development. Nat Rev Cancer. 2006;6:24–37. doi: 10.1038/nrc1782. [DOI] [PubMed] [Google Scholar]
- 41.Subbaramaiah K, Yoshimatsu K, Scherl E, Das KM, Glazier KD, Golijanin D, Soslow RA, Tanabe T, Naraba H, Dannenberg AJ. Microsomal prostaglandin E synthase-1 is overexpressed in inflammatory bowel disease. Evidence for involvement of the transcription factor Egr-1. J Biol Chem. 2004;279:12647–58. doi: 10.1074/jbc.M312972200. [DOI] [PubMed] [Google Scholar]
- 42.Otani T, Yamaguchi K, Scherl E, Du B, Tai HH, Greifer M, Petrovic L, Daikoku T, Dey SK, Subbaramaiah K, Dannenberg AJ. Levels of NAD(+)-dependent 15-hydroxyprostaglandin dehydrogenase are reduced in inflammatory bowel disease: evidence for involvement of TNF-alpha. Am J Physiol Gastrointest Liver Physiol. 2006;290:G361–8. doi: 10.1152/ajpgi.00348.2005. [DOI] [PubMed] [Google Scholar]
- 43.Brown SL, Riehl TE, Walker MR, Geske MJ, Doherty JM, Stenson WF, Stappenbeck TS. Myd88-dependent positioning of Ptgs2-expressing stromal cells maintains colonic epithelial proliferation during injury. J Clin Invest. 2007;117:258–69. doi: 10.1172/JCI29159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Coffey RJ, Hawkey CJ, Damstrup L, Graves-Deal R, Daniel VC, Dempsey PJ, Chinery R, Kirkland SC, DuBois RN, Jetton TL, Morrow JD. Epidermal growth factor receptor activation induces nuclear targeting of cyclooxygenase-2, basolateral release of prostaglandins, and mitogenesis in polarizing colon cancer cells. Proc Natl Acad Sci U S A. 1997;94:657–62. doi: 10.1073/pnas.94.2.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kohno H, Suzuki R, Sugie S, Tanaka T. Suppression of colitis-related mouse colon carcinogenesis by a COX-2 inhibitor and PPAR ligands. BMC Cancer. 2005;5:46. doi: 10.1186/1471-2407-5-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ii M, Matsunaga N, Hazeki K, Nakamura K, Takashima K, Seya T, Hazeki O, Kitazaki T, Iizawa Y. A novel cyclohexene derivative, ethyl (6R)-6-[N-(2-Chloro-4-fluorophenyl)sulfamoyl]cyclohex-1-ene-1-carboxylate (TAK-242), selectively inhibits toll-like receptor 4-mediated cytokine production through suppression of intracellular signaling. Mol Pharmacol. 2006;69:1288–95. doi: 10.1124/mol.105.019695. [DOI] [PubMed] [Google Scholar]
- 47.Arber N, Eagle CJ, Spicak J, Racz I, Dite P, Hajer J, Zavoral M, Lechuga MJ, Gerletti P, Tang J, Rosenstein RB, Macdonald K, Bhadra P, Fowler R, Wittes J, Zauber AG, Solomon SD, Levin B. Celecoxib for the prevention of colorectal adenomatous polyps. N Engl J Med. 2006;355:885–95. doi: 10.1056/NEJMoa061652. [DOI] [PubMed] [Google Scholar]
- 48.Bertagnolli MM, Eagle CJ, Zauber AG, Redston M, Solomon SD, Kim K, Tang J, Rosenstein RB, Wittes J, Corle D, Hess TM, Woloj GM, Boisserie F, Anderson WF, Viner JL, Bagheri D, Burn J, Chung DC, Dewar T, Foley TR, Hoffman N, Macrae F, Pruitt RE, Saltzman JR, Salzberg B, Sylwestrowicz T, Gordon GB, Hawk ET. Celecoxib for the prevention of sporadic colorectal adenomas. N Engl J Med. 2006;355:873–84. doi: 10.1056/NEJMoa061355. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.