Abstract
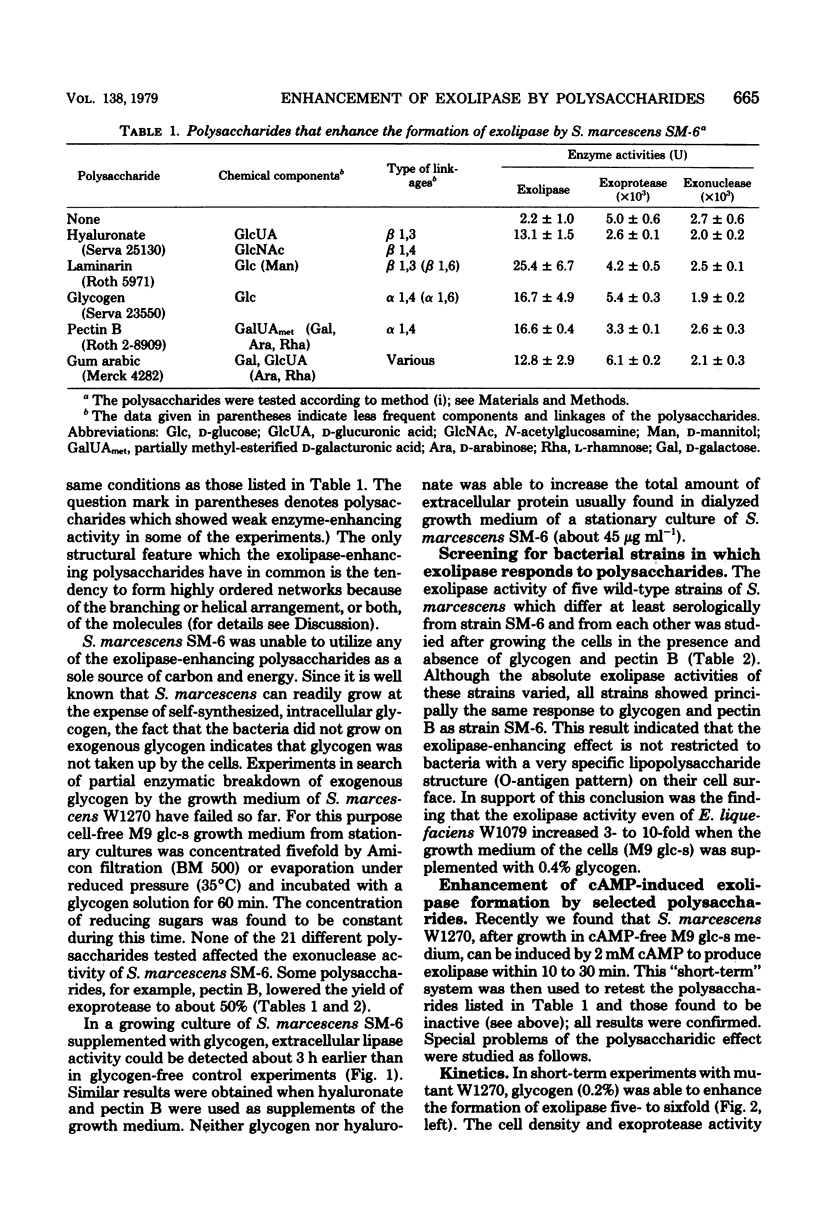
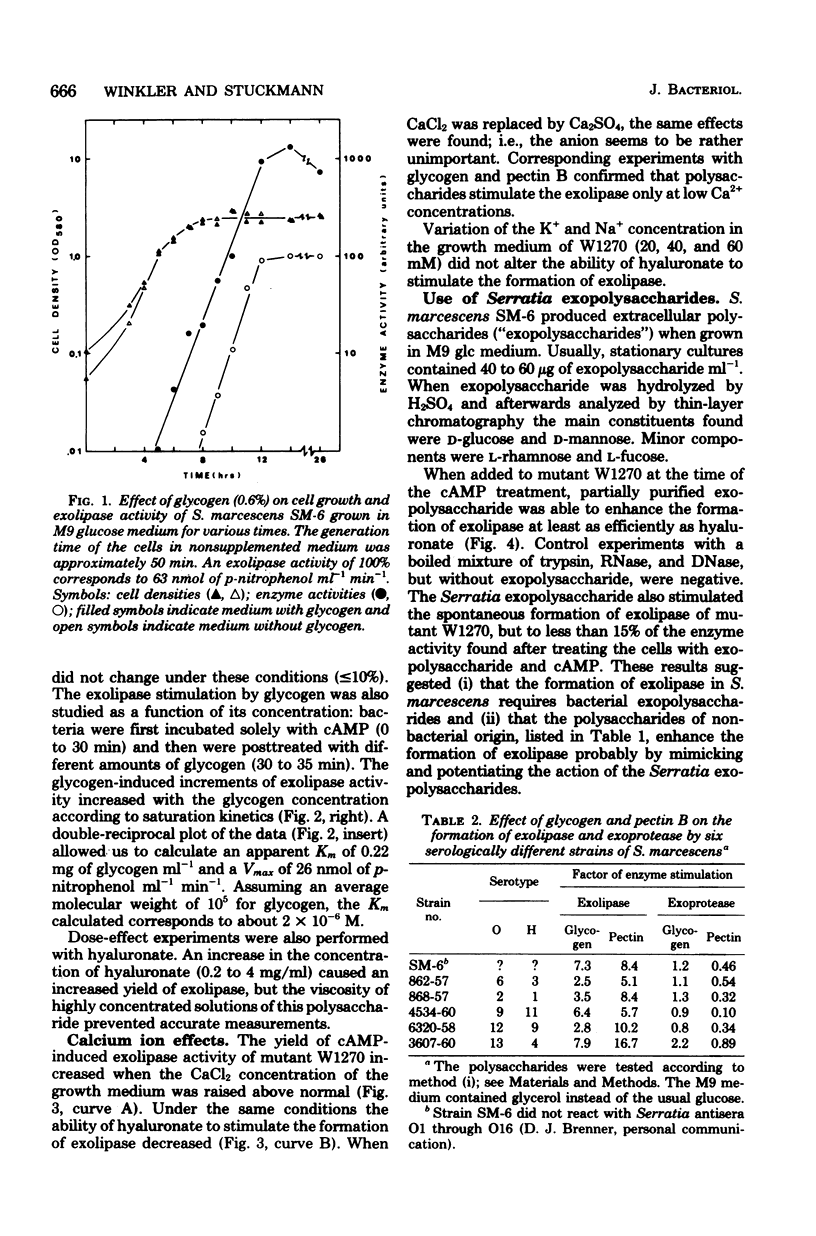
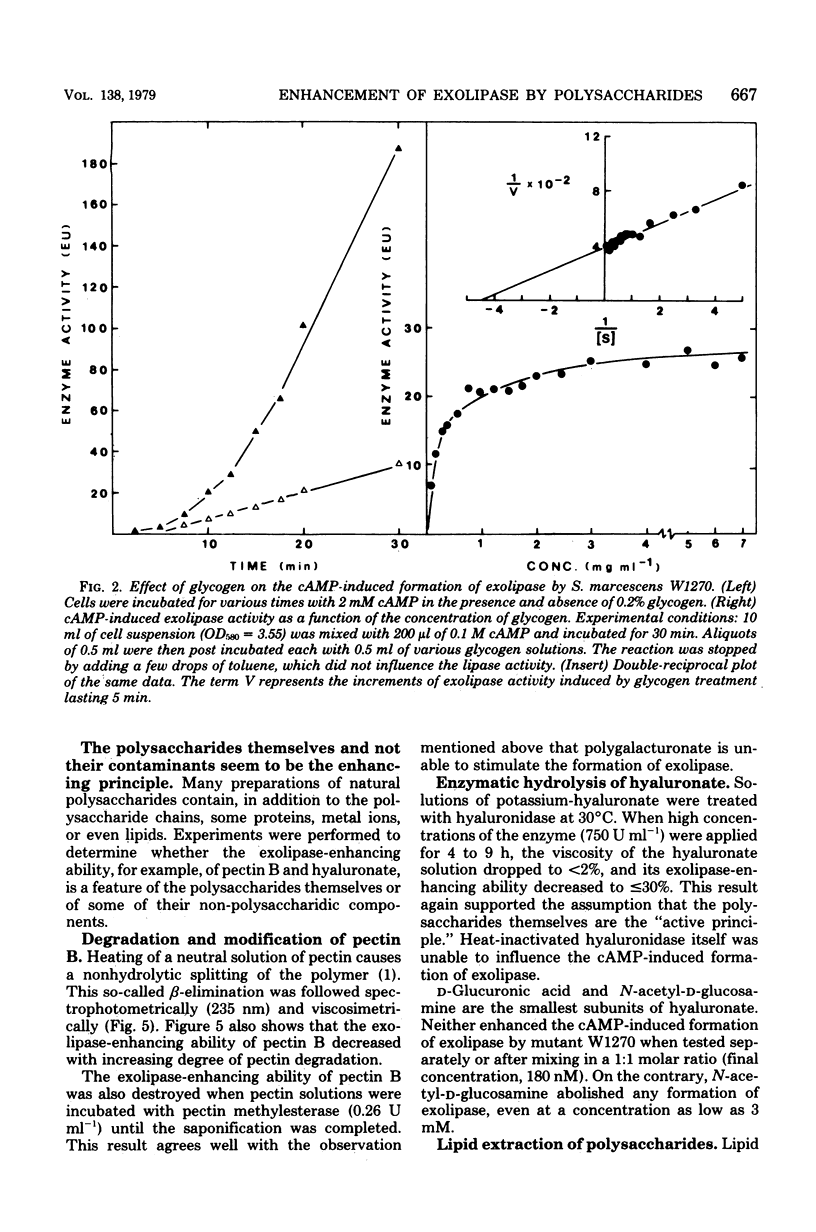
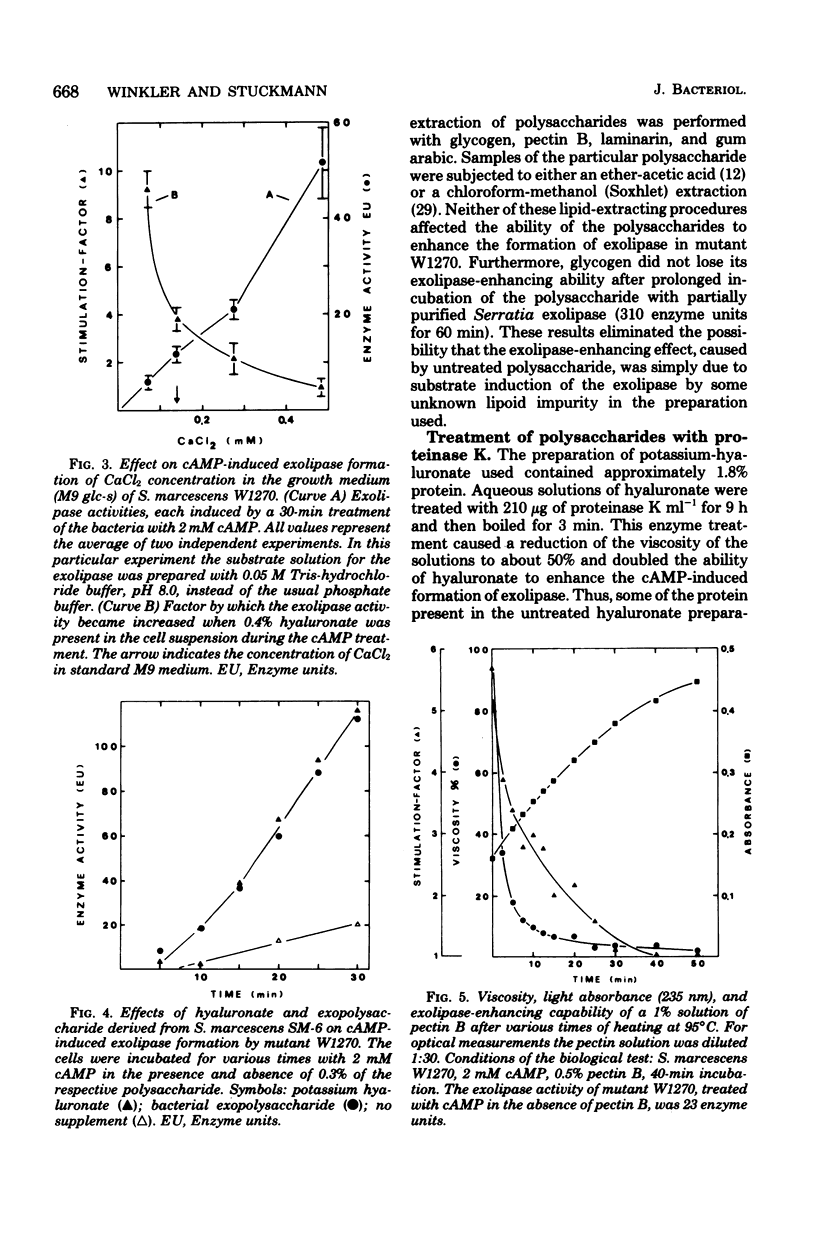
Among 21 different polysaccharides tested, 5 greatly enhanced the spontaneous and cyclic AMP-induced formation of exolipase: glycogen, hyaluronate, laminarin, pectin B, and gum arabic. These polysaccharides have in common the tendency to form highly ordered networks because of the branching or helical arrangement, or both, of their molecules. None of the polysaccharides could be utilized by the cells as the sole carbon source. Strong lipid extraction of four different polysaccharides did not reduce their exolipase-enhancing efficacy. At a constant cell density the stimulation of exolipase formation by various concentrations of glycogen followed saturation kinetics, suggesting a limited number of "sites" for the glycogen to act. The active principle present in a solution of pectin was destroyed by degradation (beta-elimination) of the polymer. Hyaluronate lost its exolipase-enhancing activity by exhaustive hydrolysis with hyaluronidase but was resistant to proteinase K. Exopolysaccharide, isolated from growth medium of Serratia marcescens SM-6, enhanced the exolipase formation as efficiently as hyaluronate. The results of this work are discussed mainly in terms of the "detachment hypothesis."
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALBERSHEIM P., NEUKOM H. DEUEL H: Splitting of pectin chain molecules in neutral solutions. Arch Biochem Biophys. 1960 Sep;90:46–51. doi: 10.1016/0003-9861(60)90609-3. [DOI] [PubMed] [Google Scholar]
- Braatz J. A., Heath E. C. The role of polysaccharide in the secretion of protein by Micrococcus sodonensis. J Biol Chem. 1974 Apr 25;249(8):2536–2547. [PubMed] [Google Scholar]
- Chajek T., Stein O., Stein Y. Lipoprotein lipase of cultured mesenchymal rat heart cells. I. Synthesis, secretion and releasability by heparin. Biochim Biophys Acta. 1978 Mar 30;528(3):456–465. doi: 10.1016/0005-2760(78)90035-8. [DOI] [PubMed] [Google Scholar]
- Dea I. C., Moorhouse R., Rees D. A., Arnott S., Guss J. M., Balazs E. A. Hyaluronic acid: a novel, double helical molecule. Science. 1973 Feb 9;179(4073):560–562. doi: 10.1126/science.179.4073.560. [DOI] [PubMed] [Google Scholar]
- Eaves G. N., Jeffries C. D. ISOLATION AND PROPERTIES OF AN EXOCELLULAR NUCLEASE OF SERRATIA MARCESCENS. J Bacteriol. 1963 Feb;85(2):273–278. doi: 10.1128/jb.85.2.273-278.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elyakova L. A., Zvyagintseva T. N. A study of the laminarins of some Far-Eastern, brown seaweeds. Carbohydr Res. 1974 Jun;34(2):241–248. doi: 10.1016/s0008-6215(00)82899-2. [DOI] [PubMed] [Google Scholar]
- LARNER J., ILLINGWORTH B., CORI G. T., CORI C. F. Structure of glycogens and amylopectins. II. Analysis by stepwise enzymatic degradation. J Biol Chem. 1952 Dec;199(2):641–651. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lampen J. O. External enzymes of yeast: their nature and formation. Antonie Van Leeuwenhoek. 1968;34(1):1–18. doi: 10.1007/BF02046409. [DOI] [PubMed] [Google Scholar]
- Laurent T. C. Interaction between proteins and glycosaminoglycans. Fed Proc. 1977 Jan;36(1):24–27. [PubMed] [Google Scholar]
- Lindahl U., Hök M. Glycosaminoglycans and their binding to biological macromolecules. Annu Rev Biochem. 1978;47:385–417. doi: 10.1146/annurev.bi.47.070178.002125. [DOI] [PubMed] [Google Scholar]
- Olivecrona T., Bengtsson G., Marklund S. E., Lindahl U., Hök M. Heparin-lipoprotein lipase interactions. Fed Proc. 1977 Jan;36(1):60–65. [PubMed] [Google Scholar]
- Park J. W., Chakrabarti B. Conformational transition of hyaluronic acid: carboxylic group participation and thermal effect. Biochim Biophys Acta. 1978 Jun 15;541(2):263–269. doi: 10.1016/0304-4165(78)90399-9. [DOI] [PubMed] [Google Scholar]
- Rees D. A. Shapely polysaccharides. The eighth Colworth medal lecture. Biochem J. 1972 Jan;126(2):257–273. doi: 10.1042/bj1260257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees D. A. Structure, conformation, and mechanism in the formation of polysaccharide gels and networks. Adv Carbohydr Chem Biochem. 1969;24:267–332. doi: 10.1016/s0065-2318(08)60352-2. [DOI] [PubMed] [Google Scholar]
- Rottem S., Leive L. Effect of variations in lipopolysaccharide on the fluidity of the outer membrane of Escherichia coli. J Biol Chem. 1977 Mar 25;252(6):2077–2081. [PubMed] [Google Scholar]
- Winkler U., Heller K. B., Folle B. Pleiotropic consequences of mutations towards antibiotic-hypersensitivity in Serratia marcescens. Arch Microbiol. 1978 Mar;116(3):259–268. doi: 10.1007/BF00417849. [DOI] [PubMed] [Google Scholar]
- Winkler U., Scholle H., Bohne L. Mutants of Serratia marcescens lacking cyclic nucleotide phosphodiesterase activity and requiring cyclic 3',5'-AMP for the utilization of various carbohydrates. Arch Microbiol. 1975 Jun 22;104(2):189–196. doi: 10.1007/BF00447323. [DOI] [PubMed] [Google Scholar]
- Wober W., Alaupović P. Studies on the protein moiety of endotoxin from gram-negative bacteria. Characterization of the protein moiety isolated by phenol treatment of endotoxin from Serratia marcescens 08 and Escherichia coli 0 141:K85(B). Eur J Biochem. 1971 Apr;19(3):340–356. doi: 10.1111/j.1432-1033.1971.tb01323.x. [DOI] [PubMed] [Google Scholar]
- van Alphen L., Verkleij A., Leunissen-Bijvelt J., Lugtenberg B. Architecture of the outer membrane of Escherichia coli. III. Protein-lipopolysaccharide complexes in intramembraneous particles. J Bacteriol. 1978 Jun;134(3):1089–1098. doi: 10.1128/jb.134.3.1089-1098.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]



