Abstract
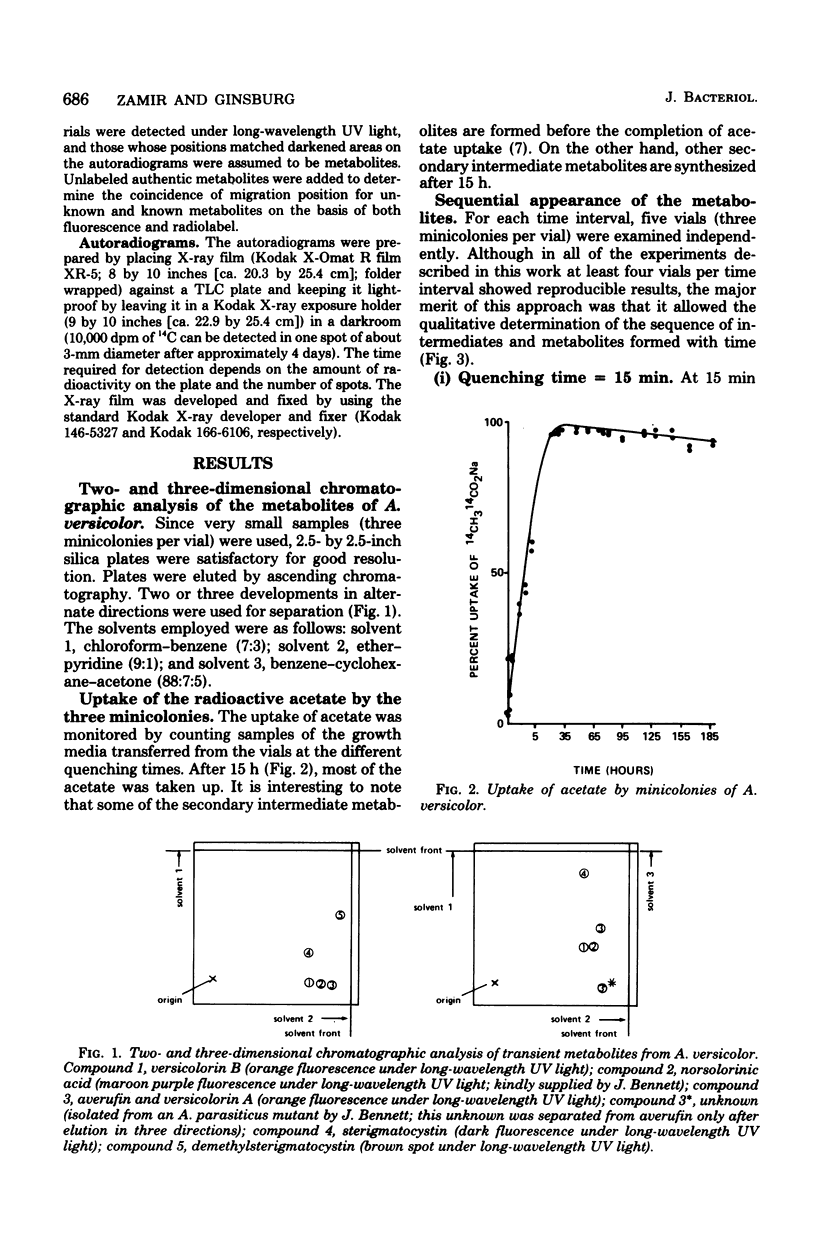
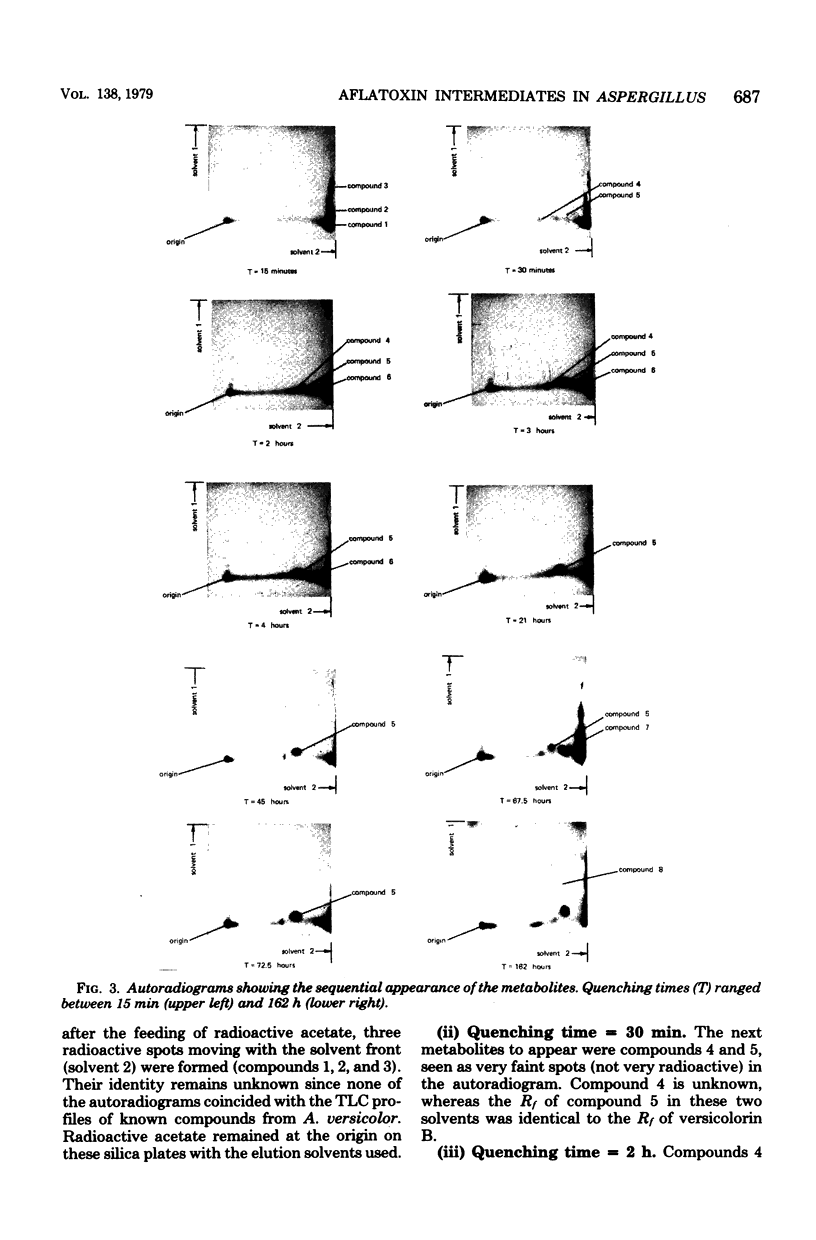
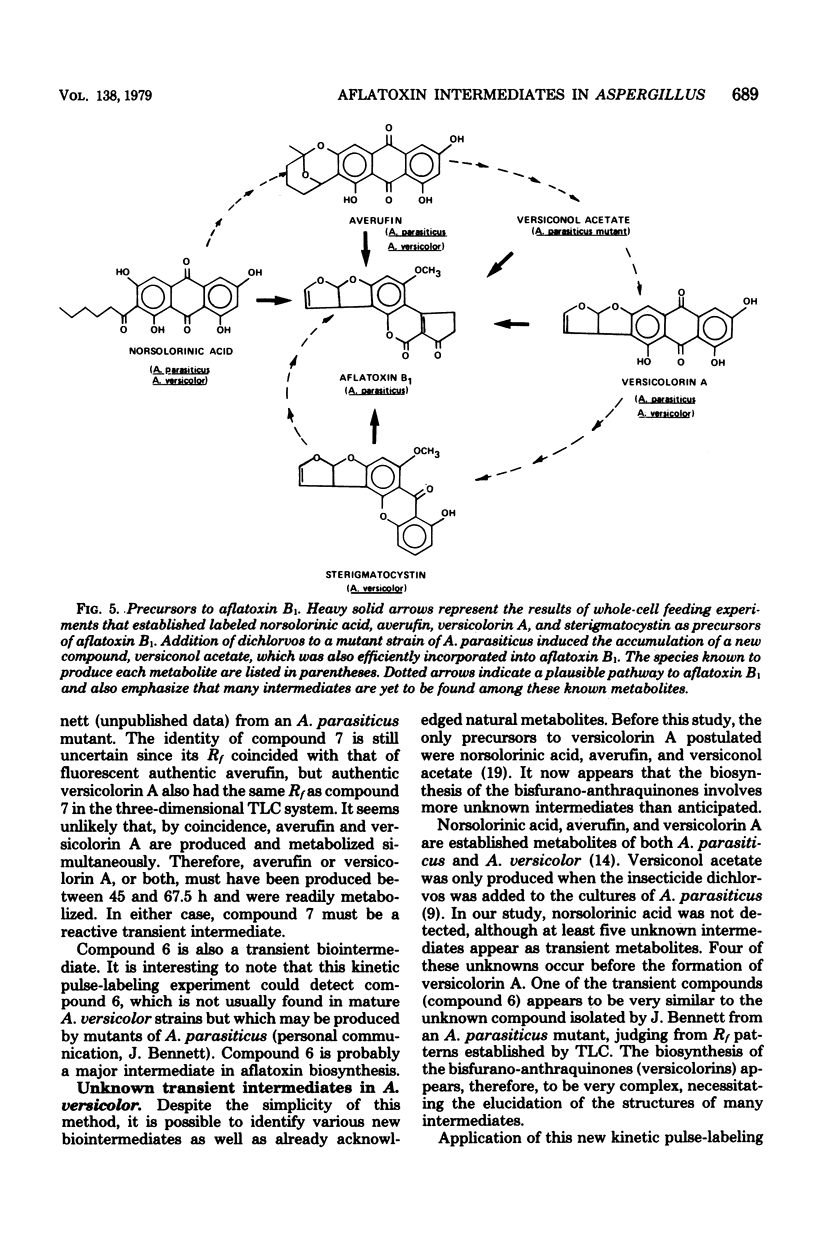
A simple technique was developed for the detection of intermediary metabolites of Aspergillus versicolor that are putative precursors of aflatoxin. Minicolony populations were allowed to metabolize [1,2-14C]acetate over various time intervals. The biosynthetic reactions were quenched by quick-freezing the minicolonies, the cells were disrupted, and the metabolites were extracted into acetone. Small silica thin-layer chromatographic plates were then used to separate any radioactive metabolites present. Elution in two or three different directions was often necessary. Radioautography of the thin-layer chromatography plates provided a sensitive assay for the appearance of the various intermediates in a timing pattern which implicated the sequence of formation. Transient intermediates were distinguished from dead-end metabolites by the rapid formation and disappearance of the former. At least five unknown precursors of versicolorin A, a dead-end metabolite, were recognized. The kinetic pulse-labeling technique should be generally applicable to other fungal species whenever the entrapment of intermediary metabolites in the mycelium poses and technical problem.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BATTAILE J., LOOMIS W. D. Biosynthesis of terpenes. II. The site and sequence of terpene formation in peppermint. Biochim Biophys Acta. 1961 Aug 19;51:545–552. doi: 10.1016/0006-3002(61)90612-6. [DOI] [PubMed] [Google Scholar]
- Biollaz M., Büchi G., Milne G. Biosynthesis of aflatoxins. J Am Chem Soc. 1968 Aug 28;90(18):5017–5019. doi: 10.1021/ja01020a042. [DOI] [PubMed] [Google Scholar]
- Biollaz M., Büchi G., Milne G. The biosynthesis of the aflatoxins. J Am Chem Soc. 1970 Feb 25;92(4):1035–1043. doi: 10.1021/ja00707a050. [DOI] [PubMed] [Google Scholar]
- Bu'Lock J. D., Shepherd D., Winstanley D. J. Regulation of 6-methylsalicylate and patulin synthesis in Penicillium urticae. Can J Microbiol. 1969 Mar;15(3):279–285. doi: 10.1139/m69-051. [DOI] [PubMed] [Google Scholar]
- Cox R. H., Churchill F., Cole R. J., Dormer J. W. Carbon-13 nuclear magnetic resonance studies of the structure and biosynthesis of versiconal acetate. J Am Chem Soc. 1977 Apr 27;99(9):3159–3161. doi: 10.1021/ja00451a049. [DOI] [PubMed] [Google Scholar]
- Forrester P. I., Gaucher G. M. Conversion of 6-methylsalicylic acid into patulin by Penicillium urticae. Biochemistry. 1972 Mar 14;11(6):1102–1107. doi: 10.1021/bi00756a025. [DOI] [PubMed] [Google Scholar]
- Hsieh D. P., Lin M. T., Yao R. C., Singh R. Biosynthesis of aflatoxin. Conversion of norsolorinic acid and other hypothetical intermediates into aflatoxin B1. J Agric Food Chem. 1976 Nov-Dec;24(6):1170–1174. doi: 10.1021/jf60208a018. [DOI] [PubMed] [Google Scholar]
- REITSEMA R. H., CRAMER F. J., SCULLY N. J., CHORNEY W. Essential oil synthesis in mint. J Pharm Sci. 1961 Jan;50:18–21. doi: 10.1002/jps.2600500104. [DOI] [PubMed] [Google Scholar]
- Singh R., Hsieh D. P. Aflatoxin biosynthetic pathway: elucidation by using blocked mutants of Aspergillus parasiticus. Arch Biochem Biophys. 1977 Jan 15;178(1):285–292. doi: 10.1016/0003-9861(77)90193-x. [DOI] [PubMed] [Google Scholar]
- Walker D. A., Crofts A. R. Photosynthesis. Annu Rev Biochem. 1970;39:389–428. doi: 10.1146/annurev.bi.39.070170.002133. [DOI] [PubMed] [Google Scholar]




