Abstract
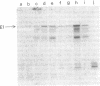
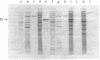
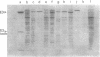
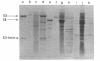
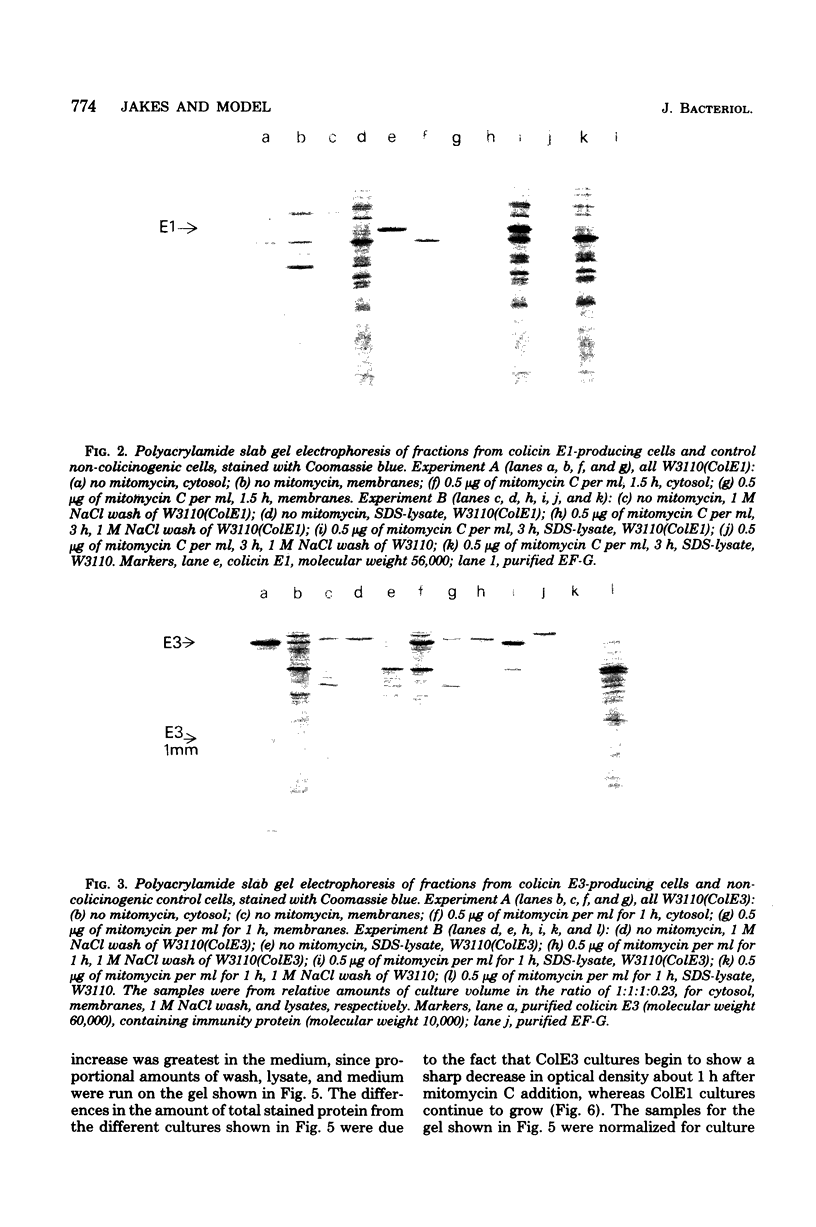
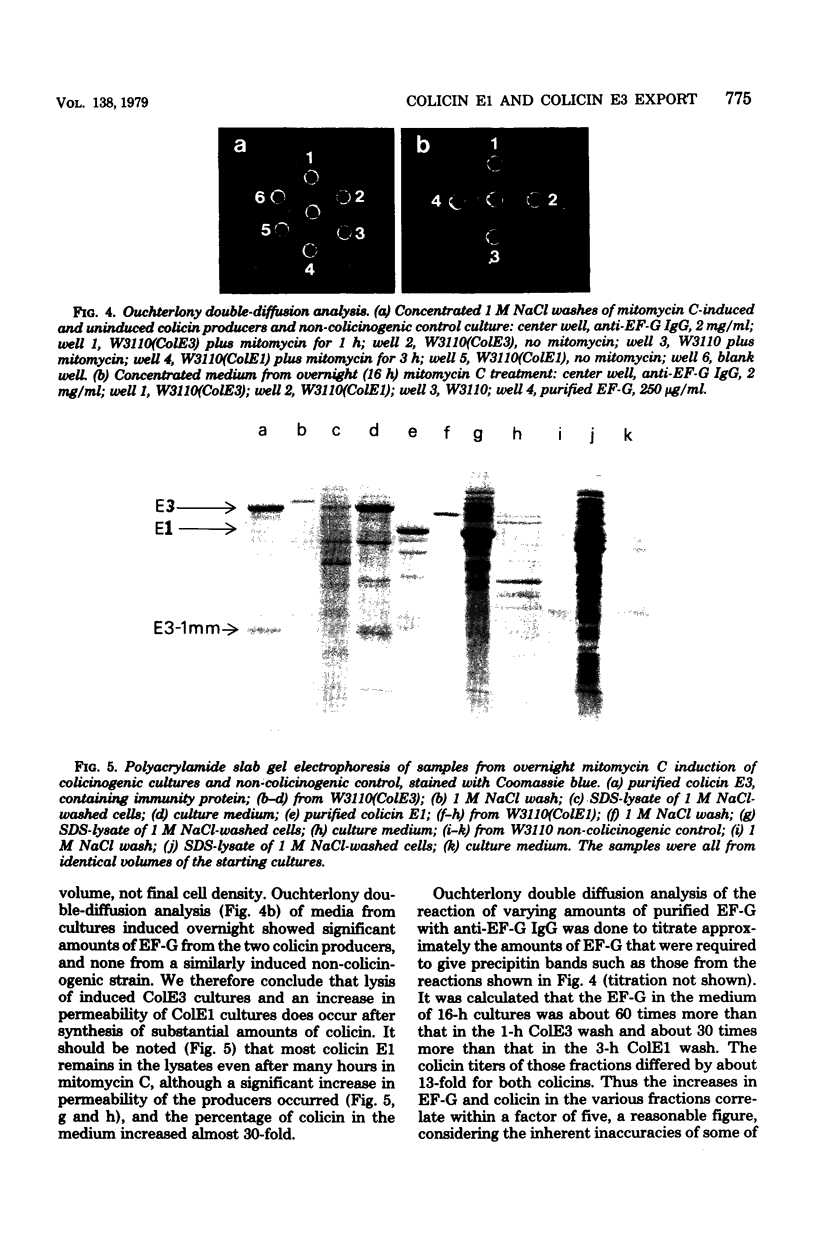
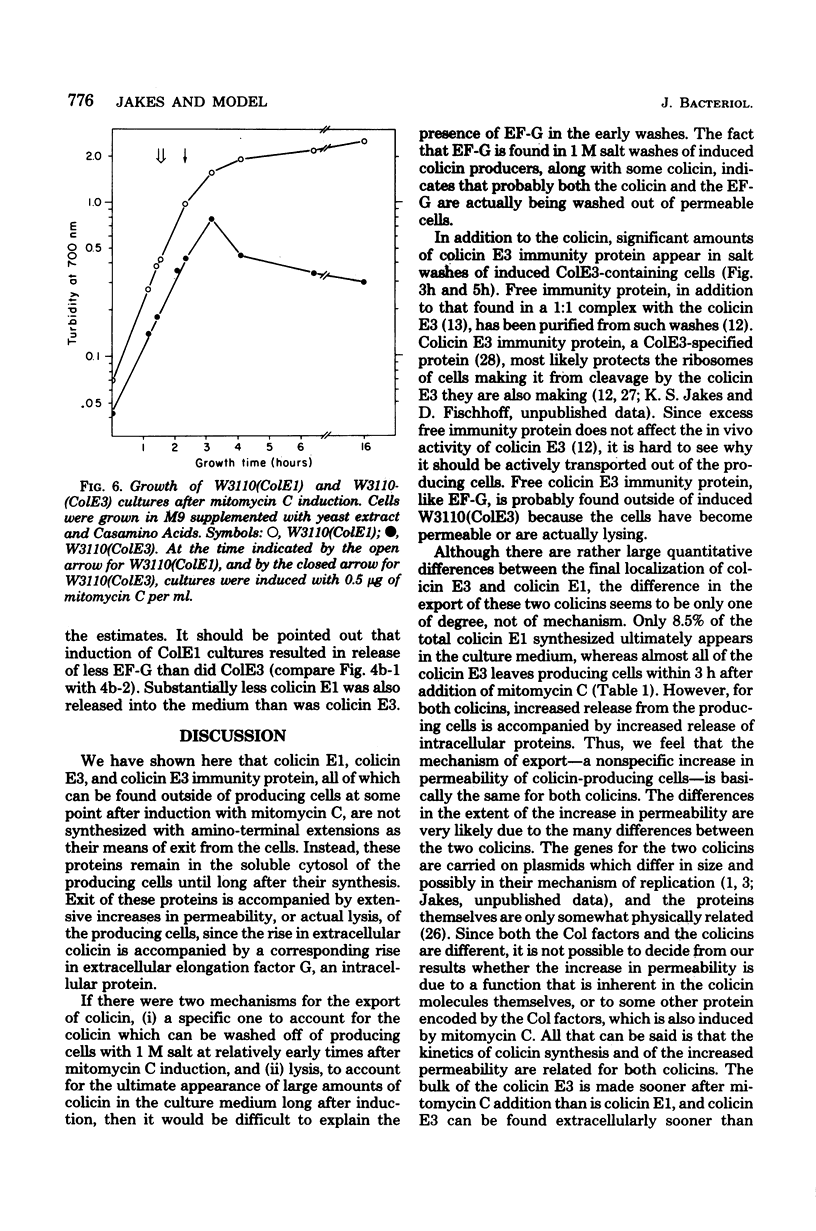
The mechanism of export of colicins E1 and E3 was examined. Neither colicin E1, colicin E3, Nor colicin E3 immunity protein appears to be synthesized as a precursor protein with an amino-terminal extension. Instead, the colicins, as well as the colicin E3 immunity protein, appear to leave the cells where they are made, long after their synthesis, by a nonspecific mechanism which results in increased permeability of the producing cells. Induction of ColE3-containing cells with mitomycin C leads to actual lysis of those cells, as some time after synthesis of the colicin E3 and its immunity protein has been completed. Induction of ColE1-containing cells results in increased permeability of the cells, but not in actual lysis, and most of the colicin E1 produced never leaves the producing cells. Intracellular proteins such as elongation factor G can be found outside of colicinogenic cells after mitomycin C induction, along with the colicin. Until substantial increases in permeability occur, most of the colicin remains cell associated, in the soluble cytosol, rather than in a membrane-associated form.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bazaral M., Helinski D. R. Circular DNA forms of colicinogenic factors E1, E2 and E3 from Escherichia coli. J Mol Biol. 1968 Sep 14;36(2):185–194. doi: 10.1016/0022-2836(68)90374-4. [DOI] [PubMed] [Google Scholar]
- Chang C. N., Blobel G., Model P. Detection of prokaryotic signal peptidase in an Escherichia coli membrane fraction: endoproteolytic cleavage of nascent f1 pre-coat protein. Proc Natl Acad Sci U S A. 1978 Jan;75(1):361–365. doi: 10.1073/pnas.75.1.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewell D. B. Nature of Col E 1 plasmid replication in Escherichia coli in the presence of the chloramphenicol. J Bacteriol. 1972 May;110(2):667–676. doi: 10.1128/jb.110.2.667-676.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devillers-Thiery A., Kindt T., Scheele G., Blobel G. Homology in amino-terminal sequence of precursors to pancreatic secretory proteins. Proc Natl Acad Sci U S A. 1975 Dec;72(12):5016–5020. doi: 10.1073/pnas.72.12.5016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FREDERICQ P. Colicins and colicinogenic factors. Symp Soc Exp Biol. 1958;12:104–122. [PubMed] [Google Scholar]
- Herschman H. R., Helinski D. R. Purification and characterization of colicin E2 and colicin E3. J Biol Chem. 1967 Nov 25;242(22):5360–5368. [PubMed] [Google Scholar]
- Horiuchi K., Vovis G. F., Enea V., Zinder N. D. Cleavage map of bacteriophage f1: location of the Escherichia coli B-specific modification sites. J Mol Biol. 1975 Jun 25;95(2):147–165. doi: 10.1016/0022-2836(75)90388-5. [DOI] [PubMed] [Google Scholar]
- Inouye H., Beckwith J. Synthesis and processing of an Escherichia coli alkaline phosphatase precursor in vitro. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1440–1444. doi: 10.1073/pnas.74.4.1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inouye S., Wang S., Sekizawa J., Halegoua S., Inouye M. Amino acid sequence for the peptide extension on the prolipoprotein of the Escherichia coli outer membrane. Proc Natl Acad Sci U S A. 1977 Mar;74(3):1004–1008. doi: 10.1073/pnas.74.3.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakes K. S., Zinder N. D. Highly purified colicin E3 contains immunity protein. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3380–3384. doi: 10.1073/pnas.71.9.3380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakes K., Zinder N. D., Boon T. Purification and properties of colicin E3 immunity protein. J Biol Chem. 1974 Jan 25;249(2):438–444. [PubMed] [Google Scholar]
- Kennedy C. K. Induction of colicin production by high temperature or inhibition of protein synthesis. J Bacteriol. 1971 Oct;108(1):10–19. doi: 10.1128/jb.108.1.10-19.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler S. W. Cell membrane antigen isolation with the staphylococcal protein A-antibody adsorbent. J Immunol. 1976 Nov;117(5 Pt 1):1482–1490. [PubMed] [Google Scholar]
- Lingappa V. R., Devillers-Thiery A., Blobel G. Nascent prehormones are intermediates in the biosynthesis of authentic bovine pituitary growth hormone and prolactin. Proc Natl Acad Sci U S A. 1977 Jun;74(6):2432–2436. doi: 10.1073/pnas.74.6.2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotz W. Effect of guanosine tetraphosphate on in vitro protein synthesis directed by E1 and E3 colicinogenic factors. J Bacteriol. 1978 Aug;135(2):707–712. doi: 10.1128/jb.135.2.707-712.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mock M., Schwartz M. Mechanism of colicin E3 production in strains harboring wild-type or mutant plasmids. J Bacteriol. 1978 Nov;136(2):700–707. doi: 10.1128/jb.136.2.700-707.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Model P., Zinder N. D. In vitro synthesis of bacteriophage f1 proteins. J Mol Biol. 1974 Feb 25;83(2):231–251. doi: 10.1016/0022-2836(74)90389-1. [DOI] [PubMed] [Google Scholar]
- Randall L. L., Hardy S. J., Josefsson L. G. Precursors of three exported proteins in Escherichia coli. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1209–1212. doi: 10.1073/pnas.75.3.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sander G. Mechanism of action of colicin E3. Effect on ribosomal elongation-factor-dependent reactions. Eur J Biochem. 1977 May 16;75(2):523–531. doi: 10.1111/j.1432-1033.1977.tb11553.x. [DOI] [PubMed] [Google Scholar]
- Schaller K., Nomura M. Colicin E2 is DNA endonuclease. Proc Natl Acad Sci U S A. 1976 Nov;73(11):3989–3993. doi: 10.1073/pnas.73.11.3989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz S. A., Helinski D. R. Purification and characterization of colicin E1. J Biol Chem. 1971 Oct 25;246(20):6318–6327. [PubMed] [Google Scholar]
- Sidikaro J., Masayasu N. In vitro synthesis of the E3 immunity protein directed by Col E3 plasmid deoxyribonucleic acid. J Biol Chem. 1975 Feb 10;250(3):1123–1131. [PubMed] [Google Scholar]
- Sidikaro J., Nomura M. E3 immunity substance. A protein from e3-colicinogenic cells that accounts for their immunity to colicin E3. J Biol Chem. 1974 Jan 25;249(2):445–453. [PubMed] [Google Scholar]