Abstract
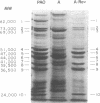
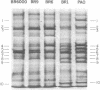
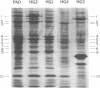
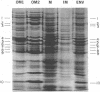
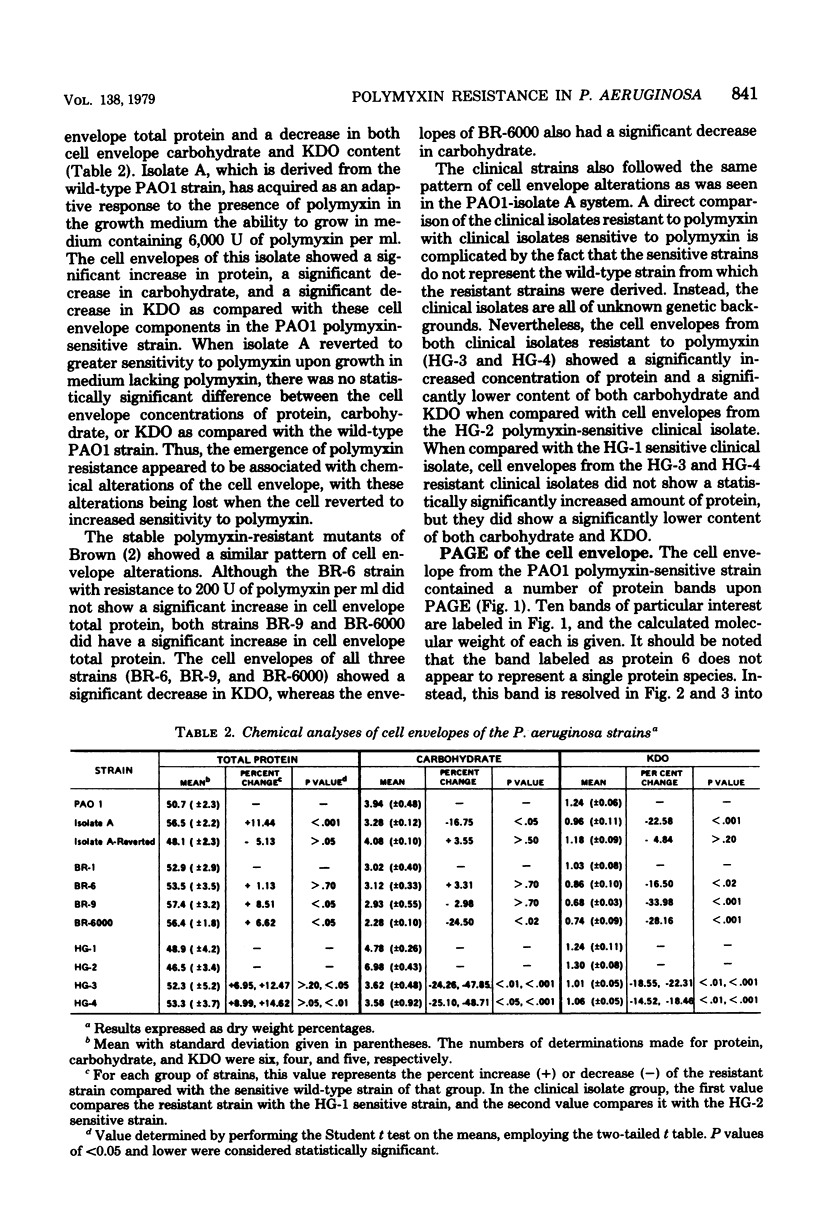
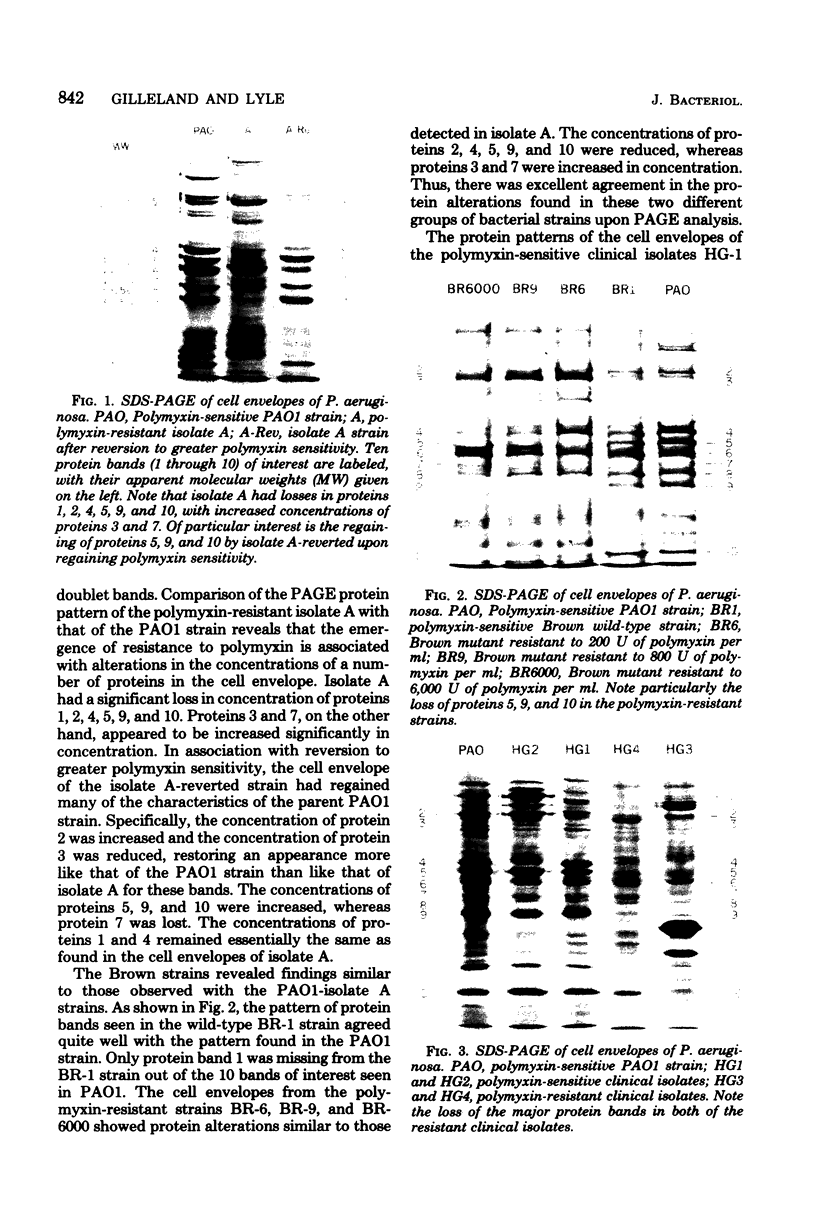
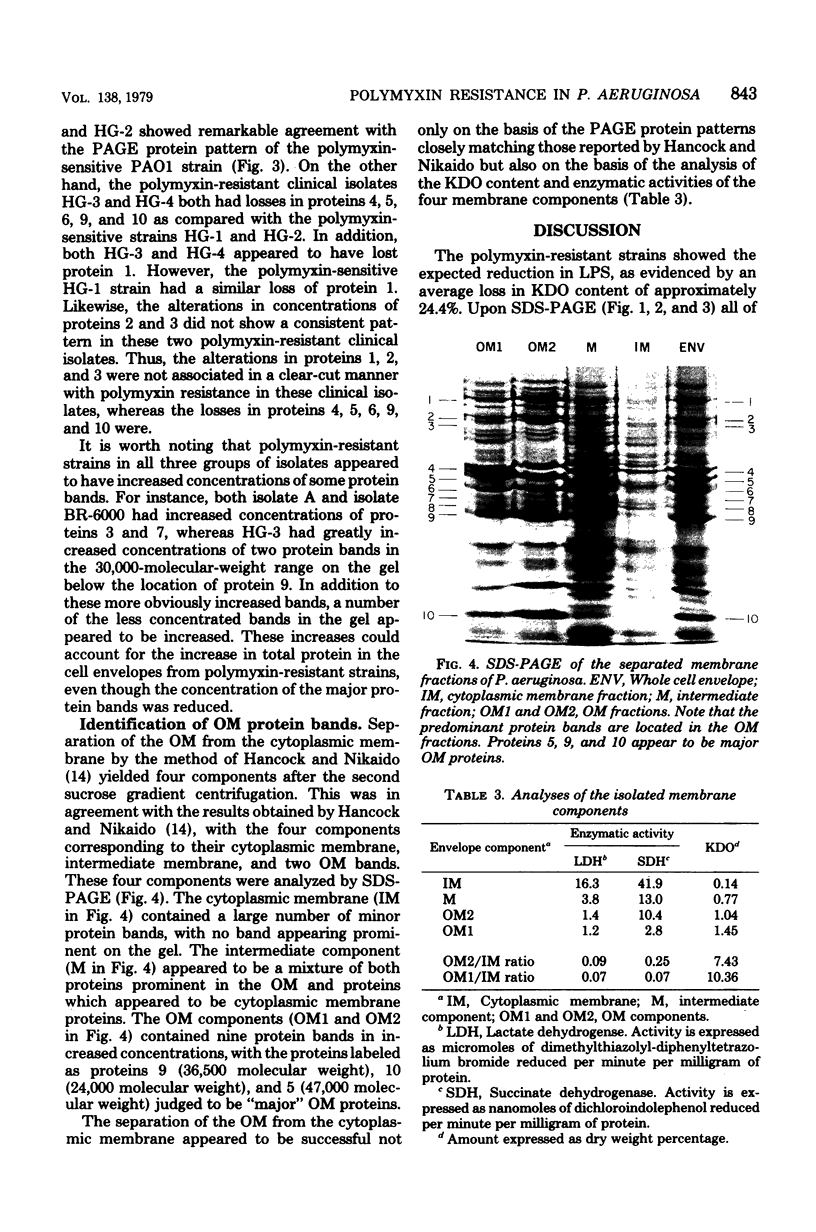
Cell envelopes from Pseudomonas aeruginosa strains resistant to polymyxin were compared with cell envelopes from polymyxin-sensitive strains as to their content of total protein, carbohydrate, and 2-keto-3-deoxyoctonate and as to their protein composition as determined by slab polyacrylamide gel electrophoresis. The cell envelopes of the polymyxin-resistant strains had reduced amounts of lipopolysaccharide, as indicated a reduction in both carbohydrate and 2-keto-3-deoxyoctonate concentrations, and a greatly altered protein composition as shown by polyacrylamide gel electrophoresis. There was a quantitative increase in total cell envelop protein in these strains. However, those protein bands identified as being major outer membrane proteins upon polyacrylamide gel electrophoresis of separated outer and cytoplasmic membranes were reduced greatly in concentration in the polymyxin-resistant cell envelopes. Thus, it appears that polymyxin resistance in these strains is associated with the alteration of the outer membrane through a loss of lipopolysaccharide and outer membrane proteins.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown M. R., Watkins W. M. Low magnesium and phospholipid content of cell wals of Pseudomonas aeruginosa resistant to polymyxin. Nature. 1970 Sep 26;227(5265):1360–1361. doi: 10.1038/2271360a0. [DOI] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Gilleland H. E., Jr, Murray R. G. Ultrastructural study of polymyxin-resistant isolates of Pseudomonas aeruginosa. J Bacteriol. 1976 Jan;125(1):267–281. doi: 10.1128/jb.125.1.267-281.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilleland H. E., Jr, Stinnett J. D., Eagon R. G. Ultrastructural and chemical alteration of the cell envelope of Pseudomonas aeruginosa, associated with resistance to ethylenediaminetetraacetate resulting from growth in a Mg2+-deficient medium. J Bacteriol. 1974 Jan;117(1):302–311. doi: 10.1128/jb.117.1.302-311.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilleland H. E., Jr, Stinnett J. D., Roth I. L., Eagon R. G. Freeze-etch study of Pseudomonas aeruginosa: localization within the cell wall of an ethylenediaminetetraacetate-extractable. J Bacteriol. 1973 Jan;113(1):417–432. doi: 10.1128/jb.113.1.417-432.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood D. The activity of polymyxins against dense populations of Escherichia coli. J Gen Microbiol. 1975 Nov;91(1):110–118. doi: 10.1099/00221287-91-1-110. [DOI] [PubMed] [Google Scholar]
- Guymon L. F., Walstad D. L., Sparling P. F. Cell envelope alterations in antibiotic-sensitive and-resistant strains of Neisseria gonorrhoeae. J Bacteriol. 1978 Oct;136(1):391–401. doi: 10.1128/jb.136.1.391-401.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton W. A. The mechanism of the bacteriostatic action of tetrachlorosalicylanilide: a Membrane-active antibacterial compound. J Gen Microbiol. 1968 Mar;50(3):441–458. doi: 10.1099/00221287-50-3-441. [DOI] [PubMed] [Google Scholar]
- Hancock R. E., Nikaido H. Outer membranes of gram-negative bacteria. XIX. Isolation from Pseudomonas aeruginosa PAO1 and use in reconstitution and definition of the permeability barrier. J Bacteriol. 1978 Oct;136(1):381–390. doi: 10.1128/jb.136.1.381-390.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartree E. F. Determination of protein: a modification of the Lowry method that gives a linear photometric response. Anal Biochem. 1972 Aug;48(2):422–427. doi: 10.1016/0003-2697(72)90094-2. [DOI] [PubMed] [Google Scholar]
- Kasahara M., Anraku Y. Succinate dehydrogenase of Escherichia coli membrane vesicles. Activation and properties of the enzyme. J Biochem. 1974 Nov;76(5):959–966. [PubMed] [Google Scholar]
- Koike M., Iida K., Matsuo T. Electron microscopic studies on mode of action of polymyxin. J Bacteriol. 1969 Jan;97(1):448–452. doi: 10.1128/jb.97.1.448-452.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lugtenberg B., Meijers J., Peters R., van der Hoek P., van Alphen L. Electrophoretic resolution of the "major outer membrane protein" of Escherichia coli K12 into four bands. FEBS Lett. 1975 Oct 15;58(1):254–258. doi: 10.1016/0014-5793(75)80272-9. [DOI] [PubMed] [Google Scholar]
- Lugtenberg B., van Boxtel R., Verhoef C., van Alphen W. Pore protein e of the outer membrane of Escherichia coli K12. FEBS Lett. 1978 Dec 1;96(1):99–105. doi: 10.1016/0014-5793(78)81071-0. [DOI] [PubMed] [Google Scholar]
- Morrison D. C., Jacobs D. M. Binding of polymyxin B to the lipid A portion of bacterial lipopolysaccharides. Immunochemistry. 1976 Oct;13(10):813–818. doi: 10.1016/0019-2791(76)90181-6. [DOI] [PubMed] [Google Scholar]
- NEWTON B. A. The properties and mode of action of the polymyxins. Bacteriol Rev. 1956 Mar;20(1):14–27. doi: 10.1128/br.20.1.14-27.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakae T. Outer membrane of Salmonella. Isolation of protein complex that produces transmembrane channels. J Biol Chem. 1976 Apr 10;251(7):2176–2178. [PubMed] [Google Scholar]
- Palva E. T. Major outer membrane protein in Salmonella typhimurium induced by maltose. J Bacteriol. 1978 Oct;136(1):286–294. doi: 10.1128/jb.136.1.286-294.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugsley A. P., Schnaitman C. A. Identification of three genes controlling production of new outer membrane pore proteins in Escherichia coli K-12. J Bacteriol. 1978 Sep;135(3):1118–1129. doi: 10.1128/jb.135.3.1118-1129.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers S. W., Gilleland H. E., Jr, Eagon R. G. Characterization of a protein-lipopolysaccharide complex released from cell walls of Pseudomonas aeruginosa by ethylenediaminetetraacetic acid. Can J Microbiol. 1969 Jul;15(7):743–748. doi: 10.1139/m69-130. [DOI] [PubMed] [Google Scholar]
- Schindler P. R., Teuber M. Action of polymyxin B on bacterial membranes: morphological changes in the cytoplasm and in the outer membrane of Salmonella typhimurium and Escherichia coli B. Antimicrob Agents Chemother. 1975 Jul;8(1):95–104. doi: 10.1128/aac.8.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu S., Iyobe S., Mitsuhashi S. Inducible high resistance to colistin in Proteus strains. Antimicrob Agents Chemother. 1977 Jul;12(1):1–3. doi: 10.1128/aac.12.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinnett J. D., Gilleland H. E., Jr, Eagon R. G. Proteins released from cell envelopes of Pseudomonas aeruginosa on exposure to ethylenediaminetetraacetate: comparison with dimethylformamide-extractable proteins. J Bacteriol. 1973 Apr;114(1):399–407. doi: 10.1128/jb.114.1.399-407.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storm D. R., Rosenthal K. S., Swanson P. E. Polymyxin and related peptide antibiotics. Annu Rev Biochem. 1977;46:723–763. doi: 10.1146/annurev.bi.46.070177.003451. [DOI] [PubMed] [Google Scholar]
- Sud I. J., Feingold D. S. Mechanism of polymyxin B resistance in Proteus mirabilis. J Bacteriol. 1970 Oct;104(1):289–294. doi: 10.1128/jb.104.1.289-294.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teuber M., Bader J. Action of polymyxin B on bacterial membranes. Binding capacities for polymyxin B of inner and outer membranes isolated from Salmonella typhimurium G30. Arch Microbiol. 1976 Aug;109(1-2):51–58. doi: 10.1007/BF00425112. [DOI] [PubMed] [Google Scholar]
- Tsang J. C., Weber D. A., Brown D. A. Evidences for complex formation between polymyxin B and lipopolysaccharides from Serratia marcescens. J Antibiot (Tokyo) 1976 Jul;29(7):735–742. doi: 10.7164/antibiotics.29.735. [DOI] [PubMed] [Google Scholar]
- Verkleij A., van Alphen L., Bijvelt J., Lugtenberg B. Architecture of the outer membrane of Escherichia coli K12. II. Freeze fracture morphology of wild type and mutant strains. Biochim Biophys Acta. 1977 Apr 18;466(2):269–282. doi: 10.1016/0005-2736(77)90224-3. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- van Alphen L., Verkleij A., Leunissen-Bijvelt J., Lugtenberg B. Architecture of the outer membrane of Escherichia coli. III. Protein-lipopolysaccharide complexes in intramembraneous particles. J Bacteriol. 1978 Jun;134(3):1089–1098. doi: 10.1128/jb.134.3.1089-1098.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Alphen W., van Seim N., Lugtenberg B. Pores in the outer membrane of Escherichia coli K12: involvement of proteins b and e in the functioning of pores for nucleotides. Mol Gen Genet. 1978 Feb 7;159(1):75–83. doi: 10.1007/BF00401750. [DOI] [PubMed] [Google Scholar]