Abstract
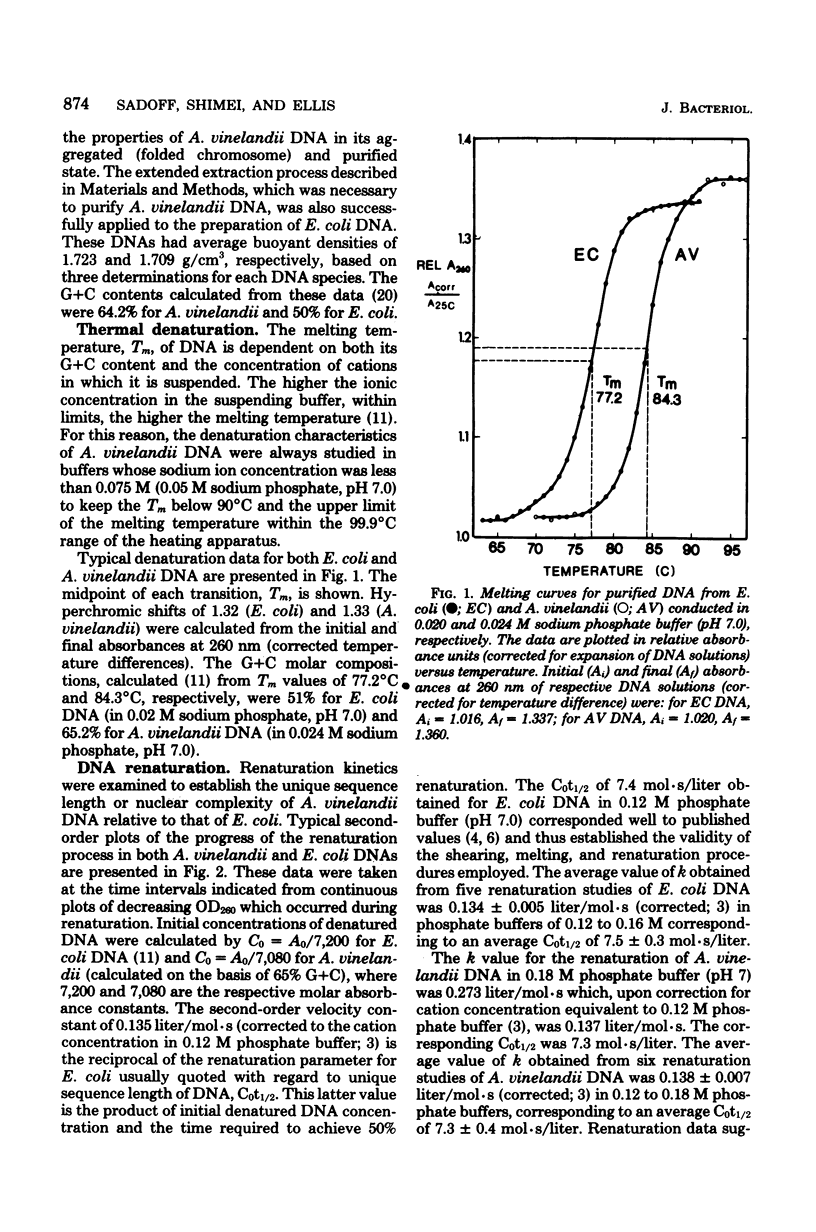
The properties of Azotobacter vinelandii deoxyribonucleic acid (DNA) and folded chromosomes were studied and compared to those of Escherichia coli as a standard. Based on melting temperature and buoyant density measurements, the guanosine + cytosine content of purified A. vinelandii DNA was 65%, whereas that of E. coli DNA was 50%. The results of renaturation studies showed that the unique DNA sequence lengths of the two organisms were similar with Cot1/2 values of 7.3 +/- 0.4 mol.s/liter and 7.5 +/- 0.3 mol.s/liter, respectively, for A. vinelandii and E. coli. Folded chromosomes of A. vinelandii sedimented in a centrifugal field at a rate identical to those derived from E. coli, 1,600 to 1,700S. Based on the DNA content per cell and the mass of a single genome, A. vinelandii contains at least 40 chromosomes per cell.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson E. H. Growth Requirements of Virus-Resistant Mutants of Escherichia Coli Strain "B". Proc Natl Acad Sci U S A. 1946 May;32(5):120–128. doi: 10.1073/pnas.32.5.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop P. E., Brill W. J. Genetic analysis of Azotobacter vinelandii mutant strains unable to fix nitrogen. J Bacteriol. 1977 May;130(2):954–956. doi: 10.1128/jb.130.2.954-956.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britten R. J., Graham D. E., Neufeld B. R. Analysis of repeating DNA sequences by reassociation. Methods Enzymol. 1974;29:363–418. doi: 10.1016/0076-6879(74)29033-5. [DOI] [PubMed] [Google Scholar]
- Britten R. J., Kohne D. E. Repeated sequences in DNA. Hundreds of thousands of copies of DNA sequences have been incorporated into the genomes of higher organisms. Science. 1968 Aug 9;161(3841):529–540. doi: 10.1126/science.161.3841.529. [DOI] [PubMed] [Google Scholar]
- Burgi A. W., Robinton J., Carlson C. L. Studies on the folded chromosome of Escherichia coli. Cold Spring Harb Symp Quant Biol. 1974;38:43–51. doi: 10.1101/sqb.1974.038.01.007. [DOI] [PubMed] [Google Scholar]
- DONDERO N. C., ZELLE M. R. Observations on the formation and behavior of conjugation cells and large bodies in Azotobacter agile. Science. 1953 Jul 10;118(3054):34–36. doi: 10.1126/science.118.3054.34. [DOI] [PubMed] [Google Scholar]
- GREEN M., ALEXANDER M., WILSON P. W. Mutants of the Azotobacter unable to use N2. J Bacteriol. 1953 Nov;66(5):623–624. doi: 10.1128/jb.66.5.623-624.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillis M., De Ley J., De Cleene M. The determination of molecular weight of bacterial genome DNA from renaturation rates. Eur J Biochem. 1970 Jan;12(1):143–153. doi: 10.1111/j.1432-1033.1970.tb00831.x. [DOI] [PubMed] [Google Scholar]
- Lin L. P., Sadoff H. L. Encystment and polymer production by Azotobacter vinelandii in the presence of beta-hydroxybutyrate. J Bacteriol. 1968 Jun;95(6):2336–2343. doi: 10.1128/jb.95.6.2336-2343.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loperfido B., Sadoff H. L. Germination of Azotobacter vinelandii cysts: sequence of macromolecular synthesis and nitrogen fixation. J Bacteriol. 1973 Feb;113(2):841–846. doi: 10.1128/jb.113.2.841-846.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARMUR J., DOTY P. Determination of the base composition of deoxyribonucleic acid from its thermal denaturation temperature. J Mol Biol. 1962 Jul;5:109–118. doi: 10.1016/s0022-2836(62)80066-7. [DOI] [PubMed] [Google Scholar]
- Müller H. P., Kern H. Strahlenresistenz, Gehalt und Basenzusammensetzung der DNA einiger strahleninduzierter Mutanten von Azotobacter chroococcum. Z Naturforsch B. 1967 Dec;22(12):1330–1336. [PubMed] [Google Scholar]
- Page W. J., Sadoff H. L. Control of transformation competence in Azotobacter vinelandii by nitrogen catabolite derepression. J Bacteriol. 1976 Mar;125(3):1088–1095. doi: 10.1128/jb.125.3.1088-1095.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page W. J., Sadoff H. L. Physiological factors affecting transformation of Azotobacter vinelandii. J Bacteriol. 1976 Mar;125(3):1080–1087. doi: 10.1128/jb.125.3.1080-1087.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettijohn D. E., Hecht R. RNA molecules bound to the folded bacterial genome stabilize DNA folds and segregate domains of supercoiling. Cold Spring Harb Symp Quant Biol. 1974;38:31–41. doi: 10.1101/sqb.1974.038.01.006. [DOI] [PubMed] [Google Scholar]
- SCHILDKRAUT C. L., MARMUR J., DOTY P. Determination of the base composition of deoxyribonucleic acid from its buoyant density in CsCl. J Mol Biol. 1962 Jun;4:430–443. doi: 10.1016/s0022-2836(62)80100-4. [DOI] [PubMed] [Google Scholar]
- SOCOLOFSKY M. D., WYSS O. Resistance of the Azotobacter cyst. J Bacteriol. 1962 Jul;84:119–124. doi: 10.1128/jb.84.1.119-124.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadoff H. L., Berke E., Loperfido B. Physiological studies of encystment in Azotobacter vinelandii. J Bacteriol. 1971 Jan;105(1):185–189. doi: 10.1128/jb.105.1.185-189.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah V. K., Davis I. C., Gordon J. K., Orme-Johnson W. H., Brill W. J. Nitrogenase. 3. Nitrogenaseless mutants of Azotobacter vinelandii: activities, cross-reactions and EPR spectra. Biochim Biophys Acta. 1973 Jan 18;292(1):246–255. doi: 10.1016/0005-2728(73)90269-7. [DOI] [PubMed] [Google Scholar]
- Shah V. K., Davis L. C., Stieghorst M., Brill W. J. Mutant of Azotobacter vinelandii that hyperproduces nitrogenase component II. J Bacteriol. 1974 Feb;117(2):917–919. doi: 10.1128/jb.117.2.917-919.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirotkin K., Wei J., Snyder L. T4 Bacteriophage-coded RNA polymerase subunit blocks host transcription and unfolds the host chromosome. Nature. 1977 Jan 6;265(5589):28–32. doi: 10.1038/265028a0. [DOI] [PubMed] [Google Scholar]
- Stonington O. G., Pettijohn D. E. The folded genome of Escherichia coli isolated in a protein-DNA-RNA complex. Proc Natl Acad Sci U S A. 1971 Jan;68(1):6–9. doi: 10.1073/pnas.68.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetmur J. G., Davidson N. Kinetics of renaturation of DNA. J Mol Biol. 1968 Feb 14;31(3):349–370. doi: 10.1016/0022-2836(68)90414-2. [DOI] [PubMed] [Google Scholar]
- Worcel A., Burgi E. On the structure of the folded chromosome of Escherichia coli. J Mol Biol. 1972 Nov 14;71(2):127–147. doi: 10.1016/0022-2836(72)90342-7. [DOI] [PubMed] [Google Scholar]


