Abstract
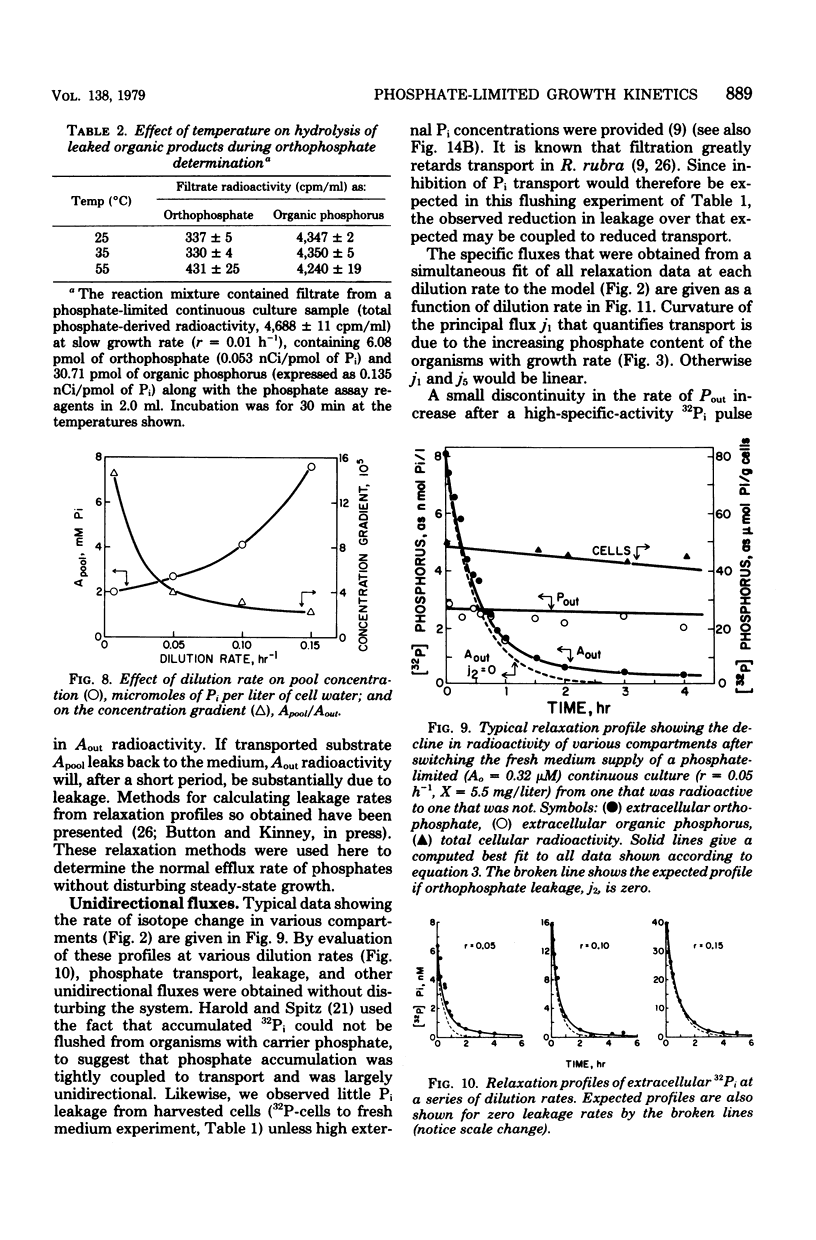
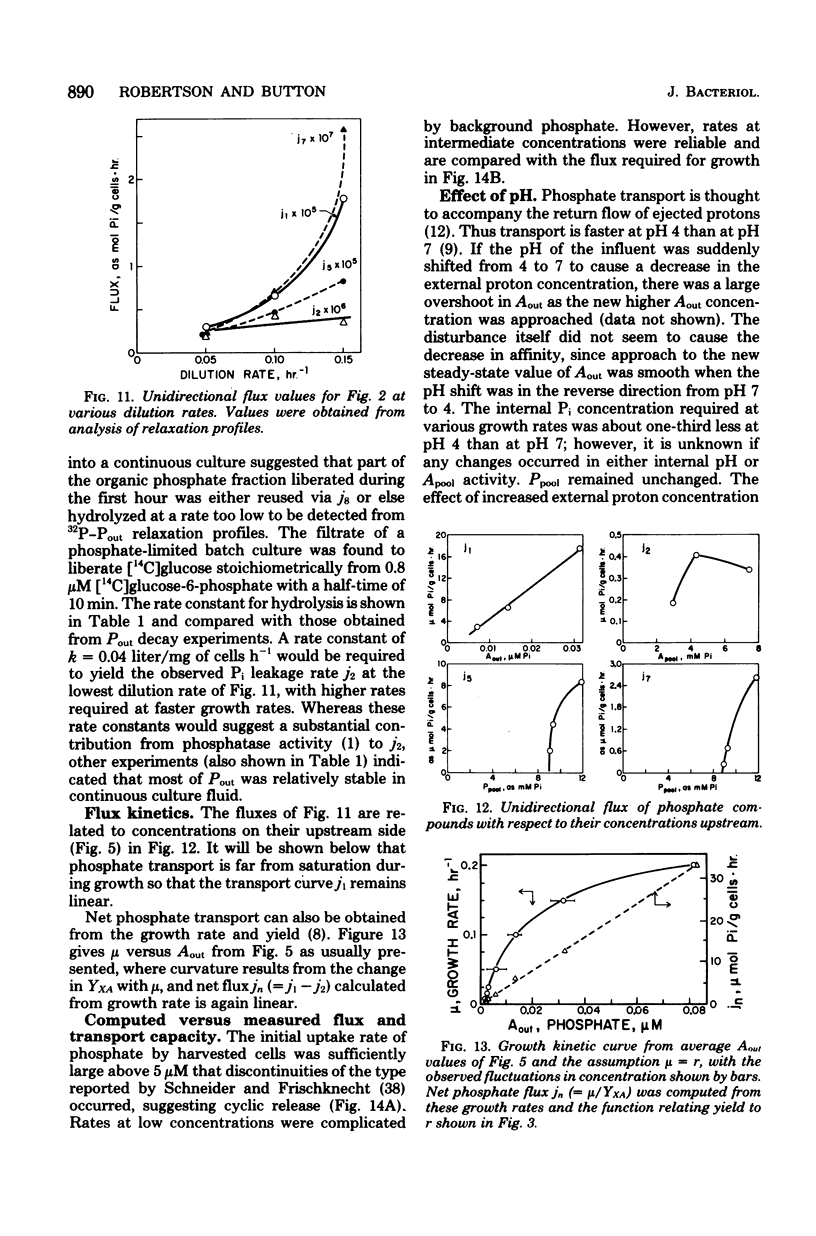
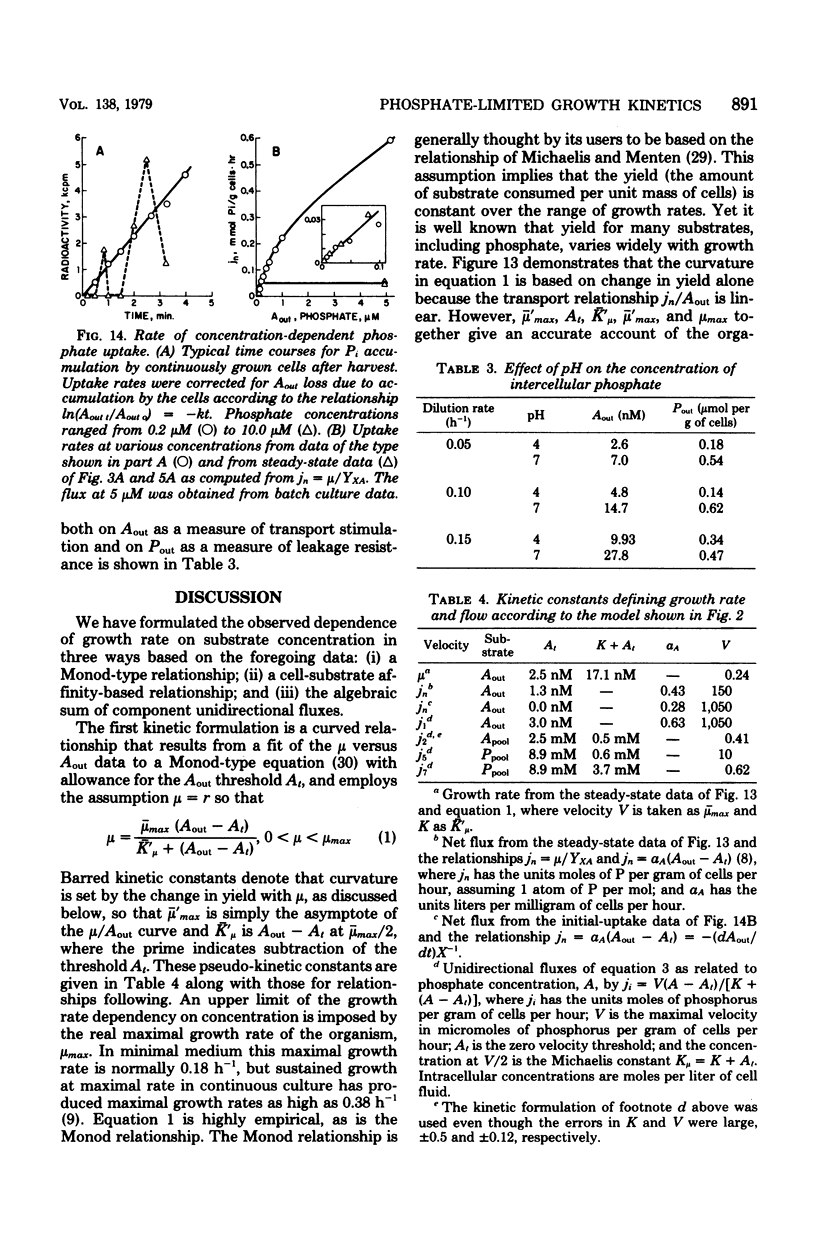
The phosphate-limited growth kinetics of Rhodotorula rubra, a small yeast of marine origin, were examined by analysis of 32P distributions in continuous cultures. Isotope relaxation procedures were used to identify unidirectional flows of Pi and organic phosphate among compartments modeled during growth. The concentrations of phosphates in these compartments at various growth rates were used, together with attendant flows, to produce a mathematical model of growth. Both Pi and phosphate-containing metabolic intermediates leaked from cells during growth. Total leakage ranged from 4 to 10% of influx and was comprised mostly of Pi. Transport capacity was at least 10 times that required for growth at saturating Pi concentrations, so that influx was linear with concentration during growth. This led to the realization that the curvature of Monod plots (Kmu = 12 nM mumax = 0.18/h, and the threshold At = 2.5 nM) is due to change in yield with growth rate. Growth rate related to Pi by the affinity, aA (= 0.43 liter/mg of cells.h) of cells for Pi and the growth rate-dependent yield. It was also specified by a series of kinetic constants that specified flow among the various compartments and equilibrium compartment concentrations as they were set by extracellular Pi. The importance of leakage by healthy cells to the organic chemistry of aquatic systems is noted.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berenblum I., Chain E. An improved method for the colorimetric determination of phosphate. Biochem J. 1938 Feb;32(2):295–298. doi: 10.1042/bj0320295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns D. J., Beever R. E. Kinetic characterization of the two phosphate uptake systems in the fungus Neurospora crassa. J Bacteriol. 1977 Nov;132(2):511–519. doi: 10.1128/jb.132.2.511-519.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Button D. K., Dunker S. S., Morse M. L. Continuous culture of Rhodotorula rubra: kinetics of phosphate-arsenate uptake, inhibition, and phosphate-limited growth. J Bacteriol. 1973 Feb;113(2):599–611. doi: 10.1128/jb.113.2.599-611.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman A. G., Fall L., Atkinson D. E. Adenylate energy charge in Escherichia coli during growth and starvation. J Bacteriol. 1971 Dec;108(3):1072–1086. doi: 10.1128/jb.108.3.1072-1086.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockburn M., Earnshaw P., Eddy A. A. The stoicheiometry of the absorption of protons with phosphate and L-glutamate by yeasts of the genus Saccharomyces. Biochem J. 1975 Mar;146(3):705–712. doi: 10.1042/bj1460705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costerton J. W., Geesey G. G., Cheng K. J. How bacteria stick. Sci Am. 1978 Jan;238(1):86–95. doi: 10.1038/scientificamerican0178-86. [DOI] [PubMed] [Google Scholar]
- Friedberg I. Phosphate transport in Micrococcus lysodeikticus. Biochim Biophys Acta. 1977 May 2;466(3):451–460. doi: 10.1016/0005-2736(77)90338-8. [DOI] [PubMed] [Google Scholar]
- HANES C. S., ISHERWOOD F. A. Separation of the phosphoric esters on the filter paper chromatogram. Nature. 1949 Dec 31;164(4183):1107-12, illust. doi: 10.1038/1641107a0. [DOI] [PubMed] [Google Scholar]
- Harold F. M., Spitz E. Accumulation of arsenate, phosphate, and aspartate by Sreptococcus faecalis. J Bacteriol. 1975 Apr;122(1):266–277. doi: 10.1128/jb.122.1.266-277.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jannasch H. W. Estimations of bacterial growth rates in natural waters. J Bacteriol. 1969 Jul;99(1):156–160. doi: 10.1128/jb.99.1.156-160.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keating K. I. Blue-green algal inhibition of diatom growth: transition from mesotrophic to eutrophic community structure. Science. 1978 Mar 3;199(4332):971–973. doi: 10.1126/science.199.4332.971. [DOI] [PubMed] [Google Scholar]
- Law A. T., Button D. K. Multiple-carbon-source-limited growth kinetics of a marine coryneform bacterium. J Bacteriol. 1977 Jan;129(1):115–123. doi: 10.1128/jb.129.1.115-123.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg H., Gerdes R. G., Chegwidden K. Two systems for the uptake of phosphate in Escherichia coli. J Bacteriol. 1977 Aug;131(2):505–511. doi: 10.1128/jb.131.2.505-511.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler D. W. Eutrophication and recovery in experimental lakes: implications for lake management. Science. 1974 May 24;184(4139):897–899. doi: 10.1126/science.184.4139.897. [DOI] [PubMed] [Google Scholar]
- Schneider K., Frischknecht K. Orthophosphate influx and efflux rates of Chlorella fusca measured in a continuous turbidostat culture with 32P under various conditions. Arch Microbiol. 1977 Dec 15;115(3):339–346. doi: 10.1007/BF00446461. [DOI] [PubMed] [Google Scholar]



