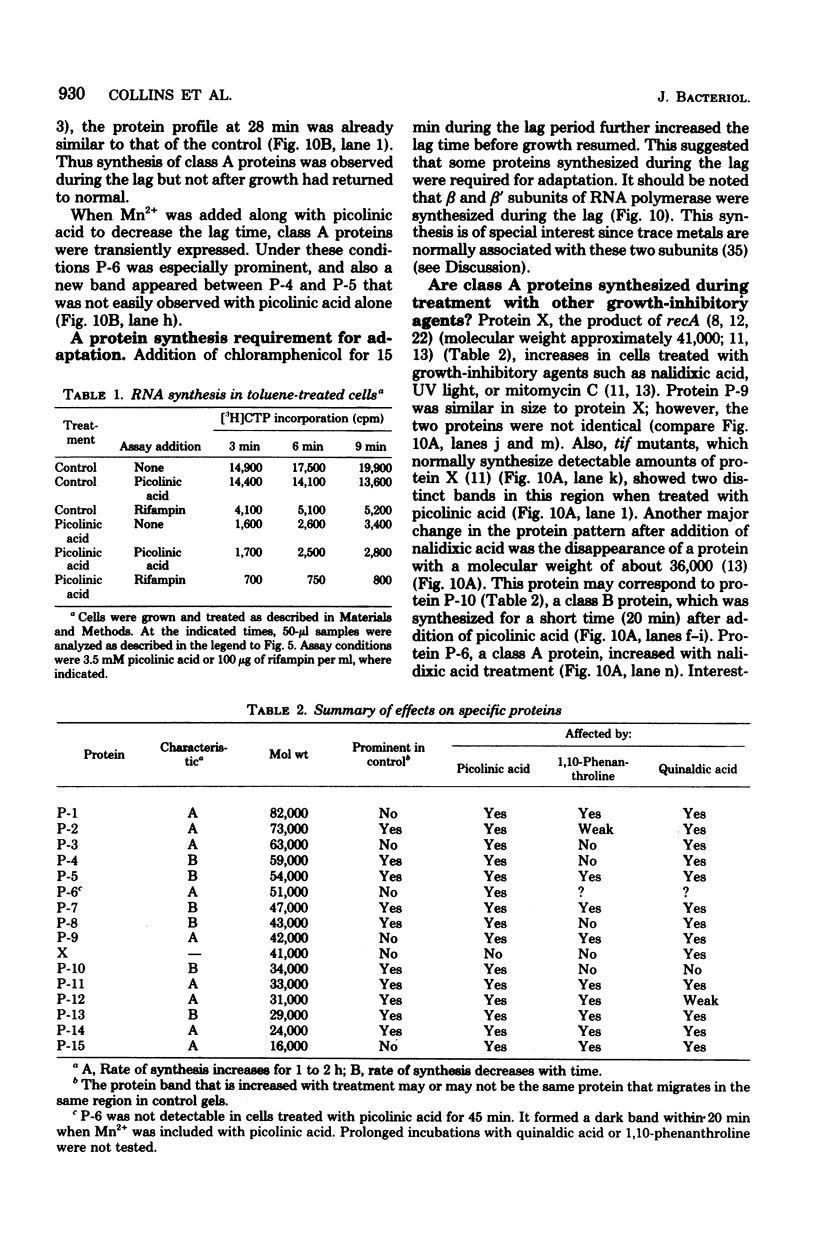
Abstract
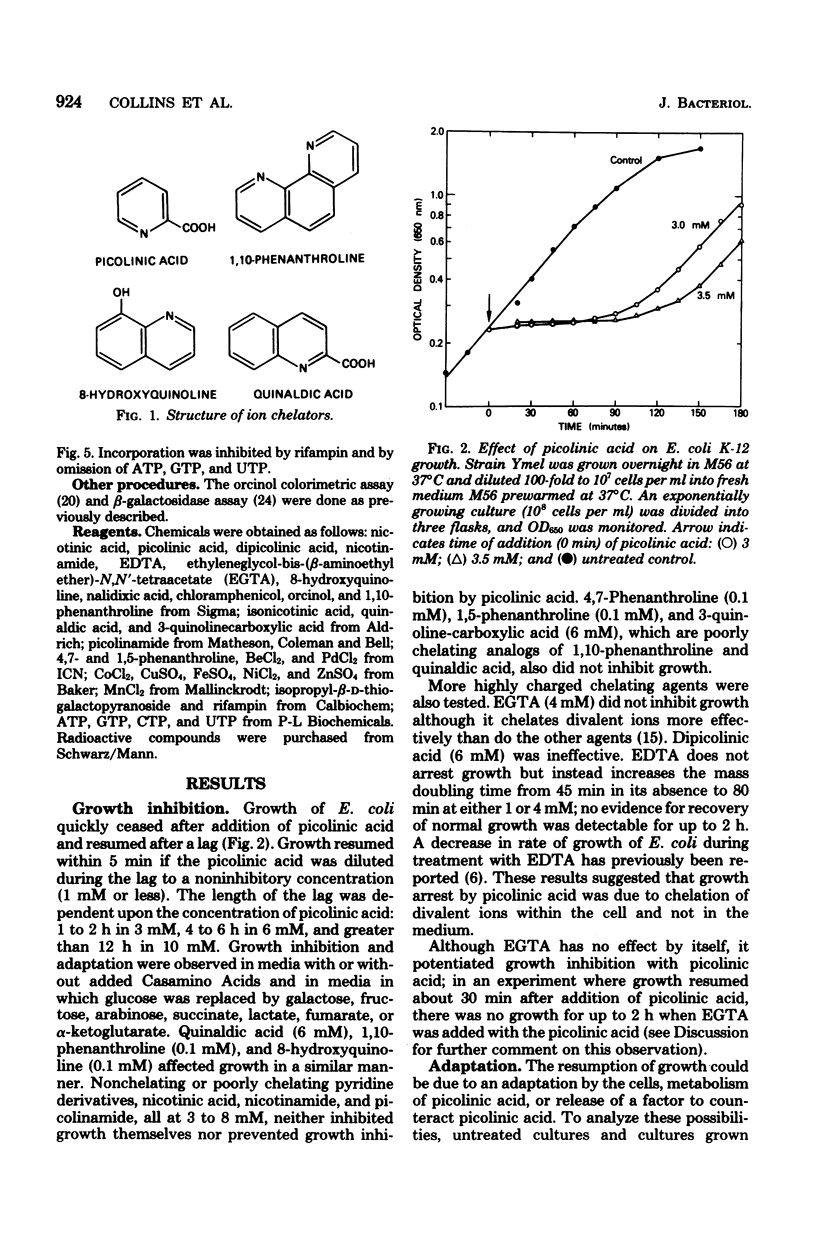
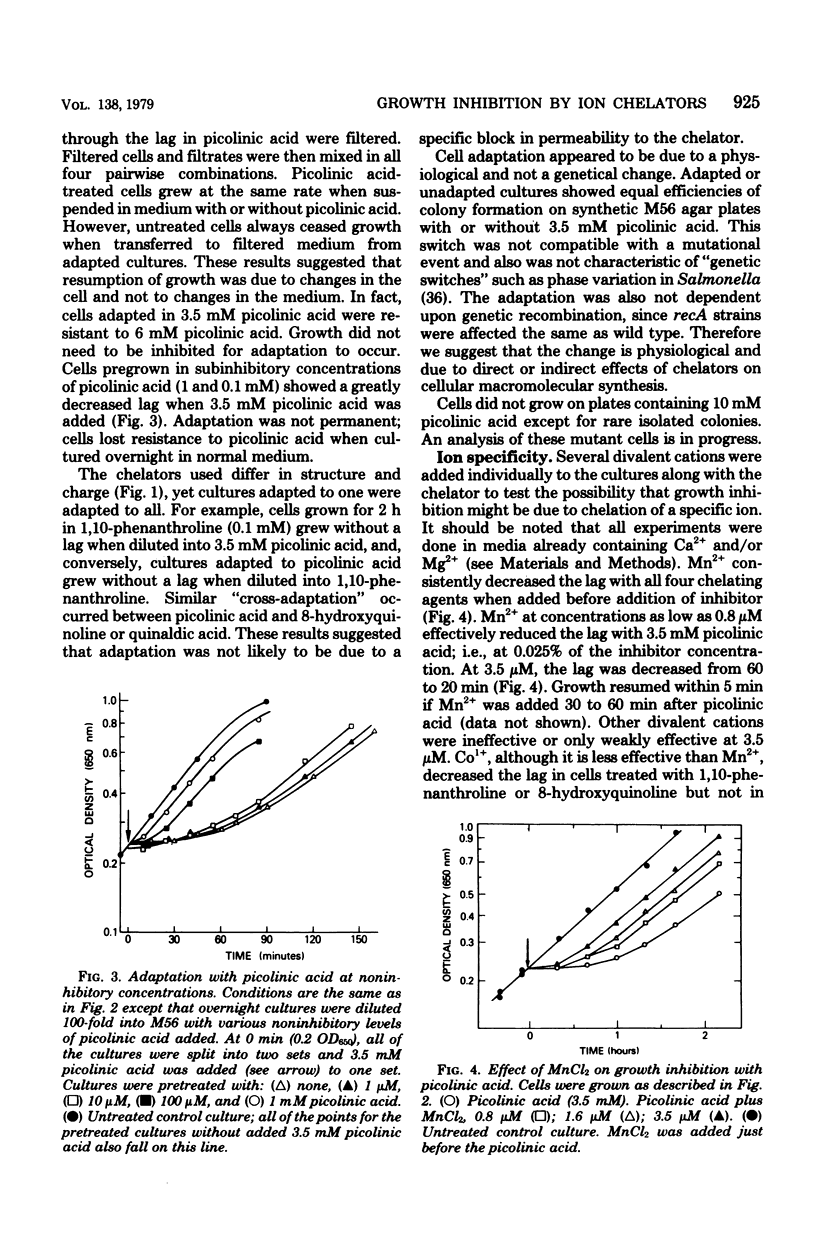
The ion chelators picolinic acid, quinaldic acid, 1,10-phenanthroline, and 8-hydroxyquinoline, but not ethylenediaminetetraacetate, ethyleneglycol-bis-(beta-aminoethyl ether)-N,N-tetraacetate, or dipicolinic acid, rapidly but transiently arrest growth of Escherichia coli K-12. Cells adapt and become resistant to growth inhibition by these agents, a process which requires protein synthesis. Mn2+, at low concentrations, decreases the time required for resumption of growth. Proteins synthesized during the lag are quantitatively and qualitatively different from those synthesized during normal growth. Inhibition of growth can explained by an effect on RNA polymerase, a known metalloenzyme.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Crane R. T., Sun I. L., Crane F. L. Lipophilic chelator inhibition of electron transport in Escherichia coli. J Bacteriol. 1975 May;122(2):686–690. doi: 10.1128/jb.122.2.686-690.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies R. C., Riordan J. F., Auld D. S., Vallee B. L. Kinetics of carboxypeptidase A. I. Hydrolysis of carbobenzoxyglycyl-l-phenylalanine, benzoylglycyl-l-phenylalanine, and hippuryl-dl-beta-phenyllactic acid by metal-substituted and acetylated carboxypeptidases. Biochemistry. 1968 Mar;7(3):1090–1099. doi: 10.1021/bi00843a029. [DOI] [PubMed] [Google Scholar]
- Dvorak H. F., Heppel L. A. Metallo-enzymes released from Escherichia coli by osmotic shock. II. Evidence that 5'-nucleotidase and cyclic phosphodiesterase are zinc metallo-enzymes. J Biol Chem. 1968 May 25;243(10):2647–2653. [PubMed] [Google Scholar]
- Dvorak H. F. Metallo-enzymes released from Escherichia coli by osmotic shock. I. Selective depression of enzymes in cells grown in the presence of ethylenediaminetetraacetate. J Biol Chem. 1968 May 25;243(10):2640–2646. [PubMed] [Google Scholar]
- Falchuk K. H., Krishan A. 1,10-Phenanthroline inhibition of lymphoblast cell cycle. Cancer Res. 1977 Jul;37(7 Pt 1):2050–2056. [PubMed] [Google Scholar]
- Fernandez-Pol J. A., Bono V. H., Jr, Johnson G. S. Control of growth by picolinic acid: differential response of normal and transformed cells. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2889–2893. doi: 10.1073/pnas.74.7.2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudas L. J., Mount D. W. Identification of the recA (tif) gene product of Escherichia coli. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5280–5284. doi: 10.1073/pnas.74.12.5280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudas L. J. The induction of protein X in DNA repair and cell division mutants of Escherichia coli. J Mol Biol. 1976 Jul 5;104(3):567–587. doi: 10.1016/0022-2836(76)90121-2. [DOI] [PubMed] [Google Scholar]
- Helmstetter C. E. Initiation of chromosome replication in Escherichia coli. I. Requirements for RNA and protein synthesis at different growth rates. J Mol Biol. 1974 Mar 25;84(1):1–19. doi: 10.1016/0022-2836(74)90209-5. [DOI] [PubMed] [Google Scholar]
- Jackson R. W., DeMoss J. A. Effects of toluene on Escherichia coli. J Bacteriol. 1965 Nov;90(5):1420–1425. doi: 10.1128/jb.90.5.1420-1425.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston G. C., Singer R. A. RNA synthesis and control of cell division in the yeast S. cerevisiae. Cell. 1978 Aug;14(4):951–958. doi: 10.1016/0092-8674(78)90349-5. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lark K. G. Evidence for the direct involvement of RNA in the initiation of DNA replication in Escherichia coli 15T. J Mol Biol. 1972 Feb 28;64(1):47–60. doi: 10.1016/0022-2836(72)90320-8. [DOI] [PubMed] [Google Scholar]
- Lazzarini R. A., Cashel M., Gallant J. On the regulation of guanosine tetraphosphate levels in stringent and relaxed strains of Escherichia coli. J Biol Chem. 1971 Jul 25;246(14):4381–4385. [PubMed] [Google Scholar]
- Lazzarini R. A., Dahlberg A. E. The control of ribonucleic acid synthesis during amino acid deprivation in Escherichia coli. J Biol Chem. 1971 Jan 25;246(2):420–429. [PubMed] [Google Scholar]
- McEntee K. Protein X is the product of the recA gene of Escherichia coli. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5275–5279. doi: 10.1073/pnas.74.12.5275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messer W. Initiation of deoxyribonucleic acid replication in Escherichia coli B-r: chronology of events and transcriptional control of initiation. J Bacteriol. 1972 Oct;112(1):7–12. doi: 10.1128/jb.112.1.7-12.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheim A. B., Katzir N., Oppenheim A. Regulation of protein synthesis in bacteriophage lambda. Restoration of gene expression in lambda N-strains by mutations in the cro gene. Virology. 1977 Jun 15;79(2):405–425. doi: 10.1016/0042-6822(77)90367-1. [DOI] [PubMed] [Google Scholar]
- PLOCKE D. J., VALLEE B. L. Interaction of alkaline phosphatase of E. coli with metal ions and chelating agents. Biochemistry. 1962 Nov;1:1039–1043. doi: 10.1021/bi00912a014. [DOI] [PubMed] [Google Scholar]
- Raué H. A., Cashel M. Regulation of RNA synthesis in Escherichia coli. III. Degradation of guanosine 5'-diphosphate 3'-diphosphate in cold-shocked cells. Biochim Biophys Acta. 1975 Mar 21;383(3):290–304. [PubMed] [Google Scholar]
- Rubin H., Koide T. Mutual potentiation by magnesium and calcium of growth in animal cells. Proc Natl Acad Sci U S A. 1976 Jan;73(1):168–172. doi: 10.1073/pnas.73.1.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scrutton M. C., Wu C. W., Goldthwait D. A. The presence and possible role of zinc in RNA polymerase obtained from Escherichia coli. Proc Natl Acad Sci U S A. 1971 Oct;68(10):2497–2501. doi: 10.1073/pnas.68.10.2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver S., Johnseine P., King K. Manganese Active Transport in Escherichia coli. J Bacteriol. 1970 Dec;104(3):1299–1306. doi: 10.1128/jb.104.3.1299-1306.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H. S., Pardee A. B. Accumulation of a protein required for division during the cell cycle of Escherichia coli. J Bacteriol. 1970 Mar;101(3):901–909. doi: 10.1128/jb.101.3.901-909.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speckhard D. C., Wu F. Y., Wu C. W. Role of the intrinsic metal in RNA polymerase from Escherichia coli. In vivo substitution of tightly bound zinc with cobalt. Biochemistry. 1977 Nov 29;16(24):5228–5234. doi: 10.1021/bi00643a011. [DOI] [PubMed] [Google Scholar]
- Springgate C. F., Mildvan A. S., Abramson R., Engle J. L., Loeb L. A. Escherichia coli deoxyribonucleic acid polymerase I, a zinc metalloenzyme. Nuclear quadrupolar relaxation studies of the role of bound zinc. J Biol Chem. 1973 Sep 10;248(17):5987–5993. [PubMed] [Google Scholar]
- Stribling D., Perham R. N. Purification and characterization of two fructose diphosphate aldolases from Escherichia coli (Crookes' strain). Biochem J. 1973 Apr;131(4):833–841. doi: 10.1042/bj1310833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C. W., Wu F. Y., Speckhard D. C. Subunit location of the intrinsic divalent metal ions in RNA polymerase from Escherichia coli. Biochemistry. 1977 Dec 13;16(25):5449–5454. doi: 10.1021/bi00644a008. [DOI] [PubMed] [Google Scholar]
- Zieg J., Silverman M., Hilmen M., Simon M. Recombinational switch for gene expression. Science. 1977 Apr 8;196(4286):170–172. doi: 10.1126/science.322276. [DOI] [PubMed] [Google Scholar]