Abstract
Nicotinic acid phosphoribosyl transferase (NAPRTase) and nicotinamide deamidase activities from Salmonella typhimurium were examined regarding their regulation by either feedback inhibition or repression mechanisms. The results indicate that neither enzyme is subject to feedback inhbition. Nicotinamide deamidase does not appear to be under repression control. NAPRTase, however, is repressed when cells are grown in minimal medium supplemented with various intermediates of the pyridine nucleotide cycle. The concentration of exogenously supplied pyridine nucleotide necessary to effect repression of NAPRTas was found to be that concentration which will result in a nadA mutant generation time of less than 60 min. Furthermore, the results presented indicate that nicotinamide adenine dinucleotide is the actual corepressor molecule. The analogs 6-aminonicotinic acid and 6-aminonicotinamide were also capable of repressing NAPRTase, but only when an intact pyridine nucleotide cycl permitted conversion to 6-aminonicotinamide adenine dinucleotide. The role of a repressible NAPRTase is discussed in relation to the overall functioning of the pyridine nucleotide cycle.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andreoli A. J., Okita T. W., Bloom R., Grover T. A. The pyridine nucleotide cycle: presence of a nicotinamide mononucleotide-specific glycohydrolase in Escherichia coli. Biochem Biophys Res Commun. 1972 Oct 6;49(1):264–269. doi: 10.1016/0006-291x(72)90039-3. [DOI] [PubMed] [Google Scholar]
- Chandler J. L., Gholson R. K. De novo biosynthesis of nicotinamide adenine dinucleotide in Escherichia coli: excretion of quinolinic acid by mutants lacking quinolinate phosphoribosyl transferase. J Bacteriol. 1972 Jul;111(1):98–102. doi: 10.1128/jb.111.1.98-102.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobb J. R., Pearcy S. C., Gholson R. K. Metabolism of 6-aminonicotinic acid in Escherichia coli. J Bacteriol. 1977 Sep;131(3):789–794. doi: 10.1128/jb.131.3.789-794.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DIETRICH L. S., FRIEDLAND I. M., KAPLAN L. A. Pyridine nucleotide metabolism: mechanism of action of the niacin antagonist, 6-aminonicotinamide. J Biol Chem. 1958 Oct;233(4):964–968. [PubMed] [Google Scholar]
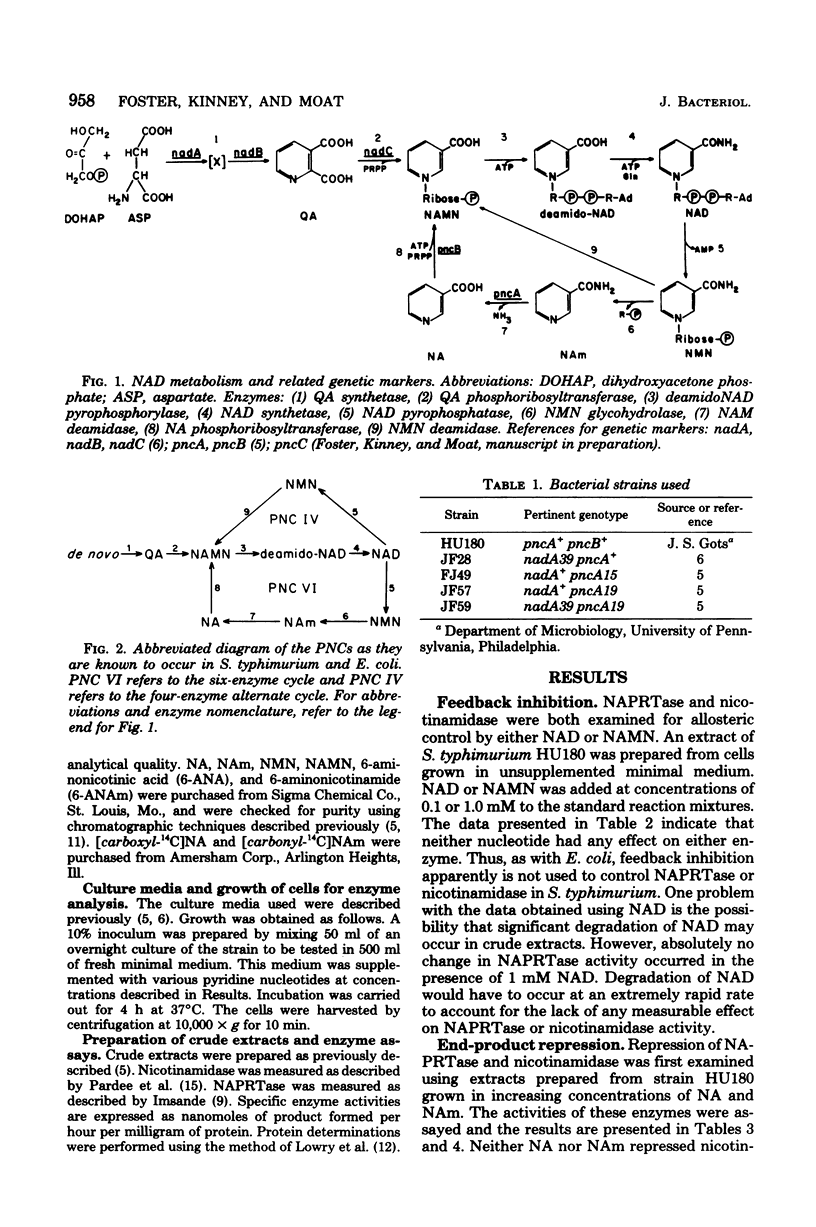
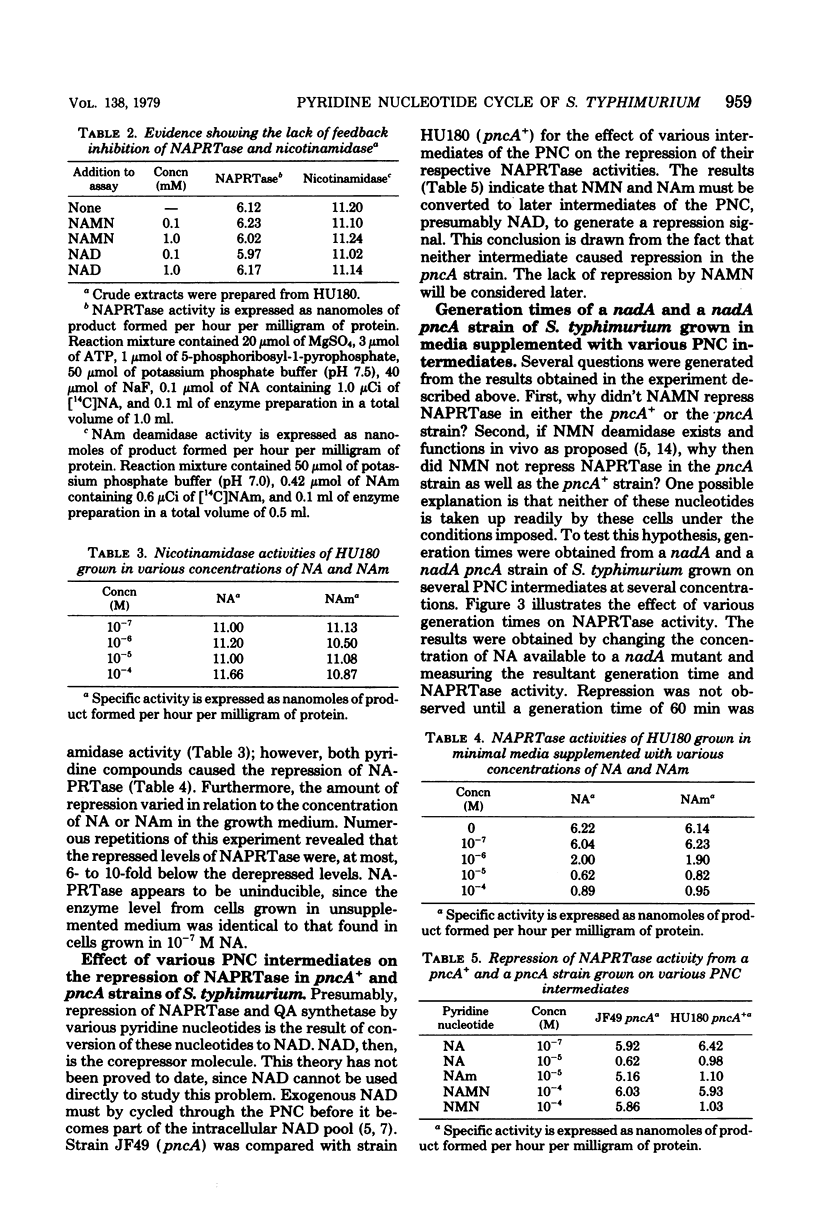
- Foster J. W., Kinney D. M., Moat A. G. Pyridine nucleotide cycle of Salmonella typhimurium: isolation and characterization of pncA, pncB, and pncC mutants and utilization of exogenous nicotinamide adenine dinucleotide. J Bacteriol. 1979 Mar;137(3):1165–1175. doi: 10.1128/jb.137.3.1165-1175.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster J. W., Moat A. G. Mapping and characterization of the nad genes in Salmonella typhimurium LT-2. J Bacteriol. 1978 Feb;133(2):775–779. doi: 10.1128/jb.133.2.775-779.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gholson R. K., Tritz G. J., Matney T. S., Andreoli A. J. Mode of nicotinamide adenine dinucleotide utilization by Escherichia coli. J Bacteriol. 1969 Sep;99(3):895–896. doi: 10.1128/jb.99.3.895-896.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith G. R., Chandler J. L., Gholson R. K. Studies on the de novo biosynthesis of NAD in Escherichia coli. The separation of the nadB gene product from the nadA gene product and its purification. Eur J Biochem. 1975 May;54(1):239–245. doi: 10.1111/j.1432-1033.1975.tb04133.x. [DOI] [PubMed] [Google Scholar]
- IMSANDE J. A COMPARATIVE STUDY OF THE REGULATION OF PYRIDINE NUCLEOTIDE FORMATION. Biochim Biophys Acta. 1964 Mar 16;82:445–453. doi: 10.1016/0304-4165(64)90436-2. [DOI] [PubMed] [Google Scholar]
- Kasărov L. B., Moat A. G. Convenient method for enzymic synthesis of 14 C-nicotinamide riboside. Anal Biochem. 1972 Mar;46(1):181–186. doi: 10.1016/0003-2697(72)90410-1. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lundquist R., Olivera B. M. Pyridine nucleotide metabolism in Escherichia coli. I. Exponential growth. J Biol Chem. 1971 Feb 25;246(4):1107–1116. [PubMed] [Google Scholar]
- PULLMAN B., PULLMAN A. On the 6-aminonicotinamide antagonism of DPN-dependent enzymatic systems. Cancer Res. 1959 Apr;19(3 Pt 1):337–338. [PubMed] [Google Scholar]
- Pardee A. B., Benz E. J., Jr, St Peter D. A., Krieger J. N., Meuth M., Trieshmann H. W., Jr Hyperproduction and purification of nicotinamide deamidase, a microconstitutive enzyme of Escherichia coli. J Biol Chem. 1971 Nov 25;246(22):6792–6796. [PubMed] [Google Scholar]
- Saxton R. E., Rocha V., Rosser R. J., Andreoli A. J., Shimoyama M., Kosaka A., Chandler J. L., Gholson R. K. A comparative study of the regulation of nicotinamide-adenine dinucleotide biosynthesis. Biochim Biophys Acta. 1968 Feb 1;156(1):77–84. doi: 10.1016/0304-4165(68)90106-2. [DOI] [PubMed] [Google Scholar]


