Abstract
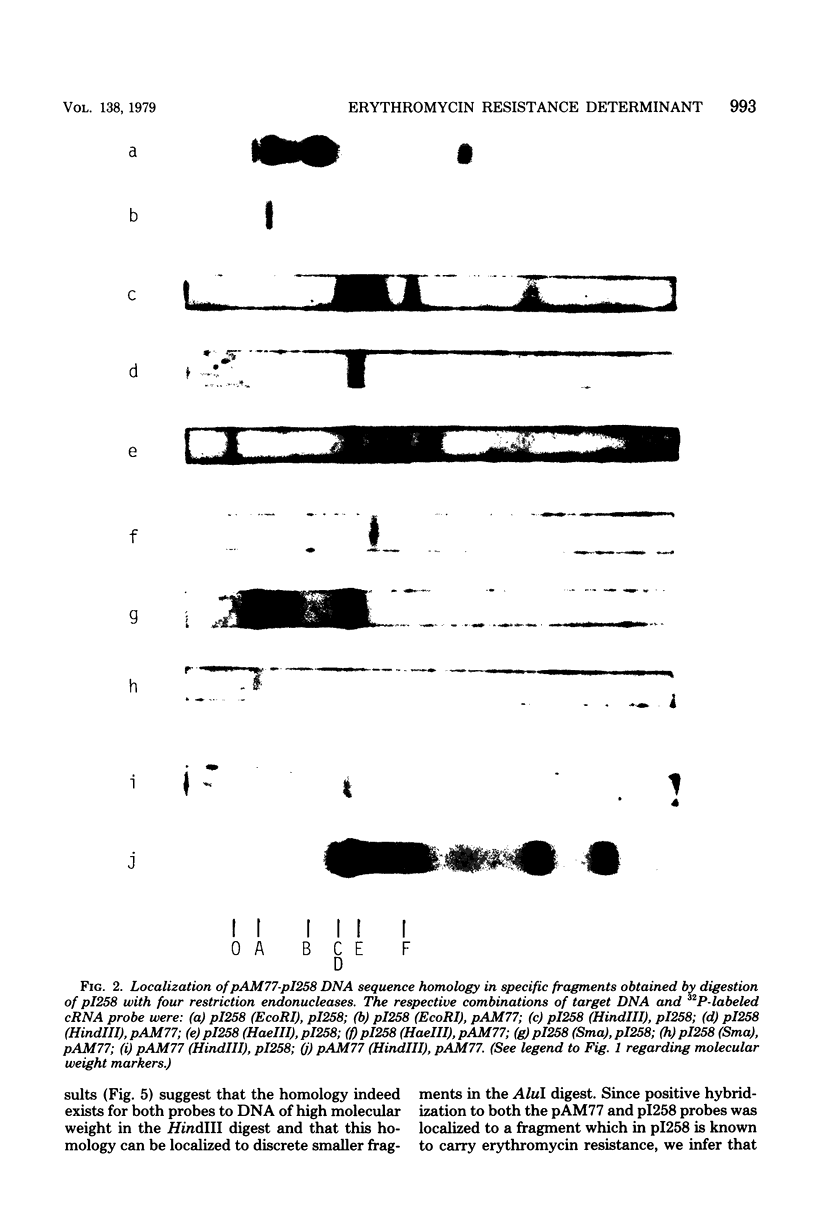
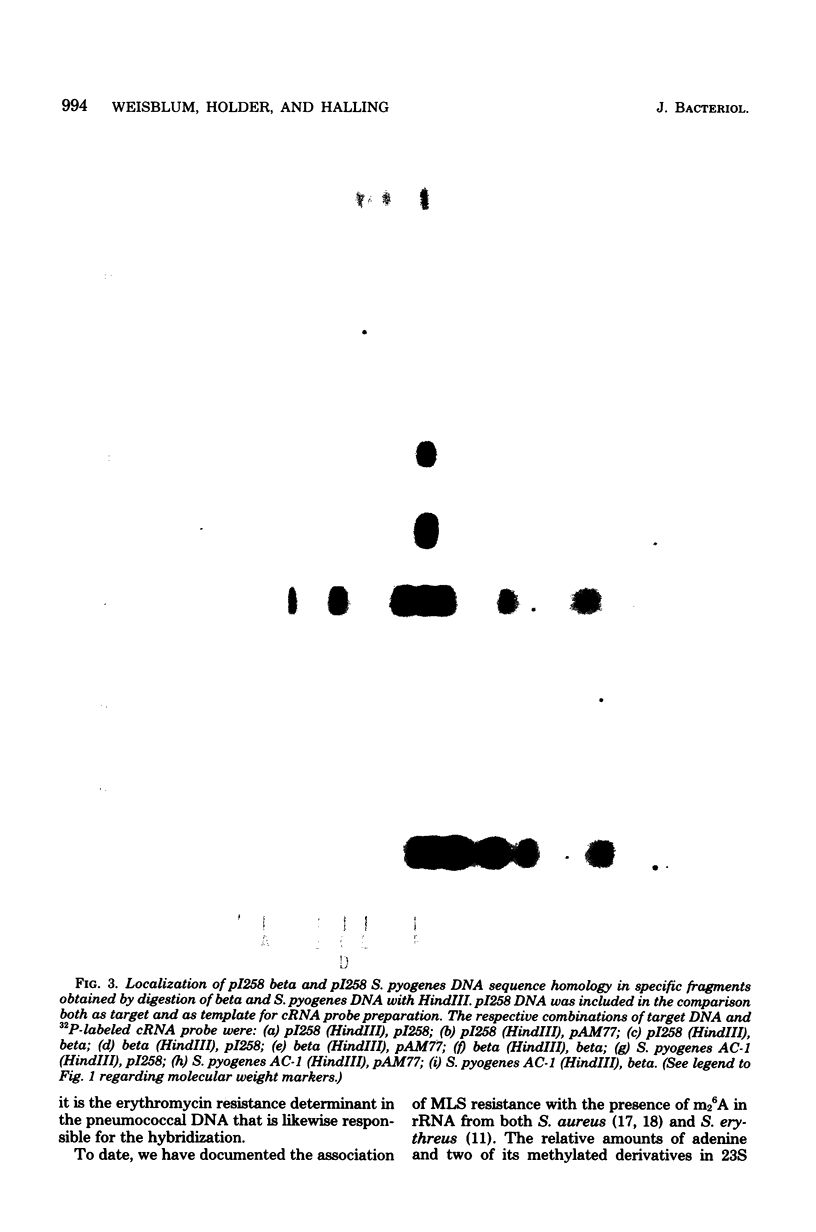
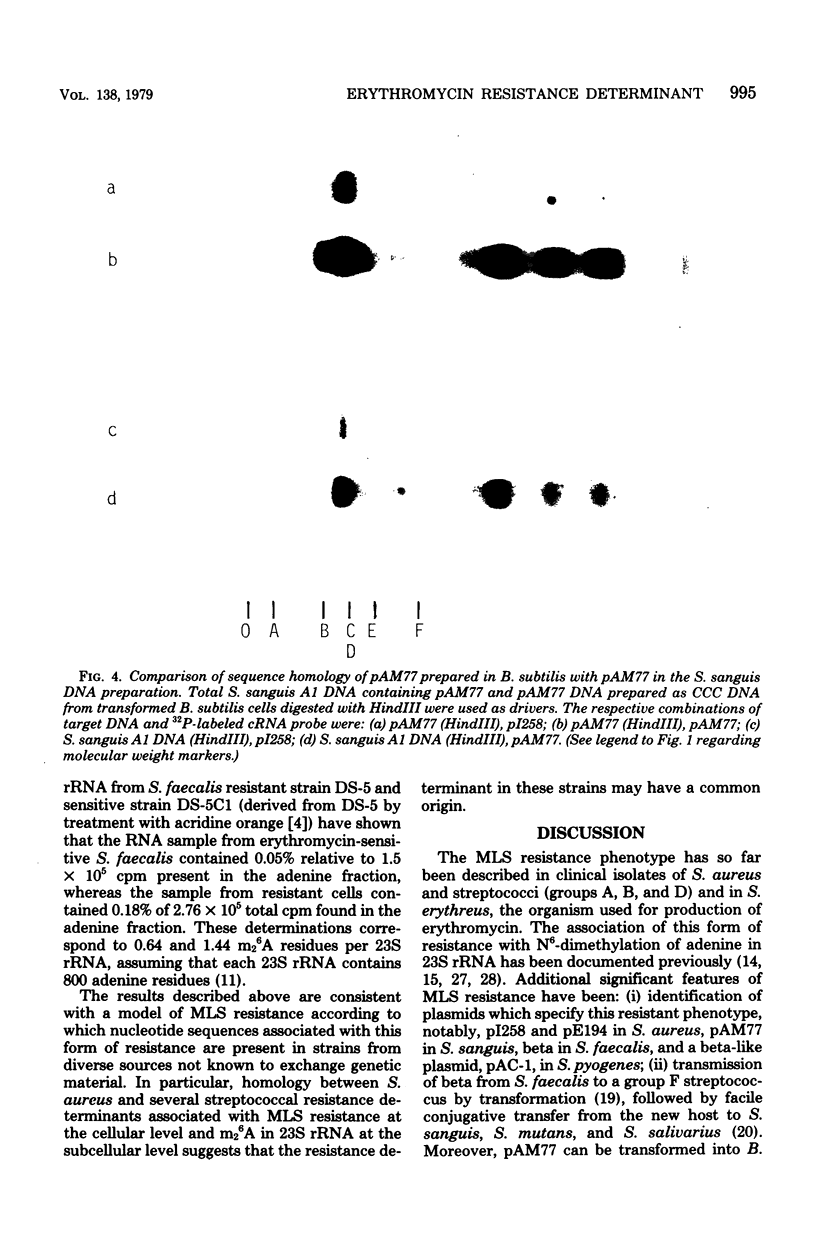
Plasmids from erythromycin-resistant Staphylococcus aureus, Streptococcus sanguis, and Streptococcus faecalis show deoxyribonucleic acid sequence homology. The homologous sequences can be localized to specific restriction endonuclease fragments, which in the case of S. aureus plasmid pI258 involves a single fragment from either EcoRI or HindIII digest known to contain the erythromycin resistance determinant. Complementary ribonucleic acid probes prepared from S. aureus plasmid pI258 and S. sanguis plasmid pAM77 also hybridize to specific fragments in restriction endonuclease digests of deoxyribonucleic acid from erythromycin-resistant Streptococcus progenes and Streptococcus pneumoniae. These studies suggest a common origin for a class of erythromycin resistance determinants in unrelated strains of pathogenic bacteria for which exchange of genetic material has not been demonstrated.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benveniste R., Davies J. Aminoglycoside antibiotic-inactivating enzymes in actinomycetes similar to those present in clinical isolates of antibiotic-resistant bacteria. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2276–2280. doi: 10.1073/pnas.70.8.2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHABBERT Y. Antagonisme in vitro entre l'érythromycine et la spiramycine. Ann Inst Pasteur (Paris) 1956 Jun;90(6):787–790. [PubMed] [Google Scholar]
- Clewell D. B., Franke A. E. Characterization of a plasmid determining resistance to erythromycin, lincomycin, and vernamycin Balpha in a strain Streptococcus pyogenes. Antimicrob Agents Chemother. 1974 May;5(5):534–537. doi: 10.1128/aac.5.5.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewell D. B., Yagi Y., Dunny G. M., Schultz S. K. Characterization of three plasmid deoxyribonucleic acid molecules in a strain of Streptococcus faecalis: identification of a plasmid determining erythromycin resistance. J Bacteriol. 1974 Jan;117(1):283–289. doi: 10.1128/jb.117.1.283-289.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courvalin P. M., Carlier C., Chabbert Y. A. Plasmid-linked tetracycline and erythromycin resistance in group D "streptococcus". Ann Inst Pasteur (Paris) 1972 Dec;123(6):755–759. [PubMed] [Google Scholar]
- Courvalin P. M., Carlier C., Croissant O., Blangy D. Identification of two plasmids determining resistance to tetracycline and to erythromycin in group D streptococcus. Mol Gen Genet. 1974;132(3):181–192. doi: 10.1007/BF00269391. [DOI] [PubMed] [Google Scholar]
- Dixon J. M., Lipinski A. E. Infections with beta-Hemolytic Streptococcus resistant to lincomycin and erythromycin and observations on zonal-pattern resistance to lincomycin. J Infect Dis. 1974 Oct;130(4):351–356. doi: 10.1093/infdis/130.4.351. [DOI] [PubMed] [Google Scholar]
- Dixon J. M., Lipinski A. E. Resistance of group A beta-hemolytic streptococci to lincomycin and erythromycin. Antimicrob Agents Chemother. 1972 Apr;1(4):333–339. doi: 10.1128/aac.1.4.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunny G. M., Clewell D. B. Transmissible toxin (hemolysin) plasmid in Streptococcus faecalis and its mobilization of a noninfectious drug resistance plasmid. J Bacteriol. 1975 Nov;124(2):784–790. doi: 10.1128/jb.124.2.784-790.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham M. Y., Weisblum B. 23S ribosomal ribonucleic acid of macrolide-producing streptomycetes contains methylated adenine. J Bacteriol. 1979 Mar;137(3):1464–1467. doi: 10.1128/jb.137.3.1464-1467.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershfield V. Plasmids mediating multiple drug resistance in group B streptococcus: transferability and molecular properties. Plasmid. 1979 Jan;2(1):137–149. doi: 10.1016/0147-619x(79)90012-x. [DOI] [PubMed] [Google Scholar]
- Horodniceanu T., Bouanchaud D. H., Bieth G., Chabbert Y. A. R plasmids in Streptococcus agalactiae (group B). Antimicrob Agents Chemother. 1976 Nov;10(5):795–801. doi: 10.1128/aac.10.5.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphreys G. O., Willshaw G. A., Anderson E. S. A simple method for the preparation of large quantities of pure plasmid DNA. Biochim Biophys Acta. 1975 Apr 2;383(4):457–463. doi: 10.1016/0005-2787(75)90318-4. [DOI] [PubMed] [Google Scholar]
- Jacob A. E., Hobbs S. J. Conjugal transfer of plasmid-borne multiple antibiotic resistance in Streptococcus faecalis var. zymogenes. J Bacteriol. 1974 Feb;117(2):360–372. doi: 10.1128/jb.117.2.360-372.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs M. R., Koornhof H. J., Robins-Browne R. M., Stevenson C. M., Vermaak Z. A., Freiman I., Miller G. B., Witcomb M. A., Isaäcson M., Ward J. I. Emergence of multiply resistant pneumococci. N Engl J Med. 1978 Oct 5;299(14):735–740. doi: 10.1056/NEJM197810052991402. [DOI] [PubMed] [Google Scholar]
- Lai C. J., Dahlberg J. E., Weisblum B. Structure of an inducibly methylatable nucleotide sequence in 23S ribosomal ribonucleic acid from erythromycin-resistant Staphylococcus aureus. Biochemistry. 1973 Jan 30;12(3):457–460. doi: 10.1021/bi00727a015. [DOI] [PubMed] [Google Scholar]
- Lai C. J., Weisblum B. Altered methylation of ribosomal RNA in an erythromycin-resistant strain of Staphylococcus aureus. Proc Natl Acad Sci U S A. 1971 Apr;68(4):856–860. doi: 10.1073/pnas.68.4.856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBlanc D. J., Hassell F. P. Transformation of Streptococcus sanguis Challis by plasmid deoxyribonucleic acid from Streptococcus faecalis. J Bacteriol. 1976 Oct;128(1):347–355. doi: 10.1128/jb.128.1.347-355.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBlanc D. J., Hawley R. J., Lee L. N., St Martin E. J. "Conjugal" transfer of plasmid DNA among oral streptococci. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3484–3487. doi: 10.1073/pnas.75.7.3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malke H. Genetics of resistance to macrolide antibiotics and lincomycin in natural isolates of Streptococcus pyogenes. Mol Gen Genet. 1974;135(4):349–367. doi: 10.1007/BF00271149. [DOI] [PubMed] [Google Scholar]
- Malke H., Jacob H. E., Störl K. Characterization of the antibiotic resistance plasmid ERL1 from Streptococcus pyogenes. Mol Gen Genet. 1976 Mar 30;144(3):333–338. doi: 10.1007/BF00341732. [DOI] [PubMed] [Google Scholar]
- Novick R. P., Bouanchaud D. The problems of drug-resistant pathogenic bacteria. Extrachromosomal nature of drug resistance in Staphylococcus aureus. Ann N Y Acad Sci. 1971 Jun 11;182:279–294. doi: 10.1111/j.1749-6632.1971.tb30664.x. [DOI] [PubMed] [Google Scholar]
- Novick R. P., Edelman I., Schwesinger M. D., Gruss A. D., Swanson E. C., Pattee P. A. Genetic translocation in Staphylococcus aureus. Proc Natl Acad Sci U S A. 1979 Jan;76(1):400–404. doi: 10.1073/pnas.76.1.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick R. P., Murphy E., Gryczan T. J., Baron E., Edelman I. Penicillinase plasmids of Staphylococcus aureus: restriction-deletion maps. Plasmid. 1979 Jan;2(1):109–129. doi: 10.1016/0147-619x(79)90010-6. [DOI] [PubMed] [Google Scholar]
- Radloff R., Bauer W., Vinograd J. A dye-buoyant-density method for the detection and isolation of closed circular duplex DNA: the closed circular DNA in HeLa cells. Proc Natl Acad Sci U S A. 1967 May;57(5):1514–1521. doi: 10.1073/pnas.57.5.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Weisblum B., Graham M. Y., Gryczan T., Dubnau D. Plasmid copy number control: isolation and characterization of high-copy-number mutants of plasmid pE194. J Bacteriol. 1979 Jan;137(1):635–643. doi: 10.1128/jb.137.1.635-643.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson C. R., Baldwin N. J. Characterization and construction of molecular cloning vehicles within Staphylococcus aureus. J Bacteriol. 1978 Oct;136(1):402–413. doi: 10.1128/jb.136.1.402-413.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yag Y., Franke A. E., Clewell D. B. Plasmid-determined resistance to erythromycin: comparison of strains of streptococcus faecalis and streptococcus pyogenes with regard to plasmid hmology and resistance inducibility. Antimicrob Agents Chemother. 1975 Jun;7(6):871–873. doi: 10.1128/aac.7.6.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagi Y., McLellan T. S., Frez W. A., Clewell D. B. Characterization of a small plasmid determining resistance to erythromycin, lincomycin, and vernamycin Balpha in a strain of Streptococcus sanguis isolated from dental plaque. Antimicrob Agents Chemother. 1978 May;13(5):884–887. doi: 10.1128/aac.13.5.884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Embden J. D., Engel H. W., van Klingeren B. Drug resistance in group D streptococci of clinical and nonclinical origin: prevalence, transferability, and plasmid properties. Antimicrob Agents Chemother. 1977 Jun;11(6):925–932. doi: 10.1128/aac.11.6.925. [DOI] [PMC free article] [PubMed] [Google Scholar]