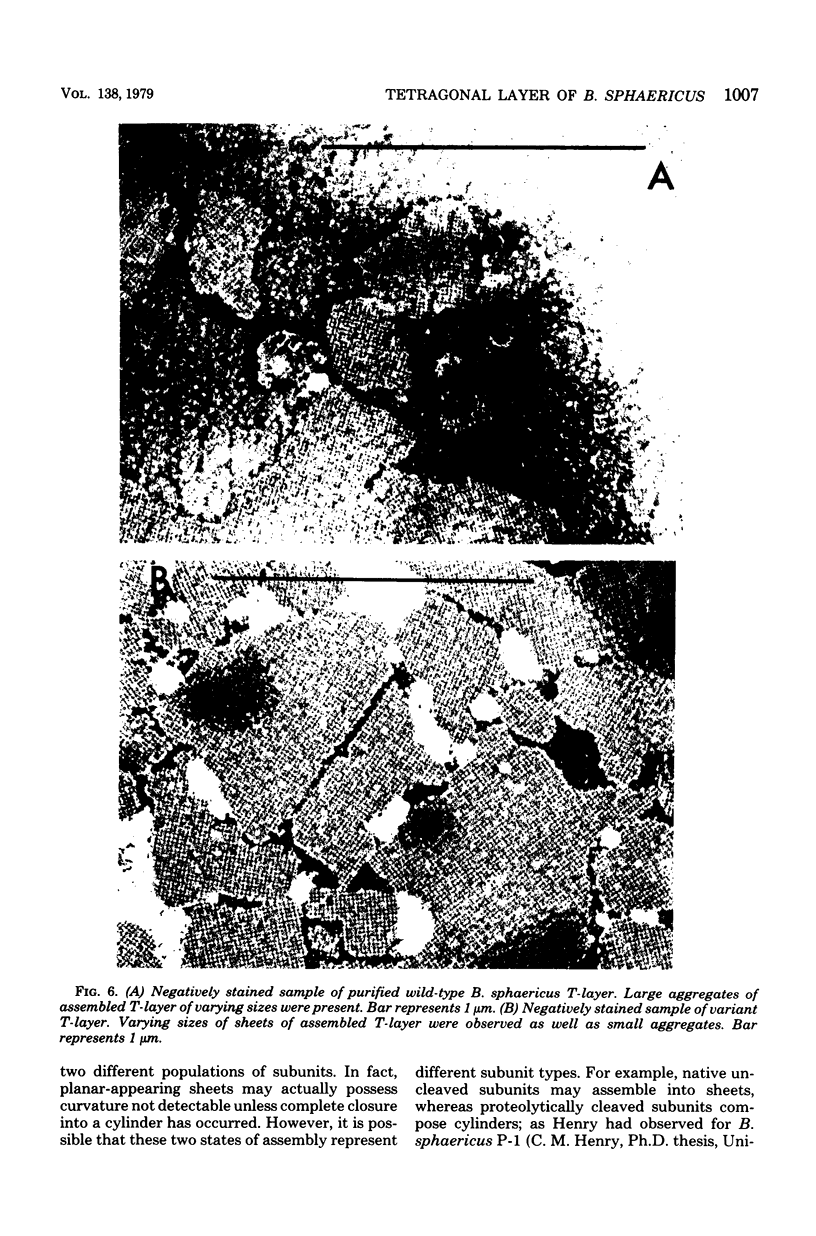
Abstract
The tetragonally arranged cell wall layer (T-layer) of Bacillus sphaericus NTCC 9602 was isolated and characterized. Parallel studies were made on a spontaneous variant of the wild-type strain which had a T-layer subunit of altered molecular weight. A purification method for the T-layers was devised which involved separation of the cell walls from the cytoplasmic contents, urea dissociation of the T-layer from the cell walls, removal of soluble contaminants by differential centrifugation, and finally selective adsorption of uncleaved subunits to sacculi. The purified subunits retained the capacity to form an assembly in vitro with the same lattice parameters as that observed on whole cells or cell walls and could readsorb to the cell walls from which they had been extracted. Both the wild-type and the variant subunits behaved as single, homogeneous polypeptide chains. Carbohydrate assay and isoelectric point determinations revealed that both subunit types were acidic glycoproteins. Values obtained for thebuoyant density, isoelectric point, and extinction coefficient differed minimally; major differences were observed in the molecular weight and the characterisitc width of cylinders formed by in vitro-assembled T-layer of the wild-type and variant. Assembled T-layer was subject to alkaline or acid dissociation and in acid titration dissociated at its isoelectric point.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beaman T. C., Pankratz H. S., Gerhardt P. Paracrystalline sheets reaggregated from solubilized exosporium of Bacillus cereus. J Bacteriol. 1971 Jul;107(1):320–324. doi: 10.1128/jb.107.1.320-324.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckmire F. L., Murray R. G. Studies on the cell wall of Spirillum serpens. 1. Isolation and partial purification of the outermost cell wall layer. Can J Microbiol. 1970 Oct;16(10):1011–1022. doi: 10.1139/m70-171. [DOI] [PubMed] [Google Scholar]
- Buckmire F. L., Murray R. G. Studies on the cell wall of Spirillum serpens. II. Chemical characterization of the outer structured layer. Can J Microbiol. 1973 Jan;19(1):59–66. doi: 10.1139/m73-009. [DOI] [PubMed] [Google Scholar]
- Buckmire F. L., Murray R. G. Substructure and in vitro assembly of the outer, structured layer of Spirillum serpens. J Bacteriol. 1976 Jan;125(1):290–299. doi: 10.1128/jb.125.1.290-299.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chester I. R., Murray R. G. Protein-lipid-lipopolysaccharide association in the superficial layer of Spirillum serpens cell walls. J Bacteriol. 1978 Feb;133(2):932–941. doi: 10.1128/jb.133.2.932-941.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischman D. A., Weinbaum G. Hexagonal pattern in cell walls of Escherichia coli B. Science. 1967 Jan 27;155(3761):472–474. doi: 10.1126/science.155.3761.472. [DOI] [PubMed] [Google Scholar]
- Hastie A. T., Brinton C. C., Jr Specific interaction of the tetragonally arrayed protein layer of Bacillus sphaericus with its peptidoglycan sacculus. J Bacteriol. 1979 Jun;138(3):1010–1021. doi: 10.1128/jb.138.3.1010-1021.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henning U. L. Determination of cell shape in bacteria. Annu Rev Microbiol. 1975;29:45–60. doi: 10.1146/annurev.mi.29.100175.000401. [DOI] [PubMed] [Google Scholar]
- Howard L., Tipper D. J. A polypeptide bacteriophage receptor: modified cell wall protein subunits in bacteriophage-resistant mutants of Bacillus sphaericus strain P-1. J Bacteriol. 1973 Mar;113(3):1491–1504. doi: 10.1128/jb.113.3.1491-1504.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hungerer K. D., Tipper D. J. Cell wall polymers of Bacillus sphaericus 9602. I. Structure of the vegetative cell wall peptidoglycan. Biochemistry. 1969 Sep;8(9):3577–3587. doi: 10.1021/bi00837a013. [DOI] [PubMed] [Google Scholar]
- KUSHNER D. J., BAYLEY S. T., BORING J., KATES M., GIBBONS N. E. MORPHOLOGICAL AND CHEMICAL PROPERTIES OF CELL ENVELOPES OF THE EXTREME HALOPHILE, HALOBACTERIUM CUTIRUBRUM. Can J Microbiol. 1964 Jun;10:483–497. doi: 10.1139/m64-058. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Low R. B., Wool I. G., Martin T. E. Skeletal muscle ribosomal proteins: general characteristics and effect of diabetes. Biochim Biophys Acta. 1969 Nov 11;194(1):190–202. doi: 10.1016/0005-2795(69)90194-9. [DOI] [PubMed] [Google Scholar]
- Marshall C. L., Wicken A. J., Brown A. D. The outer layer of the cell envelope of Halobacterium halobium. Can J Biochem. 1969 Jan;47(1):71–74. doi: 10.1139/o69-013. [DOI] [PubMed] [Google Scholar]
- Mescher M. F., Strominger J. L. Structural (shape-maintaining) role of the cell surface glycoprotein of Halobacterium salinarium. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2687–2691. doi: 10.1073/pnas.73.8.2687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nermut M. V., Murray R. G. Ultrastructure of the cell wall of Bacillus polymyxa. J Bacteriol. 1967 Jun;93(6):1949–1965. doi: 10.1128/jb.93.6.1949-1965.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid M. S., Bieleski R. L. A simple apparatus for vertical flat-sheet polyacrylamide gel electrophoresis. Anal Biochem. 1968 Mar;22(3):374–381. doi: 10.1016/0003-2697(68)90278-9. [DOI] [PubMed] [Google Scholar]
- Righetti P., Drysdale J. W. Isoelectric focusing in polyacrylamide gels. Biochim Biophys Acta. 1971 Apr 27;236(1):17–28. doi: 10.1016/0005-2795(71)90144-9. [DOI] [PubMed] [Google Scholar]
- Rosenbusch J. P. Characterization of the major envelope protein from Escherichia coli. Regular arrangement on the peptidoglycan and unusual dodecyl sulfate binding. J Biol Chem. 1974 Dec 25;249(24):8019–8029. [PubMed] [Google Scholar]
- Schwarz U., Asmus A., Frank H. Autolytic enzymes and cell division of Escherichia coli. J Mol Biol. 1969 May 14;41(3):419–429. doi: 10.1016/0022-2836(69)90285-x. [DOI] [PubMed] [Google Scholar]
- Sleytr U. B., Glauert A. M. Ultrastructure of the cell walls of two closely related clostridia that possess different regular arrays of surface subunits. J Bacteriol. 1976 May;126(2):869–882. doi: 10.1128/jb.126.2.869-882.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleytr U. B. Regular arrays of macromolecules on bacterial cell walls: structure, chemistry, assembly, and function. Int Rev Cytol. 1978;53:1–62. doi: 10.1016/s0074-7696(08)62240-8. [DOI] [PubMed] [Google Scholar]
- Sleytr U. B., Thorne K. J. Chemical characterization of the regularly arranged surface layers of Clostridium thermosaccharolyticum and Clostridium thermohydrosulfuricum. J Bacteriol. 1976 Apr;126(1):377–383. doi: 10.1128/jb.126.1.377-383.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steven A. C., Heggeler B., Müller R., Kistler J., Rosenbusch J. P. Ultrastructure of a periodic protein layer in the outer membrane of Escherichia coli. J Cell Biol. 1977 Feb;72(2):292–301. doi: 10.1083/jcb.72.2.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorne K. J., Thornley M. J., Glauert A. M. Chemical analysis of the outer membrane and other layers of the cell envelope of Acinetobacter sp. J Bacteriol. 1973 Oct;116(1):410–417. doi: 10.1128/jb.116.1.410-417.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornley M. J., Thorne K. J., Glauert A. M. Detachment and chemical characterization of the regularly arranged subunits from the surface of an Acinetobacter. J Bacteriol. 1974 May;118(2):654–662. doi: 10.1128/jb.118.2.654-662.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vesterberg O. Isoelectric focusing of proteins in polyacrylamide gels. Biochim Biophys Acta. 1972 Jan 26;257(1):11–19. doi: 10.1016/0005-2795(72)90248-6. [DOI] [PubMed] [Google Scholar]
- Wrigley N. G. The lattice spacing of crystalline catalase as an internal standard of length in electron microscopy. J Ultrastruct Res. 1968 Sep;24(5):454–464. doi: 10.1016/s0022-5320(68)80048-6. [DOI] [PubMed] [Google Scholar]
- Zacharius R. M., Zell T. E., Morrison J. H., Woodlock J. J. Glycoprotein staining following electrophoresis on acrylamide gels. Anal Biochem. 1969 Jul;30(1):148–152. doi: 10.1016/0003-2697(69)90383-2. [DOI] [PubMed] [Google Scholar]