Abstract
Species-specific adhesion of sperm to the egg during sea urchin fertilization involves the interaction of the sperm adhesive protein,bindin, and a complementary receptor on the egg surface,and serves to restrict the gene pool to individuals of the same species. We used PCR representation difference analysis to clone the species-specific egg receptor for bindin, EBR1, from Strongylocentrotus franciscanus (Sf) and S. purpuratus (Sp). Sf-EBR1 contains a novel ADAMTS-like N-terminal domain followed by ∼19 tandem EBR repeats consisting of alternating CUB and thrombospondin type 1 (TSP-1) domains where the last 10 EBR repeats are species-specific and highly conserved. Recombinant protein corresponding to the species-specific EBR repeat displays species-specific sperm adhesion and bindin-binding activity. The Sp-EBR1 ortholog has the same ADAMTS (a disintegrin and metalloprotease with thrombospondin type-1 modules) core region followed by eight and one-half tandem egg bindin receptor (EBR) repeats that share 88% identity with the Sf-EBR1 repeats,but has an entirely different species-specific domain consisting of hyalin-like (HYR) repeats. Thus,the species-specific domains of egg bindin receptor 1 (EBR1) from both species function as the egg surface receptor to mediate species-specific sperm adhesion.
Keywords: EBR1, ADAMTS, TSP-1, Hyalin, sea urchin, species specificity
Sperm–egg interaction typically exhibits species specificity in most animals. Several molecules from both sperm and eggs that are thought to mediate gamete interaction have been identified in many animals. In abalone, the acrosomal protein of sperm, lysin, interacts with the egg vitelline envelope receptor for lysin (VERL) and creates a hole on the extracellular matrix (ECM) of the egg with species selectivity (Swanson and Vacquier 1997). In mammals, numerous molecules, such as galactosyltransferase, ADAM (a disintegrin and metalloprotease), and Sp56, have been identified from sperm and are proposed to interact with such as egg zona pellucida proteins Zp2, Zp3, or Integrins on the egg. However, a clear understanding of the specificity of these interactions and the regulation of species specificity is not yet known (Primakoff and Myles 2002; He et al. 2003).
Acrosome-reacted sea urchin sperm adhere to glycoprotein receptors that are components of the vitelline envelope (VE) known as the ECM of the egg. It has been known for several decades that the protein bindin on the acrosome-reacted sea urchin sperm head mediates the species-specific adhesion of sperm to the VE during fertilization (Summers and Hylander 1975; Glabe and Vacquier 1977b; Vacquier and Moy 1977; Glabe et al. 1982). The egg ECM also serves as a major barrier for interspecific fertilization in mammalian systems (Yanagimachi 1981, 1994). In sea urchins, the complementary receptor for bindin was shown to be an ∼350-kD protein on the VE of the egg (Glabe and Vacquier 1978; Carroll et al. 1986; Ruiz-Bravo and Lennarz 1986; Ohlendieck et al. 1993). However, the identity of this receptor has remained elusive, and attempts to solubilize and purify the active receptor from the VE of the egg have not been successful (Glabe and Lennarz 1981; Ruiz-Bravo et al. 1986). Therefore, we used a novel molecular approach, representation difference analysis (RDA), to amplify species-specific cDNA fragments from ovaries of the sea urchin Strongylocentrotus franciscanus (Sf) that are distinct from cDNAs fragments in another species of the same genus, S. purpuratus (Sp), to identify genes involved in determining species specificity (Hubank and Schatz 1994; Kamei et al. 2000).
Here we report the identification and characterization of the species-specific egg bindin receptor (EBR1) from two sea urchin species, S. franciscanus and S. purpuratus, along with their unique domain structures. The biological activity of recombinant species-specific egg bindin receptor (EBR) and hyalin-like (HYR) repeats indicate that they serve as species-specific ligands for the sperm adhesive protein bindin.
Results and Discussion
Characterization of the species-specific cDNA clone, Sf-H2
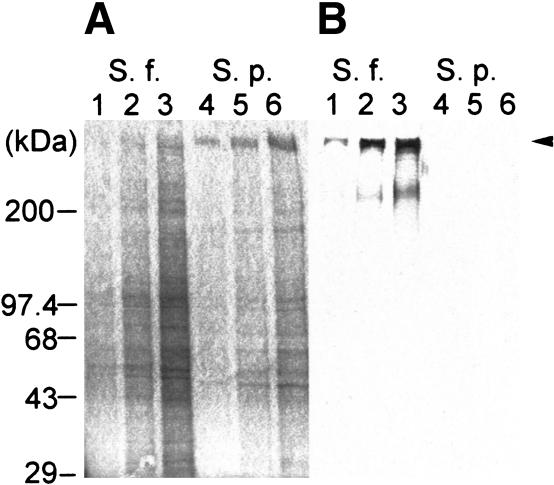
We isolated four species-specific cDNA clones from S. franciscanus ovary mRNA, as previously described (Kamei et al. 2000). One of the clones (Sf-H2) was a candidate for the egg-bindin receptor because it hybridizes species-specifically to an ∼14–15-kb mRNA (see below) and therefore is sufficiently large to encode the 350-kD egg receptor protein. We raised polyclonal antibodies against recombinant Sf-H2 protein, and the antibodies detect a protein of ∼350 kD on purified VEs from S. franciscanus, but not S. purpuratus eggs (Fig. 1A,B). Thus, the Sf-H2 cDNA probe and antibody detect molecules of the size predicted for the egg receptor.
Figure 1.
Species specificity of Sf-H2. (A) Coomassie blue staining of purified VEs from S. franciscanus and S. purpuratus separated on a 4%–20% SDS-PAGE gel. (Lanes 1–3) S. franciscanus VE. (Lanes 4–6) S. purpuratus VE. (B) Western blot analysis of VE from S. franciscanus and S. purpuratus separated on a 4%–20% SDS-PAGE gel (shown in A) using anti-Sf-H2 antibody. The arrow highlights the ∼350-kD protein detected by the anti-Sf-H2 antibody. (Lanes 1–3) S. franciscanus VE. (Lanes 4–6) S. purpuratus VE. Lanes 1 and 4 are 2 μg of VE protein, lanes 2 and 5 are 5 μg of VE protein, and lanes 3 and 6 are 10 μg of VE protein.
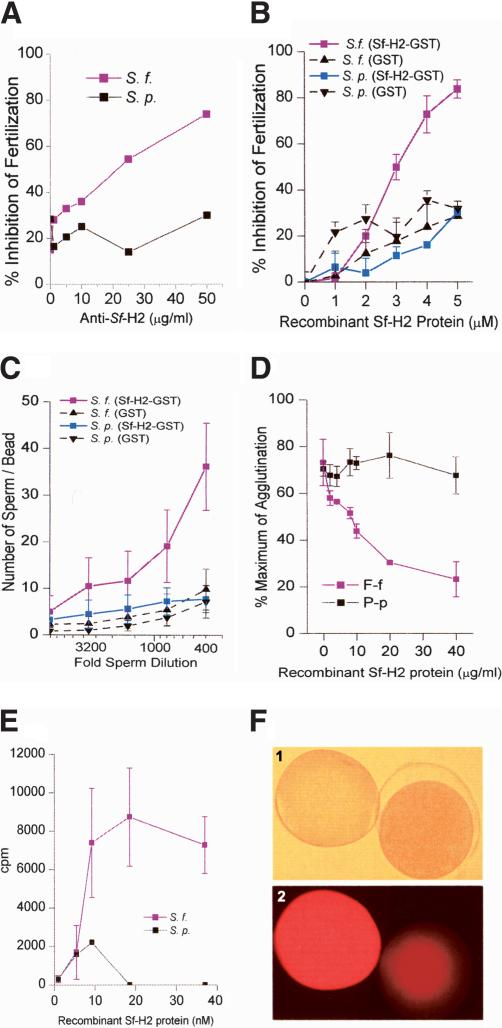
Because the Sf-H2 transcript encodes a species-specific VE protein of the size and location expected for the receptor, we determined whether Sf-H2 displayed species-specific adhesive activity by using several different assays. We first examined the effect of anti-Sf-H2 antibody or recombinant Sf-H2 protein on fertilization. Anti-Sf-H2 polyclonal antibody inhibited fertilization of S. franciscanus eggs in a concentration-dependent manner, but had no effect on the fertilization of S. purpuratus eggs (Fig. 2A). Similarly, recombinant Sf-H2 protein only inhibited S. franciscanus fertilization, but not S. purpuratus fertilization (Fig. 2B). These results suggest that anti-Sf-H2 antibodies block the receptor on the egg, and the recombinant Sf-H2 protein competitively inhibits the binding of sperm to the VE receptor. We also directly examined sperm adhesive activity of Sf-H2 by using a recombinant glutathione S transferase (GST) fusion protein immobilized on glutathione agarose beads to assess sperm adhesion to the beads. We found that acrosome-reacted S. franciscanus sperm specifically bound to beads containing immobilized Sf-H2 GST fusion protein, but not to control beads containing GST alone (Fig. 2C). These data are consistent with the interpretation that Sf-H2 encodes a species-specific sperm receptor.
Figure 2.
Inhibition of fertilization by anti-Sf-H2 antibody and recombinant Sf-H2 protein. (A) Species-specific inhibition of S. franciscanus fertilization by anti-Sf-H2 antibody. Increasing concentrations of the antibody only inhibit the fertilization of homologous eggs. Red line indicates S. franciscanus gametes; black line indicates S. purpuratus gametes. (B) Inhibition of fertilization by recombinant Sf-H2. Increasing concentrations of Sf-H2 inhibits only the fertilization of homologous eggs. Both red and blue lines show the percentage of inhibition of fertilization by using recombinant Sf-H2–GST. Both broken lines indicate control samples GST only. (C) Species-specific binding of acrosome-reacted sperm to immobilized recombinant Sf-H2 protein. The graph shows the number of sperm binding to the beads. Red line indicates S. franciscanus sperm; blue line, S. purpuratus sperm. Both broken lines show the binding to GST controls. (D) Species-specific inhibition of bindin-mediated egg agglutination by recombinant Sf-H2 protein. The percentage of agglutinated eggs is shown as a function of increasing recombinant Sf-H2 protein. Red line indicates S. franciscanus eggs and S. franciscanus bindin; black line indicates S. purpuratus eggs and S. purpuratus bindin. (E) Species-specific binding of recombinant iodinated Sf-H2 to purified bindin particles. Red line indicates S. franciscanus bindin; black line, S. purpuratus bindin. (F) Immunostaining of unfertilized and fertilized S. franciscanus eggs with anti-Sf-H2 antibody. Panel 1 shows the phase contrast image; panel 2, the bright-field immunofluorescence staining with anti-Sf-H2 antibody. The egg on the left is unfertilized, and the one on the right is fertilized.
The sperm adhesive protein bindin is commonly purified as insoluble acrosomal granules, and these bindin granules were shown to species-specifically agglutinate eggs of the same species (Glabe and Lennarz 1979). This agglutination activity of bindin granules can be used to measure the adhesive interaction between bindin and the egg surface (Glabe and Lennarz 1979). Recombinant Sf-H2 protein specifically inhibits agglutination of S. franciscanus eggs by homologous bindin, but does not inhibit the agglutination of S. purpuratus eggs by S. purpuratus bindin (Fig. 2D). We also directly measured the interaction of iodinated recombinant Sf-H2 protein to purified bindin granules using a sedimentation assay. Recombinant Sf-H2 protein bound only to S. franciscanus bindin granules in a concentration-dependent and saturable manner (Fig. 2E). Therefore, Sf-H2 satisfies a number of different functional criteria for the egg surface adhesive receptor for bindin.
The egg surface receptor for sperm is either released from the VE or destroyed due to the action of proteases that are released from the cortical granules upon fertilization (Vacquier et al. 1973; Glabe and Vacquier 1977a). We examined the localization of the Sf-H2 on whole mounts of fertilized and unfertilized eggs by immunofluorescence staining with anti-Sf-H2 antibodies. Bright staining was detected on the unfertilized egg surface, but this immunoreactivity was greatly diminished on the fertilization envelope and egg surface after fertilization (Fig. 2F). The absence of anti-Sf-H2 staining in the fertilization envelope of fertilized eggs is consistent with the known release or destruction of the receptor from the elevating fertilization envelope after fertilization.
The Sf-H2 gene product meets all of the known structural and functional criteria for the egg receptor, including size, VE localization and modification after fertilization, species-specific sperm, and bindin-binding activity. Thus, from these data, we conclude that Sf-H2 is the egg receptor for sperm, and we have named this gene product egg bindin receptor (EBR1).
Comparison of the sequences of EBR1 from both S. franciscanus and S. purpuratus
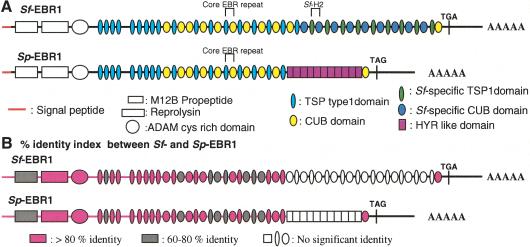
The deduced amino acid sequence of Sf-EBR1 consists of 4595 amino acids (GenBank accession no. AY138966). Starting at the N terminus, the protein contains a 30-residue signal peptide followed by a putative propeptide, metalloprotease domain, cis-rich domain, and eight thrombospondin type 1 (TSP-1) domains, indicating that EBR1 shares several significant structural features with members of the ADAMTS family of proteins (a disintegrin and metalloprotease with thrombospondin type-1 modules; Tang and Hong 1999; Tang 2001). However, Sf-EBR1 lacks a disintegrin-like domain that typically follows the catalytic domain in ADAMTS family members. Sf-EBR1 also contains an additional C-terminal region containing ∼19 repeats of a novel motif (EBR repeats) located immediately after the ADAMTS-like core sequence (Fig. 3A). These novel motifs contain a TSP-1 and a CUB (C1s/C1r, uEGF and bone morphogenic protein) subdomain (Bork and Beckmann 1993; Adams 1997). Each TSP-1 domain is from 53 to 59 amino acids, and each CUB domain is ∼104–123 amino acid residues. Thus, each EBR repeat is ∼513 bp or 171 amino acids. The nine EBR repeats that follow immediately after the ADAMTS-like core region of Sf-EBR1 are significantly more variable than the last 10 EBR repeats and are defined as core EBR repeats because they also occur in Sp-EBR1 (TSP, thin light blue ovals; CUB, yellow ovals; Fig. 3A). The last 10 EBR repeats of Sf-EBR1 are homogeneous (TSP, thin green ovals; CUB, dark blue ovals), and each domain shows 85%–100% identity with the other repeats in this region. The last 10 homogeneous repeats are also species-specific, because they do not hybridize to S. purpuratus mRNA and are absent in Sp-EBR1 (see below). The original cDNA fragment isolated by RDA, Sf-H2, corresponds to one of these species-specific EBR repeats. In contrast, the TSP-1 domains outside of the EBR repeats in the ADAMTS-like core region and the TSP-1 subdomains present in the first nine core EBR repeats (thin light blue ovals) contain only 12%–50% identity with the TSP-1 subdomains in the species-specific EBR repeats (green ovals). Similarly, the first nine and the last CUB subdomains that are in the EBR repeat (yellow ovals) display only 11%–20% identity with the CUB subdomains in the species-specific EBR repeats (dark blue ovals; Fig. 3A). The second core EBR repeat for S. franciscanus (also for S. purpuratus; see Fig. 3A) has a duplicated CUB domain in contrast with all other EBR repeats, which have one CUB domain.
Figure 3.
Domain structures and homology index of Sf-EBR1 and Sp-EBR1. (A) The horizontal red line shows signal peptides; the vertical line at the right after the last CUB domain shows the stop codon. From the left, the boxes show propeptide, reprolysin, ADAM cis-rich domain, TSP-1 domain repeats, core EBR repeats (alternative TSP-1 and CUB domain repeats; light blue and yellow ovals), and species-specific EBR repeats (dark blue and green ovals; for the Sf-EBR1) or HYR-like domain repeats (purple boxes; for the Sp-EBR1). An EBR repeat represents a pair of TSP-1 and CUB (e.g., the set of thin green oval and dark blue oval) of Sf-EBR1. (B) Graphic representation of the homology between Sf-EBR1 and Sp-EBR1. Domains shown in pink represent >80% amino acid identity; those shown in gray represent 60%–80% identity between S. franciscanus and S. purpuratus EBR1 at the amino acid level. No significant identity was observed in the white-colored region.
Although the species-specific Sf-EBR1 repeats do not hybridize with S. purpuratus DNA, S. purpuratus has a homologous EBR1 gene because the 3′ UTR of Sf-EBR1 hybridizes to the same size fragment on genomic Southern blots of S. purpuratus DNA(data not shown). The EBR1 gene was cloned and sequenced from an S. purpuratus ovary cDNA library by using the 3′ UTR of Sf-EBR1 as an initial probe and the same cloning and sequencing strategy used for Sf-EBR1 (GenBank accession no. AY341264). The gene encodes a protein with a strikingly similar repeat structure but with some notable differences compared with Sf-EBR1. The deduced amino acids sequence of Sp-EBR1 consists of 3713 amino acid residues. Starting at the N terminus, the protein contains a 30-residue signal peptide followed by a putative propeptide, metalloprotease domain, cis-rich domain, eight TSP-1 domains, and eight and one-half core EBR repeats. Thus, Sp-EBR1 shares the same domain structure as Sf-EBR1 for the first ∼8 kb of sequence and shares 88% nucleotide identity with Sf-EBR1 in this region (Fig. 3B). This includes the first eight and one-half core EBR repeats, which have 80%–90% identity with the corresponding EBR repeats in Sf-EBR1. The last CUB domain of both Sf-EBR1 and Sp-EBR1 also shares 86% identity (Fig. 3B). However, the region of Sp-EBR1 corresponding to the species-specific Sf-EBR1 domains (dark blue and green ovals or white ovals) contains 11 repeats of a HYR domain (purple boxes or white boxes) and not the homogeneous EBR repeats observed in Sf-EBR1 (Fig. 3A,B). Each HYR repeat is 243 bp or 81 amino acids, and each repeat, except the first and the last HYR repeat, shows ∼95% sequence identity. The HYR repeat domain in Sp-EBR1 is distinct from the previously reported hyalin gene containing 12 HYR repeats in S. pupuratus (Wessel et al. 1998), with <40% amino acid identity when compared with HYR-like domains in Sp-EBR1. We examined the adhesive function of the Sp-HYR by measuring the adhesion of sperm to recombinant Sp-HYR expressed as a GST fusion protein. S. purpuratus sperm bind specifically to Sp-HYR compared with S. franciscanus sperm and GST controls (Fig. 4). Therefore, the species-unique HYR domain of Sp-EBR1 functions as a species-specific receptor for sperm adhesion analogous to the species-specific EBR repeats of Sf-EBR1 (Fig. 2C). The deduced EBR1 sequences from both species lack an identifiable transmembrane domain, but EBR1 would be expected to be incorporated into the VE ECM based on its similarity to other ADAMTS proteins (Kuno and Matsushima 1998).
Figure 4.
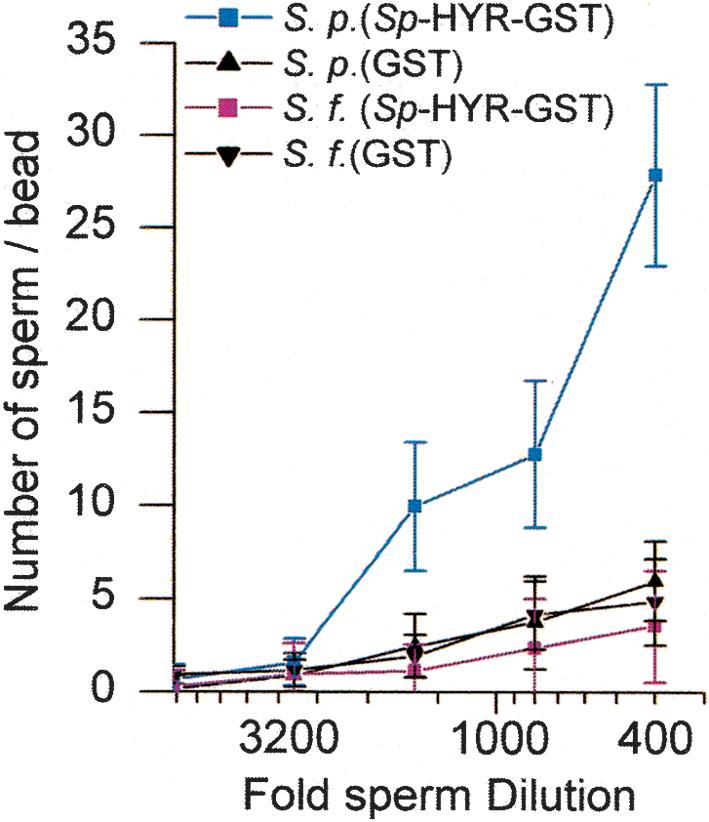
Species-specific binding of acrosome-reacted sperm to immobilized recombinant Sp-HYR protein. The graph shows the number of sperm binding to the beads. Blue line indicates S. purpuratus sperm; red line, S. franciscanus sperm. Both black lines show the binding to GST controls.
Northern blot analyses were performed with both common and species-specific probes to examine if the probes hybridize to the same size transcript. The common probe derived from the 5′ region of Sf-EBR1 detected a single mRNA species of ∼14–15 kb for S. franciscanus and 12–13 kb for S. purpuratus. Each species-specific region of EBR1 hybridized to the same size transcript identified with the 5′ common region probe, but in a species-specific manner (Fig. 5). Reverse transcriptase PCR (RT–PCR) analysis also showed that the EBR1 gene that was originally cloned from ovary cDNA library exists in unfertilized eggs but is not detected in sperm (data not shown).
Figure 5.
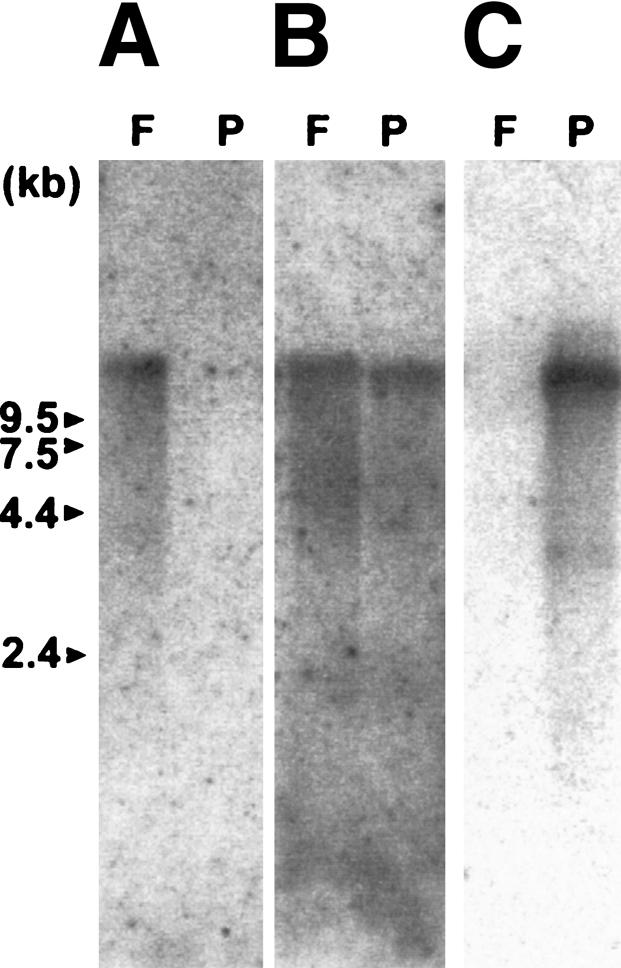
Northern blot analysis of S. franciscanus and S. purpuratus ovary mRNAs. (F) S. franciscanus ovary mRNA; (P) S. purpuratus ovary mRNA (1 μg/lane). The probes are as follows: Sf-EBR repeat (H2) DNA(A), 5′region (TSP-1 repeats through the first TSP-CUB domains) of Sf-EBR1 (B), and Sp-HYR-like domain DNA(C).
We have cloned and sequenced the gene encoding the species-specific egg bindin receptor, EBR1, from both S. franciscanus and S. purpuratus. These genes are novel ADAMTS-like proteins and share a long repetitive architecture with species-specific domains unique to S. franciscanus and S. purpuratus that are situated near the 3′ end. We have also demonstrated that the species-unique EBR1 repeats of S. franciscanus and S. purpuratus display species-specific adhesive activity by using a variety of different assays. The finding that recombinant EBR1 protein displays species-specific sperm adhesion and bindin-binding activity indicates that protein, but not carbohydrate, is important for determining the species specificity of sperm–egg adhesion. Based on the lack of an identifiable transmembrane domain and the similarity to ADAMTS, it is likely that EBR1 is an ECM protein, which is consistent with the fact that sperm bind to the VE, which represents the ECM of the egg (Glabe and Vacquier 1978; Hirohashi and Lennarz 1998). The repeated structure of the adhesive domain may be important for determining the strength of the adhesive interaction by providing multivalency. This correlates well with the structure of bindin, which has a complementary repeated structure (Minor et al. 1991).
EBR1 also has a variant ADAMTS domain at its 5′ end consisting of a signal peptide, propeptide domain, and zinc metalloprotease domain, but it lacks a disintigrin-like domain. Instead, an ADAM cysteine-rich (ACR) domain, which is typical of ADAM and snake venom proteases, as well as ADAMTS, immediately follows the zinc metalloprotease domain. The zinc metalloprotease domain contains a catalytic site consensus sequence identified in snake venom and matrix metalloproteinases (Bode et al. 1993). This suggests that EBR1 may have protease activity, but further study is required to determine whether this is the case. Although EBR1 lacks a disintrigin-like domain, other ADAMTS family members, such as ADAMTS1 and ADAMTS5, also lack disintigrin domains (Adams and Tucker 2000). Like other ADAMTS members, EBR1 has TSP-1 repeats after the ACR domain. The TSP-1 repeats are found in “matricellular” proteins (Bornstein 2001) and are believed to be involved in a variety of cellular processes, including cell and matrix adhesion, cell migration, and sulfated polysaccharide binding. TSP-1 repeats fold into a novel, antiparallel, three-stranded fold capped by disulfide bonds on each end (Tan et al. 2002). After the TSP-1 repeats of EBR1, there are a series of alternating CUB and TSP-1 repeats. Although CUB and TSP-1 domains occur together in other proteins, this is the first instance in which they have been found in a tandemly repeated fashion that we refer to as an EBR repeat. Both the CUB subdomain of the EBR repeat and the HYR domains are predicted to fold as immunoglobulin-like β-sandwich structures (Bork and Beckmann 1993; Callebaut et al. 2000). The CUB domain protein, spermadhesin, has been implicated in mammalian sperm adhesion by binding to the zona pellucida (Romero et al. 1997). The known adhesive function of both the TSP-1 and CUB domains in other proteins is consistent with their proposed role as specific receptors for bindin in sea urchin fertilization. In Sp-EBR1, the last 10 species-specific EBR repeats of Sf-EBR1 are replaced with 11 HYR repeats. Further functional dissection of the individual EBR1 domains may provide some insight into the roles that EBR1 plays in fertilization.
Material and methods
Animal collection
Sea urchins, S. franciscanus and S. purpuratus, were obtained from Marinus, Inc.
Generation of Sf-H2 polyclonal antibody
Polyclonal antibodies were produced in female rabbits (New Zealand white) by subcutaneous injection of purified Sf-H2 GST fusion protein. Anti-Sf-H2 antibodies were purified with protein A Sepharose column chromatography and then applied on a GST column to remove anti-GST antibodies.
Northern blot analysis
Northern blot analysis was performed with isolated S. franciscanus and S. purpuratus ovary mRNA probed with 32P-labeled Sf-H2, 5′region (TSP-1 repeats through the first TSP-CUB domains) of Sf-EBR1, and Sp-HYR domain DNA(Kamei et al. 2000).
Western blot analysis
VEs were prepared from both species of sea urchin eggs (Glabe and Vacquier 1977a; Hirohashi and Lennarz 1998) and separated by electrophoresis on 4%–20% SDS-PAGE gradient gels. Each sample was loaded at 2, 5, and 10 μg per lane and detected with 1:2500 dilution of anti-Sf-H2 polyclonal antiserum as the primary and 1:25,000 dilution of HRP conjugated rabbit F(ab′)2 antibody as the secondary antibody.
Inhibition of fertilization
The inhibition of fertilization assay was performed with S. franciscanus and S. purpuratus eggs fertilized with sperm of the same species. After a 10-min preincubation with each concentration of antibody or recombinant protein, samples were inseminated with a concentration of sperm adjusted to give ∼70%–80% fertilization in the absence of inhibitor. The fertilization reaction was stopped after 5 min by the dilution of the sperm with filtered seawater. The fertilized eggs were scored by counting with a stereo microscope (Minor et al. 1993).
Sperm–beads adhesion assay
The sperm-binding assay was performed as previously described (Stears and Lennarz 1997) with glutathione agarose beads loaded with Sf-H2–GST fusion protein. GST fusion protein alone was used as a control. The numbers of attached sperm to at least 30 beads were counted by observation with a microscope.
Egg agglutination assay
Egg agglutination assays were performed as previously described (Glabe and Vacquier 1977b; Glabe and Lennarz 1979) by using recombinant Sf-H2 protein as an inhibitor. Agglutination of both species of eggs by homologous bindin was performed with increasing concentrations (0–40 μg/mL) of recombinant Sf-H2 and a constant concentration of bindin, which gave at least 75% egg agglutination.
Receptor ligand binding using iodinated EBR1
Recombinant 125I-labeled Sf-H2 protein was iodinated by using Iodogen (Pierce) according to the manufacturer's instructions and was incubated with purified bindin particles for 10 min in filtered seawater-Tris (pH 7.5) at room temperature. The sample was layered on a 20% sucrose pad in a 0.4-mL Eppendorf tube and centrifuged at 15,000 × g for 10 min to separate the iodinated protein bound to bindin particles from the unbound protein. The pellet and supernatant fractions were counted by using a γ counter. The nonspecific background was determined by adding an excess of cold recombinant Sf-H2 protein.
Localization of receptor protein on the egg
For antibody staining, 2.5% paraformaldehyde/1% glutaraldehyde-fixed unfertilized and fertilized eggs were used. Fixed eggs were washed with Tris-buffered saline (TBS; pH 7.5) several times, and then blocked with 1% IgG free BSAin TBS. The samples were incubated with anti-Sf-H2 antiserum at a 1:10 dilution for 1 h, washed three times with 0.1% Tween-TBS (pH 7.5), and then placed in blocking solution a second time. The TRITC-labeled goat F(ab′)2 antirabbit F(ab′)2 antibody was treated at a 1:1000 dilution for 1 h and washed with 0.1% Tween-TBS (pH 7.5) thoroughly, and samples examined under the fluorescence microscope.
cDNA library screening and isolation of the complete cDNA
S. franciscanus cDNA libraries from sea urchin ovaries were constructed by using λ ZAP (Stratagene) with oligo dT primer or random primer as recommended by the manufacturer. The S. purpuratus library was kindly provided by Dr. Wessel (Brown University, Providence, RI). The library was screened by using 32P-labeled Sf-H2 or unique 5′ and 3′ DNA fragments as probes. The complete gene sequence of the cDNAs was obtained by cDNA primer walking and the determination of 5′ end of cDNA by the RACE (rapid amplification cDNA ends) analysis for both S. franciscanus and S. purpuratus. The open reading frame and domain structure was assembled and analyzed by using Lasergene analysis software (DNASTAR Inc; Burland 2000) and SMART search (Letunic et al. 2002).
Acknowledgments
We thank Dr. V.D. Vacquier for thoughtful discussions and advice, and Dr. G. Wessel for kindly providing the S. purpuratus ovary cDNA library. We also thank Dr. J. Claypool for experimental advice and discussions. We thank Young Min Lee and Chae Lee for assistance in screening of the cDNA libraries.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Article and publication are at http://www.genesdev.org/cgi/doi/10.1101/gad.1133003.
References
- Adams J.C. 1997. Thrombospondin-1. Int. J. Biochem. Cell Biol. 29: 861-865. [DOI] [PubMed] [Google Scholar]
- Adams J.C. and Tucker, R.P. 2000. The thrombospondin type 1 repeat (TSR) superfamily: Diverse proteins with related roles in neuronal development. Dev. Dyn. 218: 280-299. [DOI] [PubMed] [Google Scholar]
- Bode W., Gomis-Ruth, F.X., and Stockler, W. 1993. Astacins, serralysins, snake venom and matrix metalloproteinases exhibit identical zinc-binding environments (HEXXHXXGXXH and Met-turn) and topologies and should be grouped into a common family, the `metzincins.' FEBS. Lett. 331: 134-140. [DOI] [PubMed] [Google Scholar]
- Bork P. and Beckmann, G. 1993. The CUB domain: Awidespread module in developmentally regulated proteins. J. Mol. Biol. 231: 539-545. [DOI] [PubMed] [Google Scholar]
- Bornstein P. 2001. Thrombospondins as matricellular modulators of cell function. J. Clin. Invest. 107: 929-934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burland T.G. 2000. DNASTAR's Lasergene sequence analysis software. Methods Mol. Biol. 132: 71-91. [DOI] [PubMed] [Google Scholar]
- Callebaut I., Gilges, D., Vigon, I., and Mornon, J.P. 2000. HYR, an extracellular module involved in cellular adhesion and related to the immunoglobulin-like fold. Protein Sci. 9: 1382-1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll E.J.J., Acevedo-Duncan, M., Justice, R.W., and Santiago, L. 1986. Structure, assembly and function of the surface envelope (fertilization envelope) from eggs of the sea urchin, Strongylocentrotus purpuratus. Adv. Exp. Med. Biol. 207: 261-291. [DOI] [PubMed] [Google Scholar]
- Glabe C.G. and Lennarz, W.J. 1979. Species-specific sperm adhesion in sea urchins: Aquantitative investigation of bindin-mediated egg agglutination. J. Cell. Biol. 83: 595-604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glabe C.G. and Lennarz, W.J. 1981. Isolation of a high-molecular-weight glycoconjugate derived from the surface of S. purpuratus eggs that is implicated in sperm adhesion. J. Supra. Struct. Cell. Biochem. 15: 387-394. [DOI] [PubMed] [Google Scholar]
- Glabe C.G. and Vacquier, V.D. 1977a. Isolation and characterization of the vitelline layer of sea urchin eggs. J. Cell. Biol. 75: 410-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glabe C.G. and Vacquier, V.D. 1977b. Species specific agglutination of eggs by bindin isolated from sea urchin sperm. Nature 267: 836-838. [DOI] [PubMed] [Google Scholar]
- Glabe C.G. and Vacquier, V.D. 1978. Egg surface glycoprotein receptor for sea urchin sperm bindin. Proc. Natl. Acad. Sci. 75: 881-885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glabe C.G., Lennarz, W.J., and Vacquier, V.D. 1982. Sperm surface components involved in sea urchin fertilization. Alan R. Liss, New York. [DOI] [PubMed]
- He Z.Y., Brakebusch, C., Fassler, R., Kreidberg, J.A., Primakoff, P., and Myles, D.G. 2003. None of the integrins known to be present on the mouse egg or to be ADAM receptors are essential for sperm–egg binding and fusion. Dev. Biol. 254: 226-237. [DOI] [PubMed] [Google Scholar]
- Hirohashi N. and Lennarz, W.J. 1998. The 350-kDa sea urchin egg receptor for sperm is localized in the vitelline layer. Dev. Biol. 204: 305-315. [DOI] [PubMed] [Google Scholar]
- Hubank M. and Schatz, D.G. 1994. Identifying differences in mRNA expression by representational difference analysis of cDNA. Nucleic Acids Res. 22: 5640-5648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamei N., Swanson, W.J., and Glabe, C.G. 2000. Arapidly diverging EGF protein regulates species-specific signal transduction in early sea urchin development. Dev. Biol. 225: 267-276. [DOI] [PubMed] [Google Scholar]
- Kuno K. and Matsushima, K. 1998. ADAMTS-1 protein anchors at the extracellular matrix through the thrombospondin type I motifs and its spacing region. J. Biol. Chem. 273: 13912-13917. [DOI] [PubMed] [Google Scholar]
- Letunic I., Goodstadt, L., Dickens, N.J., Doerks, T., Schultz, J., Mott, R., Ciccarelli, F., Copley, R.R., Ponting, C.P., and Bork, P. 2002. Recent improvements to the SMART domain-based sequence annotation resource. Nucleic Acids Res. 30: 242-244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minor J.E., Fromson, D.R., Britten, R.J., and Davidson, E.H. 1991. Comparison of the bindin proteins of Strongylocentrotus franciscanus, S. purpuratus, and Lytechinus variegatus: Sequences involved in the species specificity of fertilization. Mol. Biol. Evol. 8: 781-795. [DOI] [PubMed] [Google Scholar]
- Minor J.E., Britten, R.J., and Davidson, E.H. 1993. Species-specific inhibition of fertilization by a peptide derived from the sperm protein bindin. Mol. Biol. Cell. 4: 375-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlendieck K., Dhume, S.T., Partin, J.S., and Lennarz, W.J. 1993. The sea urchin egg receptor for sperm: Isolation and characterization of the intact, biologically active receptor. J. Cell Biol. 122: 887-895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Primakoff P. and Myles, D.G. 2002. Penetration, adhesion, and fusion in mammalian sperm–egg interaction. Science 296: 2183-2185. [DOI] [PubMed] [Google Scholar]
- Romero A., Romao, M.J., Varela, P.F., Kolln, I., Dias, J.M., Carvalho, A.L., Sanz, L., Topfer-Petersen, E., and Calvete, J.J. 1997. The crystal structures of two sperm adhesins reveal the CUB domain fold. Nat. Struct. Biol. 4: 783-788. [DOI] [PubMed] [Google Scholar]
- Ruiz-Bravo N. and Lennarz, W.J. 1986. Isolation and characterization of proteolytic fragments of the sea urchin sperm receptor that retain species specificity. Dev. Biol. 118: 202-208. [DOI] [PubMed] [Google Scholar]
- Ruiz-Bravo N., Earles, D., and Lennarz, W.J. 1986. Identification and partial characterization of sperm receptor associated with the newly formed fertilization envelope from sea urchin eggs. Dev. Biol. 117: 204-208. [DOI] [PubMed] [Google Scholar]
- Stears R.L. and Lennarz, W.J. 1997. Mapping sperm binding domains on the sea urchin egg receptor for sperm. Dev. Biol. 187: 200-208. [DOI] [PubMed] [Google Scholar]
- Summers R.G. and Hylander, B.L. 1975. Species-specificity of acrosome reaction and primary gamete binding in echinoids. Exp. Cell Res. 96: 63-68. [DOI] [PubMed] [Google Scholar]
- Swanson W.J. and Vacquier, V.D. 1997. The abalone egg vitelline envelope receptor for sperm lysin is a giant multivalent molecule. Proc. Natl. Acad. Sci. 94: 6724-6729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan K., Duquette, M., Liu, J.H., Dong, Y., Zhang, R., Joachimiak, A., Lawler, J., and Wang, J.H. 2002. Crystal structure of the TSP-1 type 1 repeats: Anovel layered fold and its biological implication. J. Cell Biol. 159: 373-382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang B.L. 2001. ADAMTS: A novel family of extracellular matrix proteases. Int. J. Biochem. Cell Biol. 33: 33-44. [DOI] [PubMed] [Google Scholar]
- Tang B. and Hong, W. 1999. ADAMTS: A novel family of proteases with an ADAM protease domain and thrombospondin 1 repeats. FEBS Lett. 445: 223-225. [DOI] [PubMed] [Google Scholar]
- Vacquier V.D. and Moy, G.W. 1977. Isolation of bindin: The protein responsible for adhesion of sperm to sea urchin eggs. Proc. Natl. Acad. Sci. 74: 2456-2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vacquier V.D., Tegner, M.J., and Epel, D. 1973. Protease released from sea urchin eggs at fertilization alters the vitelline layer and aids in preventing polyspermy. Exp. Cell Res. 80: 111-119. [DOI] [PubMed] [Google Scholar]
- Wessel G.M., Berg, L., Adelson, D.L., Cannon, G., and McClay, D.R. 1998. Amolecular analysis of hyalin: Asubstrate for cell adhesion in the hyaline layer of the sea urchin embryo. Dev. Biol. 193: 115-126. [DOI] [PubMed] [Google Scholar]
- Yanagimachi R. 1981. Mechanisms of fertilization in mammals. In Fertilization and embryonic development in vitro (ed. L.M.a.J.D. Biggers), pp. 81-182. Plenum Press, New York.
- Yanagimachi R. 1994. Mammalian fertilization. In The physiology of Reproduction, 2nd ed. (ed. E.K. Neill), pp. 189-317. Raven Press, New York.